Bioavailability of Cd in Plantago weldenii and Sonchus oleraceus Plants: The Effects of a Humic and Fulvic Acids-Based Biostimulant
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil and Plant Analyses
2.2.1. Non-Destructive Analyses
2.2.2. Plant Growth Measurement and Chemical Analyses
2.2.3. Indices for Cd Transfer and Translocation
of Cd in soil)
of Cd in roots)
2.3. Data Quality Control and Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Plant Growth and Physiological Parameters
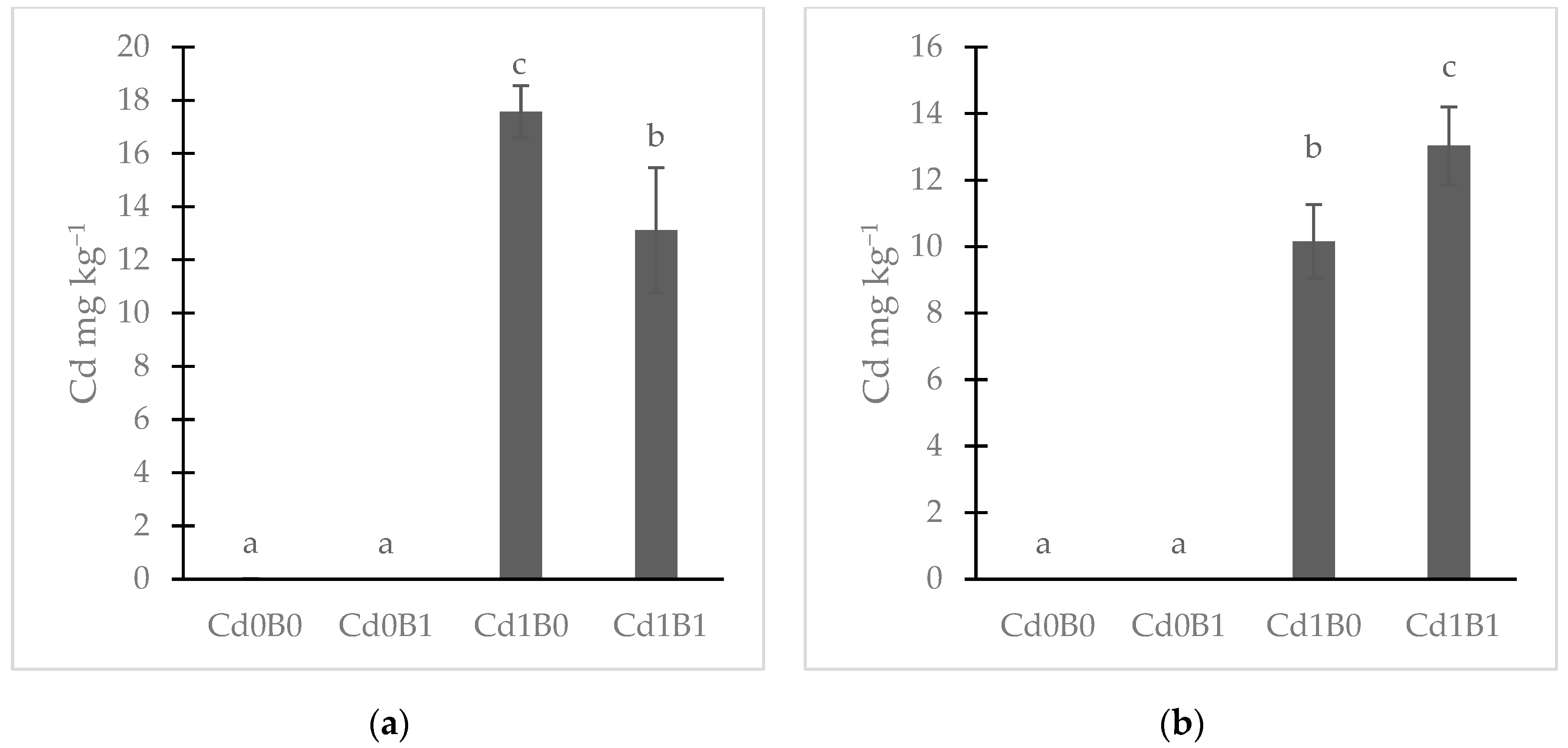
3.3. Bioavailable Concentration of Cd in Soil
3.4. Cd Concentration in Plant Tissues
3.5. Indices for the Transfer and Translocation of Cd
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, S.U.; Han, J.C.; Ahmad, M.; Gao, S.; Khan, K.A.; Li, B.; Zhou, Y.; Zhao, X.; Huang, Y. Toxic effects of lead (Pb), cadmium (Cd) and tetracycline (TC) on the growth and development of Triticum aestivum: A meta-analysis. Sci. Total Environ. 2023, 904, 166677. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Thalassinos, G.; Petropoulos, S.A.; Grammenou, A.; Antoniadis, V. Potentially Toxic Elements: A Review on Their Soil Behavior and Plant Attenuation Mechanisms against Their Toxicity. Agriculture 2023, 13, 1684. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; A Comprehensive Review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef]
- Subašić, M.; Šamec, D.; Selović, A.; Karalija, E. Phytoremediation of Cadmium Polluted Soils: Current Status and Approaches for Enhancing. Soil Syst. 2022, 6, 3. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Han, M.; Ullah, H.; Yang, H.; Yu, G.; You, S.; Liu, J.; Chen, B.; Shahab, A.; Antoniadis, V.; Shaheen, S.M.; et al. Cadmium uptake and membrane transport in roots of hyperaccumulator Amaranthus hypochondriacus L. Environ. Pollut. 2023, 331, 121846. [Google Scholar] [CrossRef]
- Saengwilai, P.; Meeinkuirt, W.; Phusantisampan, T.; Pichtel, J. Immobilization of Cadmium in Contaminated Soil Using Organic Amendments and Its Effects on Rice Growth Performance. Expo. Heal. 2020, 12, 295–306. [Google Scholar] [CrossRef]
- Danso, O.P.; Acheampong, A.; Zhang, Z.; Song, J.; Wang, Z.; Dai, J.; Zhi, T.; Yin, X.; Zhu, R. The management of Cd in rice with biochar and selenium: Effects, efficiency, and practices. Carbon Res. 2023, 2, 41. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Challenges 2021, 4, 100197. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Ali, S.; Wen, X.; Khan, K.A.; Ghramh, H.A.; Zhang, Z.; Zhang, D. Impact of Cadmium Stress on Growth and Physio-Biochemical Attributes of Eruca sativa Mill. Plants 2022, 11, 2981. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A. Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–34. