Juglans regia as Urban Trees: Genetic Diversity and Walnut Kernel Quality Assessment
Abstract
1. Introduction
2. Materials and Methods
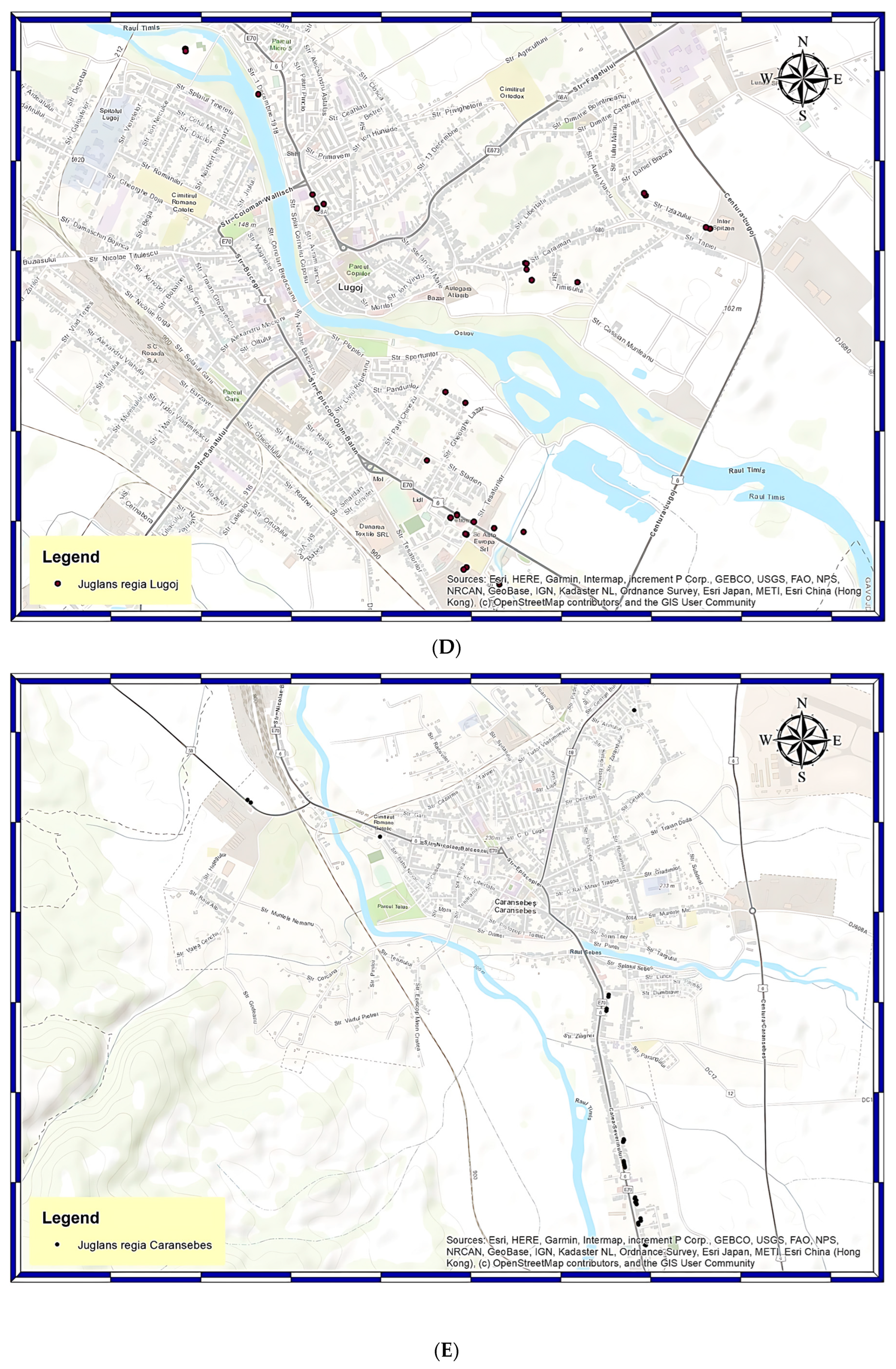
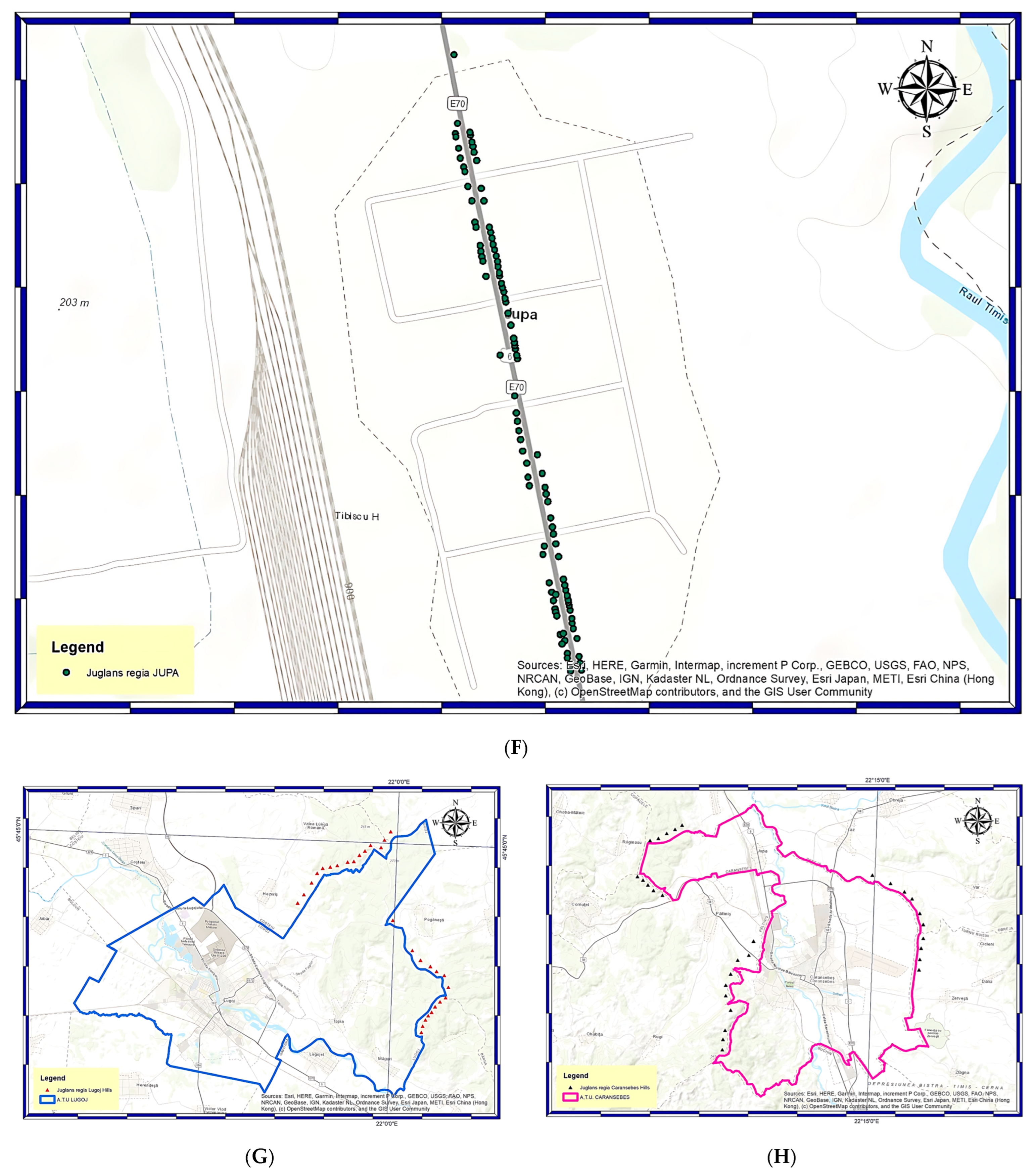
2.1. Site Description and Data Collection
2.2. Geographical Information System (G.I.S.) and Global Navigation Satellite System (GNSS) Technologies
2.3. Dendrometric Measurements
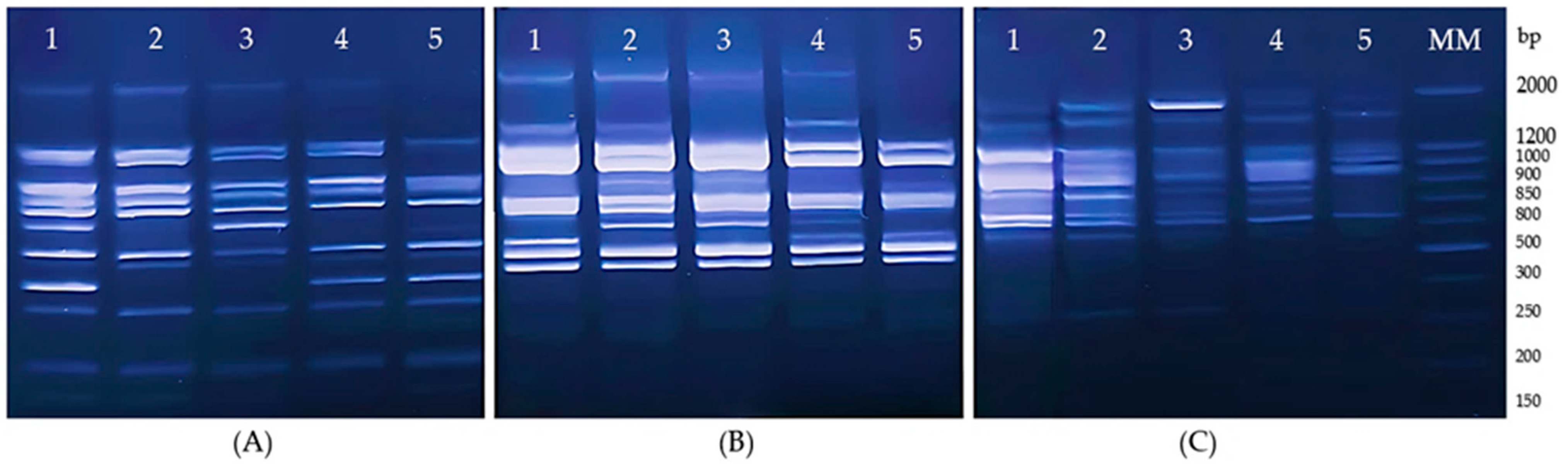
2.4. Molecular Analysis of Genetic Diversity
2.5. Determination of Kernel Chemical Composition
2.5.1. Determination of the Content of Mineral Substances Was Made According to SREN ISO 749:1999
2.5.2. Protein Determination Using the Kjeldahl Method Was Made According to SREN ISO 20483:2014
2.5.3. Determination of Total Lipids Was Conducted According to SREN ISO 659:2009
2.5.4. Determination of Total Polyphenols Was Conducted Using the Folin–Ciocalteu Method
2.5.5. Determination of Microelements and Heavy Metals
2.6. Statistical Analysis
3. Results
3.1. Statistical Analysis of J. regia Dendrometric Indices
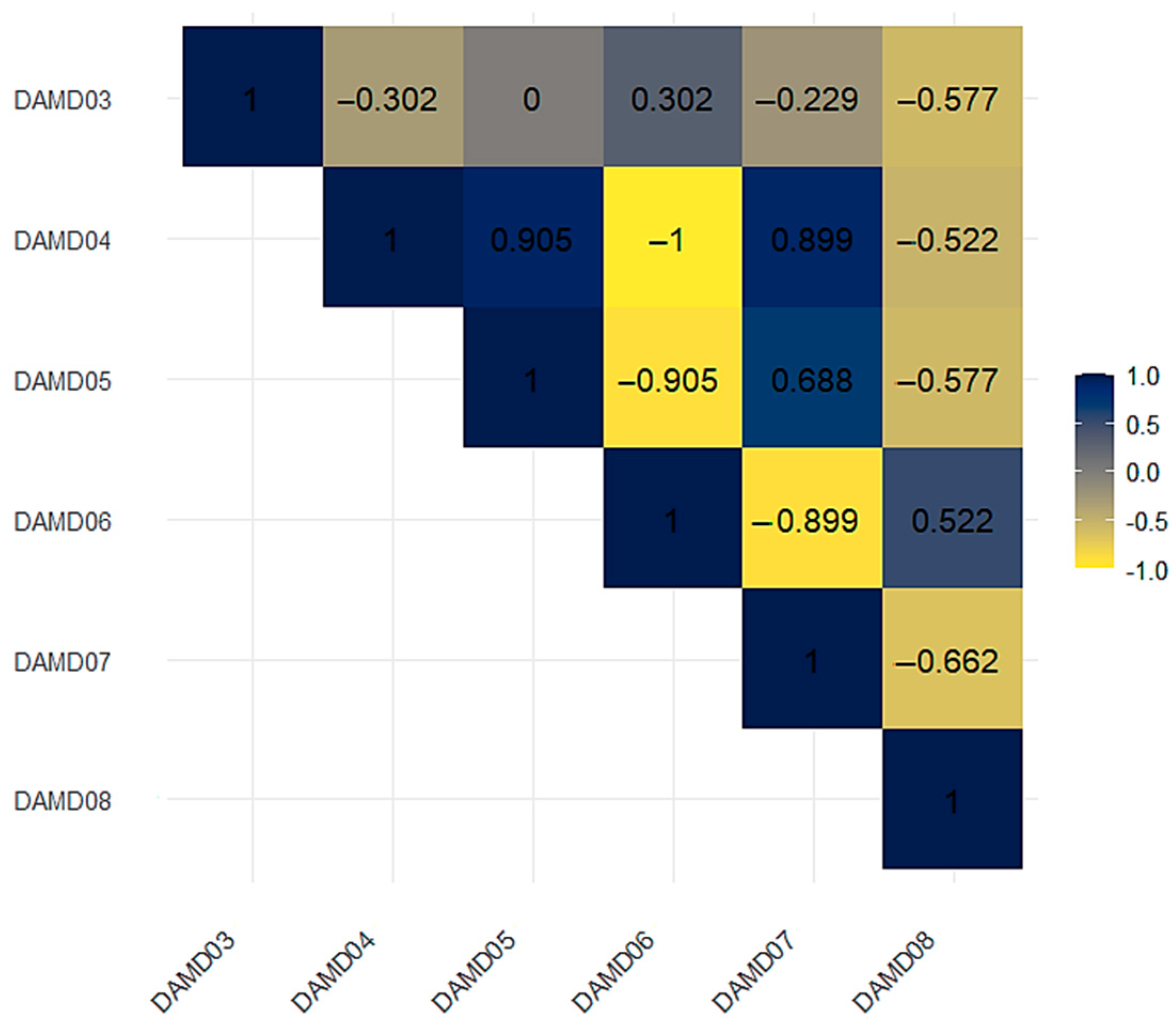
3.2. Results of the Genetic Variability of J. regia Using DAMD and SCoT Markers
3.2.1. Assessment of Genetic Diversity of J. regia Population Using DAMD Primers
3.2.2. Assessment of Genetic Diversity of J. regia Population Using SCoT Primers
3.2.3. Cluster Analysis of J. regia Populations Using DAMD and SCoT Primers
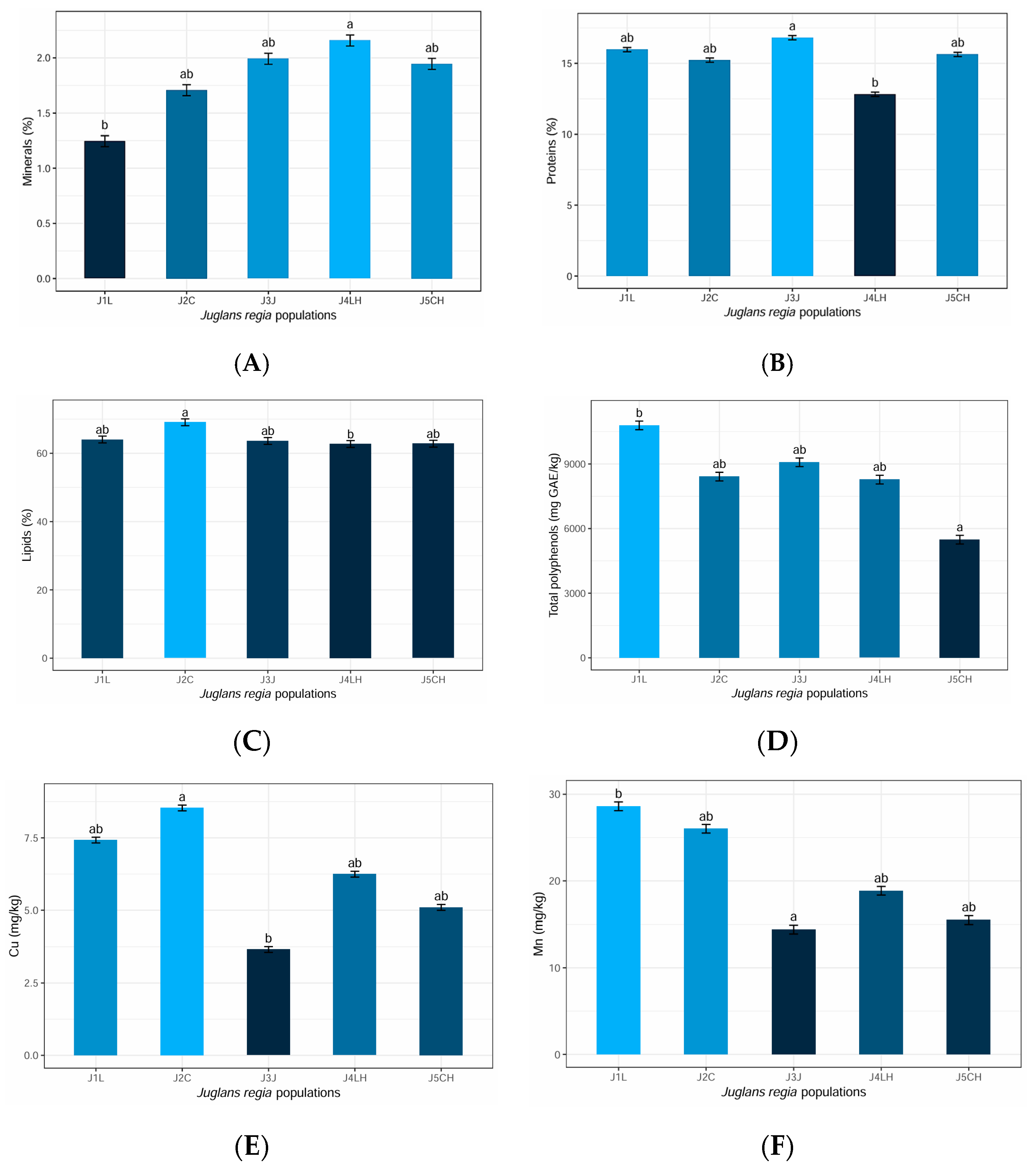
3.3. Statistical Analyses Made on Chemical Parameters of Walnut Kernels
3.3.1. Descriptive Statistics of Chemical Parameters
- the kernels of population J1L contain the following averages and standard deviations:
- -
- The average mineral content is 1.24461% with a standard deviation of 0.16085%.