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Grammenou, A.; Petropoulos, S.A.; Thalassinos, G.; Rinklebe, J.; Shaheen, S.M.; Antoniadis, V. Biostimulants in the Soil–Plant Interface: Agro-environmental Implications—A Review. Earth Syst. Environ. 2023, 7, 583–600. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review. Plants 2022, 11, 1946. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants application alleviates water stress effects on yield and chemical composition of greenhouse green bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef]
- Liava, V.; Chaski, C.; Añibarro-Ortega, M.; Pereira, A.; Pinela, J.; Barros, L.; Petropoulos, S.A. The Effect of Biostimulants on Fruit Quality of Processing Tomato Grown under Deficit Irrigation. Horticulturae 2023, 9, 1184. [Google Scholar] [CrossRef]
- Dogan, M.; Bolat, I.; Karakas, S.; Dikilitas, M.; Gutiérrez-Gamboa, G.; Kaya, O. Remediation of Cadmium Stress in Strawberry Plants Using Humic Acid and Silicon Applications. Life 2022, 12, 1962. [Google Scholar] [CrossRef]
- Khalofah, A.; Bokhari, N.A.; Migdadi, H.M.; Alwahibi, M.S. Antioxidant responses and the role of Moringa oleifera leaf extract for mitigation of cadmium stressed Lepidium sativum L. South African J. Bot. 2020, 129, 341–346. [Google Scholar] [CrossRef]
- Dobbss, L.B.; dos Santos, T.C.; Pittarello, M.; de Souza, S.B.; Ramos, A.C.; Busato, J.G. Alleviation of iron toxicity in Schinus terebinthifolius Raddi (Anacardiaceae) by humic substances. Environ. Sci. Pollut. Res. 2018, 25, 9416–9425. [Google Scholar] [CrossRef] [PubMed]
- Dziugieł, T.; Wadas, W. Possibility of increasing early crop potato yield with foliar application of seaweed extracts and humic acids. J. Cent. Eur. Agric. 2020, 21, 300–310. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Bandiera, M.; Dal Cortivo, C.; Barion, G.; Mosca, G.; Vamerali, T. Phytoremediation opportunities with alimurgic species in metal-contaminated environments. Sustainability 2016, 8, 357. [Google Scholar] [CrossRef]
- Ahmad, I.; Gul, I.; Irum, S.; Manzoor, M.; Arshad, M. Accumulation of heavy metals in wild plants collected from the industrial sites—Potential for phytoremediation. Int. J. Environ. Sci. Technol. 2023, 20, 5441–5452. [Google Scholar] [CrossRef]
- Peerzada, A.M.; O’Donnell, C.; Adkins, S. Biology, impact, and management of common sowthistle (Sonchus oleraceus L.). Acta Physiol. Plant. 2019, 41, 136. [Google Scholar] [CrossRef]
- Xiong, Z. Bioaccumulation and physiological effects of excess lead in a roadside pioneer species Sonchus oleraceus L. Environ. Pollut. 1997, 97, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.W.; Li, W.S.; Tu, S.X.; Ding, Y.Z.; Wang, R.G.; Rensing, C.; Li, Y.P.; Feng, R.W. Differences in cadmium absorption by 71 leaf vegetable varieties from different families and genera and their health risk assessment. Ecotoxicol. Environ. Saf. 2019, 184, 109593. [Google Scholar] [CrossRef] [PubMed]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Serrano, H.C.; Cotrim, H.; Pinto, M.J.; Martins-Loução, M.A.; Branquinho, C. Metal hyperaccumulation patterns within Plantago phylogeny (Plantaginaceae). Plant Soil 2017, 411, 227–241. [Google Scholar] [CrossRef]