- -
- The mean protein content is 15.97514% with a standard deviation of 0.23905%.
- -
- The mean lipid content is 64.02981% with a standard deviation of 0.01913%.
- -
- The mean of total polyphenols is 10788.4 (mg G.A.E./kg) with a standard deviation of 0 (mg G.A.E./kg);
- -
- The average Cu content is 7.423 (mg/kg) with a standard deviation of 0.018 (mg/kg);
- -
- The average Mn content is 28.618 (mg/kg) with a standard deviation of 0.021(mg/kg);
- -
- The average Zn content is 19.813 (mg/kg) with a standard deviation of 0.017(mg/kg);
- the kernels of population J2C contain the following averages and standard deviations:
- -
- The average mineral content is 1.70758% with a standard deviation of 0.19267%.
- -
- The mean protein content is 15.23205% with a standard deviation of 0.17361%.
- -
- The mean lipid content is 69.08254% with a standard deviation of 0.22905%.
- -
- The mean of total polyphenols is 8412.45 (mg G.A.E./kg) with a standard deviation of 6.639 (mg G.A.E./kg);
- -
- The average Cu content is 8.532 (mg/kg) with a standard deviation of 0.0208 (mg/kg);
- -
- The average Mn content is 26.024 (mg/kg) with a standard deviation of 0.020 (mg/kg);
- -
- The average Zn content is 46.583 (mg/kg) with a standard deviation of 0.020 (mg/kg);
- the kernels of population J3J contain the following averages and standard deviations:
- -
- The average mineral content is 1.99238% with a standard deviation of 0.21141%.
- -
- The mean protein content is 16.80667% with a standard deviation of 0.13813%.
- -
- The mean lipid content is 63.60393% with a standard deviation of 0.25422%.
- -
- The mean of total polyphenols is 9075.42 (mg G.A.E./kg) with a standard deviation of 5.75 (mg G.A.E./kg);
- -
- The average Cu content is 3.655 (mg/kg) with a standard deviation of 0.019 (mg/kg);
- -
- The average Mn content is 14.408 (mg/kg) with a standard deviation of 0.019 (mg/kg);
- -
- The average Zn content is 20.474 (mg/kg) with a standard deviation of 0.019 (mg/kg);
- -
- The average Pb content is 2.27 (mg/kg) with a standard deviation of 0.018 (mg/kg);
- -
- The average Cd content is 0.034 (mg/kg) with a standard deviation of 0.00207 (mg/kg);
- the kernels of population J4LH contain the following averages and standard deviations:
- -
- The average mineral content is 2.15819% with a standard deviation of 0.13958%.
- -
- The mean protein content is 12.81847% with a standard deviation of 0.15009%.
- -
- The mean lipid content is 62.71121% with a standard deviation of 0.15463%.
- -
- The mean of total polyphenols is 8272.57 (mg G.A.E./kg) with a standard deviation of 3.319 (mg G.A.E./kg);
- -
- The average Cu content is 6.245 (mg/kg) with a standard deviation of 0.018 (mg/kg);
- -
- The average Mn content is 18.867 (mg/kg) with a standard deviation of 0.019 (mg/kg);
- -
- The average Zn content is 20.836 (mg/kg) with a standard deviation of 0.021 (mg/kg);
- -
- The average Pb content is 1.661 (mg/kg) with a standard deviation of 0.021 (mg/kg).
- -
- The average Cd content is 0.08065 (mg/kg) with a standard deviation of 0.01152 (mg/kg).
- the kernels of population J5CH contain the following averages and standard deviations:
- -
- The average mineral content is 1.94603% with a standard deviation of 0.19136%.
- -
- The mean protein content is 15.63188% with a standard deviation of 0.25873%.
- -
- The mean lipid content is 62.81324% with a standard deviation of 0.14332%.