- Rowell, D. Soil Science: Methods and Applications; Longman Group UK Ltd.: Harlow, UK, 1994; ISBN 9780582087842. [Google Scholar]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fan, T.T.; Cui, P.X.; Sun, Q.; Zhou, D.M.; Li, C.B.; Wang, G.Q.; Lin, Y.S.; Zhang, S.T.; Yang, X.P.; et al. Binding and adsorption energy of Cd in soils and its environmental implication for Cd bioavailability. Soil Sci. Soc. Am. J. 2020, 84, 472–482. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Canellas, L.P.; Façanha, A.R. Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta 2007, 225, 1583–1595. [Google Scholar] [CrossRef]
- Nunes, R.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nogueira, F.C.S.; Canellas, L.P.; Olivares, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 2019, 9, 12019. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Spaccini, R.; Piccolo, A.; Dobbss, L.B.; Okorokova-Façanha, A.L.; Santos, G.; Olivares, F.L.; Façanha, A. Relationships Between Chemical Characteristics and Root Growth Promotion of Humic Acids Isolated From Brazilian Oxisols. Soil Sci. 2009, 174, 611–620. [Google Scholar] [CrossRef]
- Canal, S.B.; Bozkurt, M.A.; Yilmaz, H. Effects of Humic Acid and EDTA on Phytoremediation, Growth and Antioxidant Activity in Rapeseed (Brassica napus L.) Grown under Heavy Metal Stress. Polish J. Environ. Stud. 2022, 31, 4051–4060. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Weemstra, M.; Zambrano, J.; Allen, D.; Umaña, M.N. Tree growth increases through opposing above-ground and below-ground resource strategies. J. Ecol. 2021, 109, 3502–3512. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Szopiński, M.; Sitko, K.; Gieroń, Ż.; Rusinowski, S.; Corso, M.; Hermans, C.; Verbruggen, N.; Małkowski, E. Toxic effects of cd and zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front. Plant Sci. 2019, 10, 748. [Google Scholar] [CrossRef]
- Tang, L.; Ying, R.R.; Jiang, D.; Zeng, X.W.; Morel, J.L.; Tang, Y.T.; Qiu, R.L. Impaired leaf CO2 diffusion mediates Cd-induced inhibition of photosynthesis in the Zn/Cd hyperaccumulator Picris divaricata. Plant Physiol. Biochem. 2013, 73, 70–76. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2020, 39, 641–655. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S.; Zhang, Z. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef]