- -
- The mean of total polyphenols is 5484.66 (mg G.A.E./kg) with a standard deviation of 3.319 (mg G.A.E./kg);
- -
- The average Cu content is 5.098 (mg/kg) with a standard deviation of 0.017 (mg/kg);
- -
- The average Mn content is 15.496 (mg/kg) with a standard deviation of 0.018 (mg/kg);
- -
- The average Zn content is 21.547 (mg/kg) with a standard deviation of 0.022 (mg/kg);
- -
- The average Pb content is 1.204 (mg/kg) with a standard deviation of 0.019 (mg/kg);
- -
- The average Cd content is (mg/kg) with a standard deviation of (mg/kg);
3.3.2. Comparisons between Chemical Parameters
Comparisons between the Average Mineral Contents
Comparisons between the Means of Protein Content
Comparisons between the Means of Lipid Content
Comparisons between the Means of Total Polyphenols
Comparisons between the Means of Trace Elements and Heavy Metals
Comparisons between the Average Cu Contents
Comparisons between the Average Mn Contents
Comparisons between the Averages of Zn Content
Comparisons between the Averages of Pb Content
Comparisons between the Averages of Cd Content
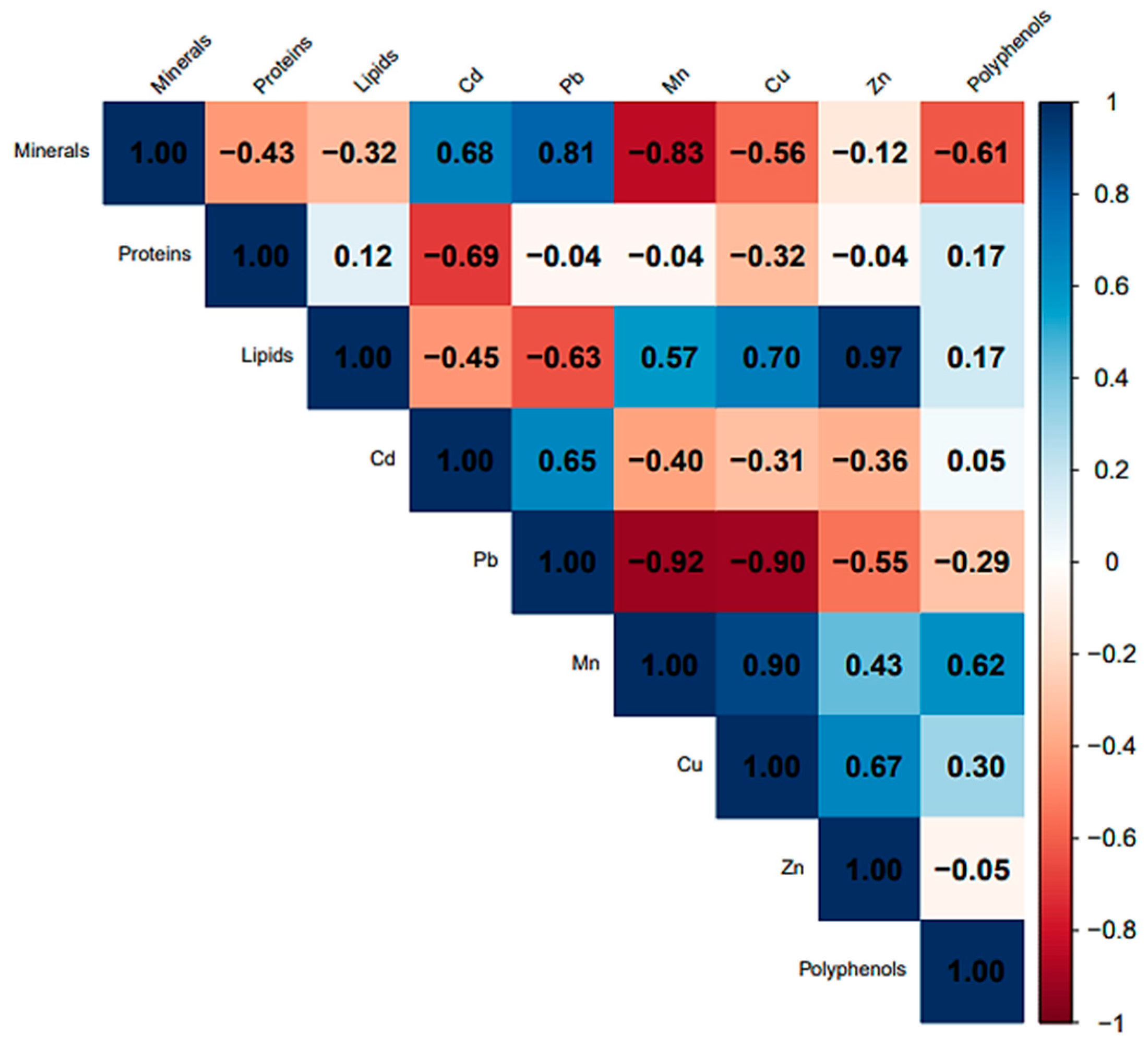
3.3.3. Correlations and Linear Regressions between Chemical Parameters
- -
- There are >0.8 statistically significant high positive correlations between the following:
- -
- minerals and Pb r (3) = 0.81, p < 0.05;
- -
- Zn and lipids (r (3) = 0.97, p = 0.0072 < 0.05, Zn = −250 + 4.2 lipids) (Figure 10A);
- -
- Cu and Mn (r (3) = 0.90, p = 0.036 < 0.05, Cu = 0.57 + 0.27 Mn) (Figure 10B).
- -
- There are <−0.8 statistically significant high negative correlations between the following:
- -
- minerals and Mn r (3) = −0.83, p < 0.05;
- -
- Pb and Mn (r (3) = −0.92, p = 0.029 < 0.05, Pb = 4 − 0.15 Mn) (Figure 10C);
- -
- Pb and Cu (r (3) = −0.90, p = 0.036 < 0.05, Pb = 4 − 0.48 Cu) (Figure 10D).
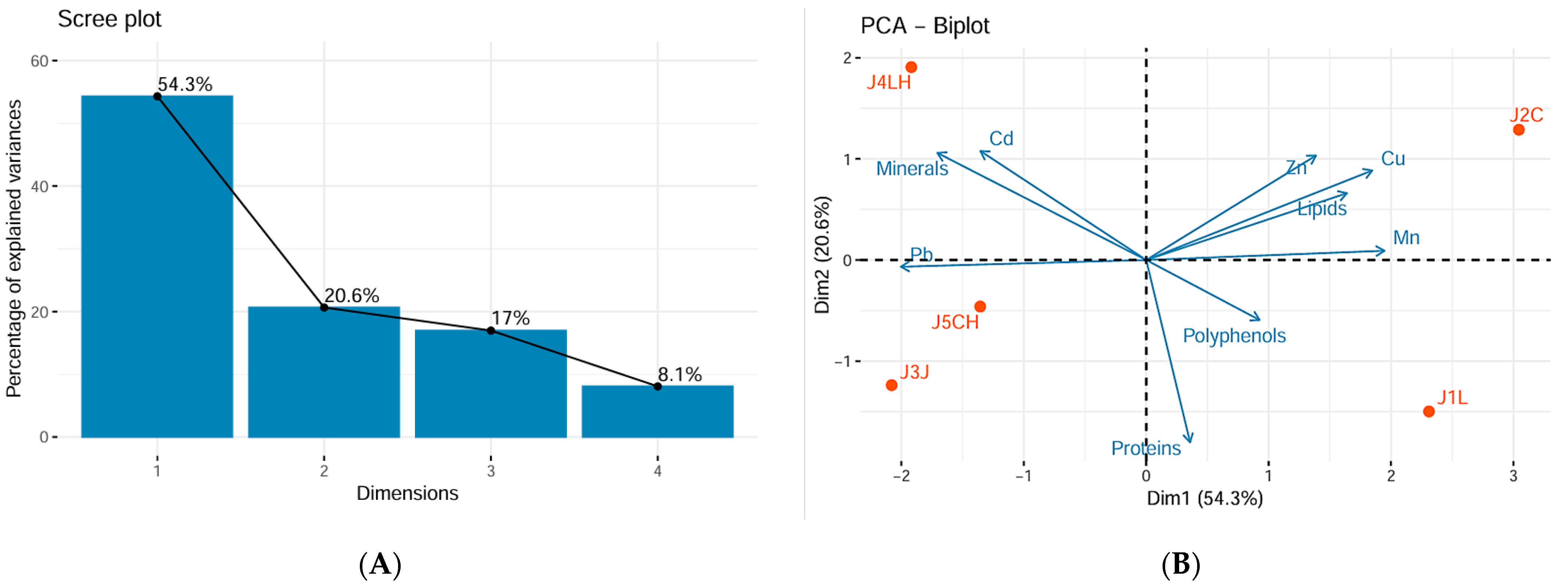
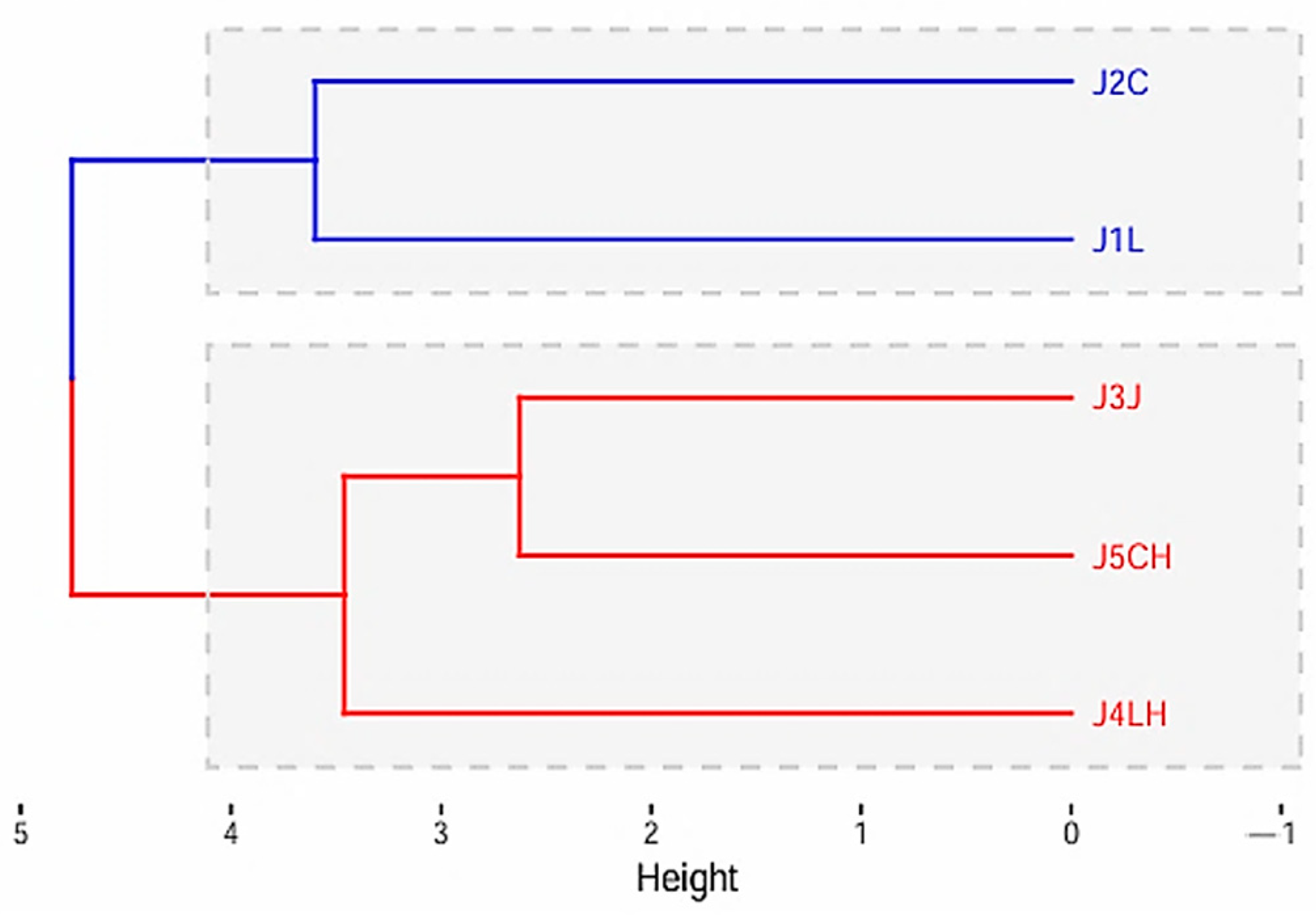