- Fang, Q.; Fan, Z.; Xie, Y.; Wang, X.; Li, K.; Liu, Y. Screening and evaluation of the bioremediation potential of Cu/Zn-resistant, autochthonous Acinetobacter sp. FQ-44 from Sonchus oleraceus L. Front. Plant Sci. 2016, 7, 1487. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Guo, S.; Wang, Q.; Chi, R. Enhanced reduction of lead bioavailability in phosphate mining wasteland soil by a phosphate-solubilizing strain of Pseudomonas sp., LA, coupled with ryegrass (Lolium perenne L.) and sonchus (Sonchus oleraceus L.). Environ. Pollut. 2021, 274, 116572. [Google Scholar] [CrossRef] [PubMed]
- Levei, L.; Kovacs, E.; Hoaghia, M.A.; Ozunu, A. Accumulation of heavy metals in plantago major grown in urban and post-industrial areas. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 87–98. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R. Element accumulation, distribution, and phytoremediation potential in selected metallophytes growing in a contaminated area. Environ. Monit. Assess. 2015, 187, 441. [Google Scholar] [CrossRef]
- Rong, Q.; Zhong, K.; Huang, H.; Li, C.; Zhang, C.; Nong, X. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco. Appl. Sci. 2020, 10, 1077. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Dar, A.A.; Wang, Z. The influence of humic and fulvic acids on Cd bioavailability to wheat cultivars grown on sewage irrigated Cd-contaminated soils. Ecotoxicol. Environ. Saf. 2020, 205, 111347. [Google Scholar] [CrossRef]
- Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L.; Meng, F.; Wang, J. A soluble humic substance for the simultaneous removal of cadmium and arsenic from contaminated soils. Int. J. Environ. Res. Public Health 2019, 16, 4999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Tang, J.; Zhou, Y.; Lin, Q.; Xiao, R.; Zhang, M. Characterization of goethite-fulvic acid composites and their impact on the immobility of Pb/Cd in soil. Chemosphere 2019, 222, 556–563. [Google Scholar] [CrossRef]
- Ding, H.; Tang, L.; Nie, Y.; Ji, H. Characteristics and interactions of heavy metals with humic acid in gold mining area soil at a upstream of a metropolitan drinking water source. J. Geochemical Explor. 2019, 200, 266–275. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Sanchis, I.; Masaguer, A.; Moliner, A. Effects of pH conditions and application rates of commercial humic substances on Cu and Zn mobility in anthropogenic mine soils. Sustainability 2019, 11, 4844. [Google Scholar] [CrossRef]
- Ekta, P.; Modi, N.R. A review of Phytoremediation. J. Pharmacogn. Phytochem. 2018, 7, 1485–1489. [Google Scholar]
- Abe, T.; Fukami, M.; Ogasawara, M. Cadmium accumulation in the shoots and roots of 93 weed species. Soil Sci. Plant Nutr. 2008, 54, 566–573. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Nemereshina, O.N.; Suliburska, J.; Gatiatulina, E.R.; Regula, J.; Nikonorov, A.A.; Skalny, A.V. Comparative Analysis of the Trace Element Content of the Leaves and Roots of Three Plantago Species. Biol. Trace Elem. Res. 2016, 173, 225–230. [Google Scholar] [CrossRef]