3.3.4. Principal Component Analysis and Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaşlı, R.; Yücedağ, C.; Ayan, S.; Simovski, B. The Role of Urban Trees in Reducing Land Surface Temperature. SilvaWorld. 2023. Available online: https://repository.ukim.mk/handle/20.500.12188/26314 (accessed on 5 September 2024).
- World Health Organization Regional Office for Europe. Urban Green Spaces and Health; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2016. [Google Scholar]
- Dangl, G.S.; Woeste, K.; Aradhya, M.K.; Koehmstedt, A.; Simon, C.; Potter, D.; Leslie, C.A.; McGranahan, G. Characterization of 14 Microsatellite Markers for Genetic Analysis and Cultivar Identification of Walnut. J. Am. Soc. Hortic. Sci. 2005, 2005, 348–354. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, I.; Woeste, K.; Fiaz, S.; Zeb, U.; Ghazy, A.I.; Azizullah, A.; Shad, S.; Malvolti, M.E.; Yue, M.; et al. Population genetics informs new insights into the phytogeographic history of Juglans regia L. Genet. Resour. Crop Evol. 2023, 70, 2263–2278. [Google Scholar] [CrossRef]
- FAO. Statistical Yearbook 2023-World Food and Agriculture-World|ReliefWeb. Available online: https://reliefweb.int/report/world/fao-statistical-yearbook-2023-world-food-and-agriculture (accessed on 6 September 2024).
- Italy, F.R. Crop and Livestock Products; Food and Agricultural Organisation of United Nations, Public FAO: Rome, Italy, 2023. [Google Scholar]
- Global Report on Food Crises (GRFC) 2024. Available online: https://www.fsinplatform.org/report/global-report-food-crises-2024/ (accessed on 10 August 2024).
- Homepage|Food Security Information Network (FSIN). Available online: https://www.fsinplatform.org/ (accessed on 18 September 2024).
- Employment Indicators (2000–2021). 2023. Statistics. Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/employment-indicators-(2000-2021).-july-2023-update/en (accessed on 10 August 2024).
- Ye, L.; Shavvon, R.S.; Qi, H.; Wu, H.; Fan, P.; Shalizi, M.N.; Khurram, S.; Davletbek, M.; Turuspekov, Y.; Liu, J. Population genetic insights into the conservation of common walnut (Juglans regia) in Central Asia. Plant Divers. 2024, 46, 600–610. [Google Scholar] [CrossRef]
- Shavvon, R.S.; Qi, H.-L.; Mafakheri, M.; Fan, P.-Z.; Wu, H.-Y.; Vahdati, F.B.; Al-Shmgani, H.S.; Wang, Y.-H.; Liu, J. Unravelling the genetic diversity and population structure of common walnut in the Iranian Plateau. BMC Plant Biol. 2023, 23, 201. [Google Scholar] [CrossRef]
- Iordănescu, O.A.; Radulov, I.; Buhan, I.P.; Cocan, I.; Berbecea, A.A.; Popescu, I.; Poșta, D.S.; Camen, D.; Lalescu, D. Physical, Nutritional and Functional Properties of Walnuts Genotypes (Juglans regia L.) from Romania. Agronomy 2021, 11, 1092. [Google Scholar] [CrossRef]
- Popa, R.-G.; Bălăcescu, A.; Popescu, L.G. Popescu, Organic Walnut Cultivation in Intensive and Super-Intensive System—Sustainable Investment. Case Study: Gorj County, Romania. Sustainability 2023, 15, 1244. [Google Scholar] [CrossRef]
- Pascota, C.; Tenche-Constantinescu, A.M.; Mircov, V.D.; Borlea, G.F. The walnut-a potential social broadleaved tree in western Romania. In Proceedings of the 3rd International Multidisciplinary Scientific Conference on Social Sciences and Arts SGEM 2016, Albena, Bulgaria, 24–30 August 2016; pp. 1155–1160. [Google Scholar]
- Pataki, D.E.; Alberti, M.; Cadenasso, M.L.; Felson, A.J.; McDonnell, M.J.; Pincetl, S.; Pouyat, R.V.; Setälä, H.; Whitlow, T.H. The Benefits and Limits of Urban Tree Planting for Environmental and Human Health. Front. Ecol. 2021, 9, 603757. [Google Scholar] [CrossRef]
- Jones, L.; Anderson, S.; Læssøe, J.; Banzhaf, E.; Jensen, A.; Bird, D.N.; Miller, J.; Hutchins, M.G.; Yang, J.; Garrett, J.; et al. A typology for urban Green Infrastructure to guide multifunctional planning of nature-based solutions. Nat. Based Solut. 2022, 2, 100041. [Google Scholar] [CrossRef]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.). Cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021, 35, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, Q.; Ni, Z.; Han, Y.; Zheng, Y.; Mo, R.; Shen, D.; Liu, Y. The Nutritional Qualities of Walnuts and Their Planted Soils from China—Level and Relationship. Forests 2023, 14, 1369. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Baciu, A.; Achim, G.; Mihai, B.O.T.U.; Trandafir, I. Mineral composition of fruits in different walnut (Juglans regia L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 156–160. [Google Scholar]
- Ioan, S.; Irina, P.; Emilian, O.; Sorina, P.; Cerasela, P.; Adriana, C.; Dorin, C.; Alina-Maria, T.-C.; Dacian, L.; Ciprian, S.; et al. Application of the Drosophila melanogaster Research Model to Evaluate the Toxicity Levels between Lead and Copper. Appl. Sci. 2024, 14, 4190. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; et al. Scientific opinion on the tolerable upper intake level for manganese. EFSA J. 2023, 21, e8413. [Google Scholar]
- Ioan, S.; Irina, P.; Dumitru, C.D.; Emilian, O.; Ramona, M.A.; Mariana, G. Analyzes Regarding the Cytotoxicity of ZnSO4 Excess on Cell Division. Pharmacophore 2023, 14, 7–14. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Shakeel, M.; Hafeez, F.; Malik, I.; Farid, A.; Ullah, H.; Ahmed, I.; Gul, H.; Mohibullah, M.; Yasin, M. Serratia marcescens strain FA-4 enhances zinc content in rice grains by activating the zinc translocating enzymes. J. Breed. Genet. 2023, 52, 495–507. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J. Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef]
- Zerizghi, T.; Guo, Q.; Tian, L.; Wei, R.; Zhao, C. An integrated approach to quantify ecological and human health risks of soil heavy metal contamination around coal mining area. Sci. Total Environ. 2021, 814, 152653. [Google Scholar] [CrossRef] [PubMed]
- GTóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L.J.E.I. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar]
- Yang, Q.; Zhang, L.; Wang, H.; Martín, J.D. Bioavailability and health risk of toxic heavy metals (As, Hg, Pb and Cd) in urban soils: A Monte Carlo simulation approach. Environ. Res. 2022, 214, 113772. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wang, Z.; Yan, X.; Yu, Y.; Liu, L.; Gao, Y.; Zhang, H.; Yang, X. Characteristics and pollution risks of Cu, Ni, Cd, Pb, Hg and As in farmland soil near coal mines. Soil Environ. Health 2023, 1, 100035. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Tian, B.; Li, J.; Luo, J.; Wang, X.; Ai, S.; Wang, X. Assessment of soil heavy metal pollution in provinces of China based on different soil types: From normalization to soil quality criteria and ecological risk assessment. J. Hazard. Mater. 2023, 441, 129891. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). 2006, Volume 364. Available online: http://data.europa.eu/eli/reg/2006/1881/oj/eng (accessed on 10 August 2024).
- Ikhsan, A.S.; Topçu, H.; Sütyemez, M.; Kafkas, S. Novel 307 Polymorphic SSR Markers from BAC-End Sequences in Walnut (Juglans regia L.): Effects of Motif Types and Repeat Lengths on Polymorphism and Genetic Diversity. Sci. Hortic. 2016, 213, 1–4. Available online: https://www.academia.edu/117172679/Novel_307_polymorphic_SSR_markers_from_BAC_end_sequences_in_walnut_Juglans_regia_L_Effects_of_motif_types_and_repeat_lengths_on_polymorphism_and_genetic_diversity (accessed on 6 September 2024). [CrossRef]
- Yildiz, E.; Pinar, H.; Uzun, A.; Yaman, M.; Sumbul, A.; Ercisli, S. Identification of genetic diversity among Juglans regia L. genotypes using molecular, morphological, and fatty acid data. Genet. Resour. Crop Evol. 2021, 68, 1425–1437. [Google Scholar] [CrossRef]
- EOrhan, E.; Eyduran, S.P.; Poljuha, D.; Akin, M.; Weber, T.; Ercisli, S. Genetic diversity detection of seed-propagated walnut (Juglans regia L.) germplasm from Eastern Anatolia using SSR markers. Folia Hortic. 2020, 32, 37–46. [Google Scholar] [CrossRef]
- Houmanat, K.; Abdellah, K.; Hssaini, L.; Razouk, R.; Hanine, H.; Jaafary, S.; Charafi, J. Molecular Diversity of Walnut (Juglans regia L.) among Two Major Areas in Morocco in Contrast with Foreign Varieties. Int. J. Fruit Sci. 2021, 21, 180–192. [Google Scholar] [CrossRef]
- Ghanbari, A.; Faraji, M.; Behnamian, M.; Estaji, A.; Pyrayesh, A.; Fahim, S. Genetic Diversity Evaluation of Some Walnut (Juglans regia L.) Genotypes in Meshkin-Shahr by ISSR Marker. J. Nuts 2019, 2, 1. [Google Scholar] [CrossRef]
- Tenche-Constantinescu, A.-M.; Lalescu, D.V.; Popescu, S.; Sarac, I.; Petrescu, I.; Petolescu, C.; Camen, D.; Horablaga, A.; Popescu, C.A.; Berar, C.; et al. Exploring the Genetic Landscape of Tilia Spp. with Molecular and Statistical Tools. Horticulturae 2024, 10, 596. [Google Scholar] [CrossRef]
- Zare-Rashnodi, N.; Erfani Moghadam, J.; Fazeli, A. The screening of Persian walnut genotypes based on the quantitative and qualitative characters and the investigation of genetic diversity among promising samples using ISSR marker. J. Plant Prod. (Agron. Breed. Hortic.) 2019, 42, 279–294. [Google Scholar]
- Tabasi, M.; Sheidai, M.; Hassani, D.; Koohdar, F. DNA fingerprinting and genetic diversity analysis with SCoT markers of Persian walnut populations (Juglans regia L.) in Iran. Genet. Resour. Crop Evol. 2020, 67, 1437–1447. [Google Scholar] [CrossRef]
- Rai, M.K. Start codon targeted (SCoT) polymorphism marker in plant genome analysis: Current status and prospects. Planta 2023, 257, 34. [Google Scholar] [CrossRef] [PubMed]
- Bidyananda, N.; Jamir, I.; Nowakowska, K.; Varte, V.; Vendrame, W.A.; Devi, R.S.; Nongdam, P. Plant Genetic Diversity Studies: Insights from DNA Marker Analyses. Int. J. Plant Biol. 2024, 15, 607–640. [Google Scholar] [CrossRef]
- Heath, D.D.; Lwama, G.K.; Devlin, R.H. PCR primed with VNTR core sequences yields species specific patterns and hypervariable probes. Nucleic Acids Res. 1993, 21, 5782–5785. [Google Scholar] [CrossRef]
- Ibrahimi, M.; Brhadda, N.; Ziri, R.; Fokar, M.; Amghar, I.; Gaboun, F.; Habach, A.; Meziani, R.; Elfadile, J.; Abdelwahd, R.; et al. Molecular Identification of Genetic Diversity and Population Structure in Moroccan Male Date Palm (Phoenix dactylifera L.) Using Inter-Simple Sequence Repeat, Direct Amplification of Minisatellite DNA, and Simple Sequence Repeat Markers. Horticulturae 2024, 10, 508. [Google Scholar] [CrossRef]
- Pinar, H.; Uzun, A.; Unlu, M.; Yaman, M. Genetic diversity in turkish banana (Musa cavendishii) genotypes with DAMD markers. Fresenius Environ. Bull. 2019, 28, 459–463. [Google Scholar]
- Zeb, U.; Dong, W.; Zhang, T.; Wang, R.; Shahzad, K.; Ma, X.; Li, Z. Comparative plastid genomics of Pinus species: Insights into sequence variations and phylogenetic relationships. J. Syst. Evol. 2020, 58, 118–132. [Google Scholar] [CrossRef]
- Law No. 24 of 15 January 2007 on the Regulation and Administration of Green Spaces in Urban Areas. FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC197145/ (accessed on 10 August 2024).
- Bihuneti, C. and Iordănescu, O.A. Evolution of walnut production worldwide. Res. J. Agric. Sci. 2023, 55. [Google Scholar]
- Li, X.; Yue, J.; Wang, S.; Luo, Y.; Su, C.; Zhou, J.; Xu, D.; Lu, H. Development of Geographic Information System Architecture Feature Analysis and Evolution Trend Research. Sustainability 2024, 16, 137. [Google Scholar] [CrossRef]
- Badaluta-Minda, C.; Herbei, M.V. Assesment of water resources using landsat satellite imagery. Environ. Eng. Manag. J. 2021, 20, 301–308. [Google Scholar]
- Repetskaya, A.I.; Petlukova, K.A.; Tabunshchik, V.A.; Vishnevski, S.O.; Savushkina, I.G. October. Application of the Field-Map software and hardware complex for creating GIS of urban green spaces and Botanical gardens collections. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 574, p. 012069. [Google Scholar]
- Jucu, I.S. Particularities of the functional profile and urban relations in post-socialist Lugoj Municipality. Rom. J. Geogr. 2010, 54, 39–49. [Google Scholar]
- Onea, D. Planificare urbană și organizarea spațiului geografic în Municipiul Caransebeș. Edit. Eurobit. Timișoara 2018, 322. ISBN 978-973-132-461-6. Available online: https://edituraeurobit.ro/2018-2/ (accessed on 25 September 2024).
- Ţenche-Constantinescu, A.M. Caransebeșul verde Concepție peisagistică și strategie de dezvoltare a sistemului de spații verzi. Edit. Eurobit. Timișoara 2017, 380. ISBN 978-973-132-408-1. Available online: https://edituraeurobit.ro/2017-2/ (accessed on 26 September 2024).
- Local Register of Green Spaces, Caransebeș Municipality. 2023. Available online: https://www.primaria-caransebes.ro/portal/CarasSeverin/Caransebes/portal.nsf/Index/100 (accessed on 25 September 2024).
- Census Results 2021: National Institute of Statistics, România, June 2023. Available online: https://ugeo.urbistat.com/AdminStat/en/ro/demografia/popolazione/municipiul-caransebes/20658433/4. (accessed on 25 September 2024).
- Rusu, R. Organizarea spaţiului geografic în Banat. Edit. Mirton Timişoara 2007, 607. ISBN 978-973-52-0201-9913(498.5). Available online: https://books.google.it/books/about/Organizarea_spa%C5%A3iului_geografic_%C3%AEn_Ban.html?id=yUbT_4Dn46QC&redir_esc=y (accessed on 26 September 2024).
- Tenche-Constantinescu, A.M.; Herbei, M.; Borlea, G.F. Restoration of Jupa Park-Landscape and Wood Species Analysis Using Gis Technology. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2018, 47, 428–437. [Google Scholar]
- Meteo Romania|Site-ul Administratiei Nationale de Meteorologie. Available online: https://www.meteoromania.ro/ (accessed on 10 August 2024).
- Şmuleac, L.; Rujescu, C.; Șmuleac, A.; Imbrea, F.; Radulov, I.; Manea, D.; Ienciu, A.; Adamov, T.; Pașcalău, R. Impact of Climate Change in the Banat Plain, Western Romania, on the Accessibility of Water for Crop Production in Agriculture. Agriculture 2020, 10, 437. [Google Scholar] [CrossRef]
- Suveti, P.F.; Batrîna, S.L.; Imbrea, F. Research regarding the influence of Banat climate conditions on rapeseed production. Res. J. Agric. Sci. 2020, 52. [Google Scholar]
- Vahdati, K. Walnut tolerance to abiotic stresses: Approaches and prospects. In Proceedings of the VII International Walnut Symposium, Shanxi, China, 20 July 2013; Volume 1050, pp. 399–406. [Google Scholar]
- Weising, K.; Nybom, H.; Pfenninger, M.; Wolff, K.; Kahl, G. DNA Fingerprinting in Plants: Principles, Methods, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Poșta, D.S.; Radulov, I.; Cocan, I.; Berbecea, A.A.; Alexa, E.; Hotea, I.; Iordănescu, O.A.; Băla, M.; Cântar, I.C.; Rózsa, S.; et al. Hazelnuts (Corylus avellana L.) from Spontaneous Flora of the West Part of Romania: A Source of Nutrients for Locals. Agronomy 2022, 12, 214. [Google Scholar] [CrossRef]
- Sze-Tao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Bouzoubaa, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 105–111, ISSN 1658-077X. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Jorhem, L.; Engman, J. Determination of Lead, Cadmium, Zinc, Copper, and Iron in Foods by Atomic Absorption Spectrometry after Microwave Digestion: NMKL Collaborative Study. J. AOAC Int. 2000, 83, 1189–1203. [Google Scholar] [CrossRef]
- Duca, A.; Alexa, E.; Dehelean, C.A.; Soica, C.; Danciu, C.; Popescu, I.; Cocan, I.; Lalescu, D.; Muntean, D.M. Assessment of lipid profile of eight propolis samples from western Romania. Farmacia 2019, 67, 126–132. [Google Scholar] [CrossRef]
- Rinovetz, A.; Alexa, E.; Cocan, I.; Popescu, I.; Rădoi, B. Considerations On Physical Separation Of A Natural Lipid Mixture By Production Of Lipid Fractions Carpathian. J. Food Sci. Technol. 2018, 10. [Google Scholar]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar] [CrossRef]
- Team, R.C. RA language and environment for statistical computing, R Foundation for Statistical. Computing 2020. [Google Scholar]
- Jardim, T.; Domingues, M.R.M.; Alves, E. An overview on lipids in nuts and oily fruits: Oil content, lipid composition, health effects, lipidomic fingerprinting and new biotechnological applications of their by-products. Crit. Rev. Food Sci. Nutr. 2023, 1–29. [Google Scholar] [CrossRef]
- Abbattista, R.; Feinberg, N.G.; Snodgrass, I.F.; Newman, J.W.; Dandekar, A.M. Unveiling the hidden quality” of the walnut pellicle: A precious source of bioactive lipids. Front. Plant Sci. 2024, 15, 1395543. [Google Scholar] [CrossRef]
- Fuentes-Soriano, P.; Bellido-Milla, D.; García-Guzmán, J.J.; Hernández-Artiga, M.P.; Gallardo-Bernal, J.J.; Palacios-Santander, J.M.; Espada-Bellido, E. A simple phosphorus determination in walnuts and assessment of the assimilable fraction. Talanta 2019, 204, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.J.; Zhang, Y.G.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Okatan, V.; Gündeşli, M.A.; Kafkas, N.E.; Attar, Ş.H.; Kahramanoğlu, İ.; Usanmaz, S.; Aşkın, M.A. Phenolic compounds, antioxidant activity, fatty acids and volatile profiles of 18 different walnut (Juglans regia L.) cultivars and genotypes. Erwerbs-Obstbau 2022, 64, 247–260. [Google Scholar] [CrossRef]
- Yin, L.L.; Qing, T.; Zhang, S.X.; Yin, K.X.; Qin, J.I.Q. Determination of Trace Elements in Edible Nuts in the Beijing Market by ICP-MS. Biomed. Environ. Sci. 2015, 28, 449–454. [Google Scholar]
- Moreda–Piñeiro, J.; Herbello–Hermelo, P.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Bioavailability Assessment of Essential and Toxic Metals in Edible Nuts and Seeds. Food Chem. 2016, 205, 146–154. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molyb-Denum, Nickel, Silicon, Vanadium, and Zinc, Panel on Micronutrients; National Academies Press: Washington, DC, USA, 2001; ISBN 10:0-309-07279-4. [Google Scholar]
- EFSA|Science, Safe Food, Sustainability. Available online: https://www.efsa.europa.eu/en (accessed on 10 August 2024).
- Measurlabs, N.M. Food Contaminant Analysis by EU Regulations|Measurlabs. Available online: https://measurlabs.com/blog/eu-regulations-on-food-contaminants/#heavy-metals (accessed on 6 September 2024).
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ross, C.W.; Hanan, N.P.; Prihodko, L.; Anchang, J.; Ji, W.; Yu, Q. Woody-biomass projections and drivers of change in sub-Saharan. Afr. Nat. Clim. Chang. 2021, 11, 449–455. [Google Scholar] [CrossRef]
- Skole, D.L.; Samek, J.H.; Dieng, M.; Mbow, C. The Contribution of Trees Outside of Forests to Landscape Carbon and Climate Change Mitigation in West Africa. Forests 2021, 12, 1652. [Google Scholar] [CrossRef]
- Janku, K.; Mana, M.; Arczyńska-Chudy, E. New locations of Persian walnut (Juglans regia L.). In Proceedings of the Urban Valleys of Watercourses in Poznań, Poznań, Poland, 13–19 May 2017. [Google Scholar]
- Paź-Dyderska, S.; Jagodziński, A.M.; Dyderski, M.K. Possible changes in spatial distribution of walnut (Juglans regia L.) in Europe under warming climate. Reg. Environ. Chang. 2021, 21, 18. [Google Scholar] [CrossRef]
- Çakir, E. Molecular Markers from Past to Present and Their Application Areas. Regen. Agric. 2023, 19. [Google Scholar]
- Hausdorf, B.; Hennig, C. Species Delimitation Using Dominant and Codominant Multilocus Markers. Syst. Biol. 2010, 59, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Cui, Z.; Shi, W.; Huang, J.; Wang, J. Development of Polymorphic Microsatellite Markers and Identification of Applications for Wild Walnut (Juglans regia L.) in Middle Asia. Diversity 2023, 15, 1073. [Google Scholar] [CrossRef]









| Markers | Primer | Primer Sequences 5′–3′ |
|---|---|---|
| DAMD | URP6R | GGCAAGCTGGTGGGAGGTAC |
| URP 9F | ATGTGTGCGATCAGTTGCTG | |
| 33.6 | GGAGGTGGGCA | |
| 14 C2 | GGCAGGATTGAAGC | |
| M 13 | GAGGGTGGCGGCTCT | |
| HBV 3 | GGTGAAGCACAGGTG | |
| SCoT | SCoT 1 | CAACAATGGCTACCACCA |
| SCoT 3 | CAACAATGGCTACCACCG | |
| SCoT 6 | CAACAATGGCTACCACGC | |
| SCoT 11 | AAGCAATGGCTACCACCA | |
| SCoT 24 | CACCATGGCTACCACCAT | |
| SCoT 36 | GCAACAATGGCTACCACC |
| Primer | Total Bands (n) | Polymorphic Bands (np) | Polymorphism (%) | PIC | SD_PIC | MI | SD_MI | Rp |
|---|---|---|---|---|---|---|---|---|
| DAMD03 | 11 | 4 | 36.36 | 0.31 | 0.05 | 1.26 | 0.22 | 3.20 |
| DAMD04 | 10 | 4 | 40.00 | 0.34 | 0.05 | 1.36 | 0.19 | 3.52 |
| DAMD05 | 11 | 6 | 54.54 | 0.35 | 0.04 | 2.09 | 0.23 | 5.44 |
| DAMD06 | 6 | 4 | 66.66 | 0.31 | 0.05 | 1.27 | 0.22 | 3.20 |
| DAMD07 | 4 | 4 | 100.00 | 0.31 | 0.05 | 1.27 | 0.22 | 3.20 |
| DAMD08 | 5 | 2 | 40.00 | 0.36 | 0.01 | 0.73 | 0.00 | 1.92 |
| Average | 7.83 | 4 | 56.26 | 0.33 | 0.04 | 1.33 | 0.18 | 3.41 |
| Primer | Total Bands (n) | Polymorphic Bands (np) | Polymorphism (%) | PIC | SD_PIC | MI | SD_MI | Rp |
|---|---|---|---|---|---|---|---|---|
| SCoT1 | 8 | 6 | 75 | 0.31 | 0.05 | 1.90 | 0.31 | 4.80 |
| SCoT3 | 8 | 4 | 50 | 0.34 | 0.05 | 1.36 | 0.19 | 3.52 |
| SCoT6 | 8 | 4 | 50 | 0.34 | 0.05 | 1.36 | 0.19 | 3.52 |
| SCoT11 | 5 | 4 | 80 | 0.31 | 0.06 | 1.26 | 0.22 | 3.20 |
| SCoT24 | 2 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SCoT36 | 4 | 1 | 25 | 0.36 | NA | 0.36 | NA | 0.96 |
| Average | 5 | 2.83 | 49.44 | 0.34 | 0.04 | 1.12 | 0.18 | 2.40 |
| Elements | Our Results: Mean Values of Heavy Metals in Walnut Kernels | Cosmulescu et al. [21] | Yin et al. [81] | Moreda–Piñeiro et al. [82] |
|---|---|---|---|---|
| Cu | 3.655–8.532 | 14.1–32.2 | 8.8 ± 0.15 | 109–3817 |
| Mn | 14.408–28.618 | 31.3–176 | 10 ± 0.1 | 7.7–84 |
| Zn | 19.813–46.583 | 19.5–36.1 | 20 ± 1.1 | 12–63 |
| Pb | 1.204–2.27 | - | - | - |
| Cd | 0.03451–0.08065 | - | 0.02 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenche-Constantinescu, A.-M.; Lalescu, D.V.; Popescu, S.; Sarac, I.; Petolescu, C.; Camen, D.; Horablaga, A.; Popescu, C.A.; Herbei, M.V.; Dragomir, L.; et al. Juglans regia as Urban Trees: Genetic Diversity and Walnut Kernel Quality Assessment. Horticulturae 2024, 10, 1027. https://doi.org/10.3390/horticulturae10101027
Tenche-Constantinescu A-M, Lalescu DV, Popescu S, Sarac I, Petolescu C, Camen D, Horablaga A, Popescu CA, Herbei MV, Dragomir L, et al. Juglans regia as Urban Trees: Genetic Diversity and Walnut Kernel Quality Assessment. Horticulturae. 2024; 10(10):1027. https://doi.org/10.3390/horticulturae10101027
Chicago/Turabian StyleTenche-Constantinescu, Alina-Maria, Dacian Virgil Lalescu, Sorina Popescu, Ioan Sarac, Cerasela Petolescu, Dorin Camen, Adina Horablaga, Cosmin Alin Popescu, Mihai Valentin Herbei, Lucian Dragomir, and et al. 2024. "Juglans regia as Urban Trees: Genetic Diversity and Walnut Kernel Quality Assessment" Horticulturae 10, no. 10: 1027. https://doi.org/10.3390/horticulturae10101027
APA StyleTenche-Constantinescu, A.-M., Lalescu, D. V., Popescu, S., Sarac, I., Petolescu, C., Camen, D., Horablaga, A., Popescu, C. A., Herbei, M. V., Dragomir, L., Popescu, G., Iordănescu, O. A., Becherescu, A., & Onisan, E. (2024). Juglans regia as Urban Trees: Genetic Diversity and Walnut Kernel Quality Assessment. Horticulturae, 10(10), 1027. https://doi.org/10.3390/horticulturae10101027