- Samadi, M.; Torbati, S.; Alipour, S. Metal(loid) uptake of Sonchus oleraceus grown around Cheshmeh-Konan copper deposit, NW Iran. J. Min. Environ. 2019, 10, 517–528. [Google Scholar] [CrossRef]

| P. weldenii | S. oleraceus | ||
|---|---|---|---|
| pH | Cd0B0 | 8.58 a | 8.46 a |
| Cd0B1 | 8.65 a | 8.54 a | |
| Cd1B0 | 8.67 a | 8.51 a | |
| Cd1B1 | 8.69 a | 8.53 a | |
| Significance | p = 0.084 NS | p = 0.112 NS | |
| OM (%) | Cd0B0 | 2.11 a | 1.99 a |
| Cd0B1 | 2.23 a | 2.28 a | |
| Cd1B0 | 2.18 a | 2.12 a | |
| Cd1B1 | 1.86 a | 2.25 a | |
| Significance | p = 0.377 NS | p = 0.097 NS |
| P. weldenii | S. oleraceus | ||
|---|---|---|---|
| Rosette diameter (cm) | Cd0B0 | 17.9 a | 30.5 ab |
| Cd0B1 | 19.7 b | 32.5 b | |
| Cd1B0 | 18.9 ab | 29.7 ab | |
| Cd1B1 | 21.3 c | 28.0 a | |
| Significance | p < 0.001 *** | p = 0.077 NS | |
| Aboveground FW (g) | Cd0B0 | 6.28 a | 9.35 a |
| Cd0B1 | 7.36 a | 9.41 a | |
| Cd1B0 | 6.87 a | 9.63 a | |
| Cd1B1 | 9.78 b | 10.03 a | |
| Significance | p < 0.001 *** | p = 0.744 NS | |
| Aboveground DW (g) | Cd0B0 | 0.76 a | 0. a |
| Cd0B1 | 1.20 b | 1.07 a | |
| Cd1B0 | 0.83 a | 1.01 a | |
| Cd1B1 | 0.95 a | 1.04 a | |
| Significance | p < 0.005 ** | p = 0.487 NS | |
| Roots (g) | Cd0B0 | 0.20 a | 0.66 ab |
| Cd0B1 | 0.49 b | 0.91 c | |
| Cd1B0 | 0.25 a | 0.71 b | |
| Cd1B1 | 0.28 a | 0.56 a | |
| Significance | p < 0.001 *** | p < 0.001 *** | |
| Number of leaves | Cd0B0 | 24.6 a | 15.2 b |
| Cd0B1 | 30.4 b | 13.7 a | |
| Cd1B0 | 27.1 ab | 13.2 a | |
| Cd1B1 | 29.2 ab | 14.3 ab | |
| Significance | p = 0.171 NS | p = 0.023 * |
| S. oleraceus | ||
|---|---|---|
| Leaf area (cm2) | Cd0B0 | 283 a |
| Cd0B1 | 279 a | |
| Cd1B0 | 279 a | |
| Cd1B1 | 296 a | |
| Significance | p = 0.797 NS | |
| Specific leaf area (m2 kg−1) | Cd0B0 | 30.1 b |
| Cd0B1 | 26.3 a | |
| Cd1B0 | 27.8 ab | |
| Cd1B1 | 28.5 ab | |
| Significance | p = 0.032 * | |
| Total chlorophyll concentration | Cd0B0 | 15.45 b |
| Cd0B1 | 13.47 ab | |
| Cd1B0 | 13.17 a | |
| Cd1B1 | 12.15 a | |
| Significance | p = 0.024 * |
| PAR (0 w m−2) | PAR (400 w m−2) | PAR (800 w m−2) | PAR (1200 w m−2) | ||
|---|---|---|---|---|---|
| Photosynthetic rate | Cd0B0 | −0.41 b | 6.43 a | 7.94 a | 8.72 a |
| (µmol CO2 m−2 s−1) | Cd0B1 | −1.14 a | 8.18 ab | 9.44 ab | 10.06 ab |
| Cd1B0 | −0.82 ab | 8.25 ab | 9.76 ab | 9.45 ab | |
| Cd1B1 | −0.57 b | 10.27 b | 11.96 b | 13.14 b | |
| Significance | p = 0.027 * | p = 0.047 * | p = 0.077 NS | p = 0.096 NS |
| P. weldenii | S. oleraceus | ||
|---|---|---|---|
| Shoots (dry weight) | Cd0B0 | 0 a | 0 a |
| Cd0B1 | 0.11 ab | 0 a | |
| Cd1B0 | 0.12 ab | 8.29 b | |
| Cd1B1 | 0.54 b | 9.16 b | |
| Significance | p = 0.046 * | p < 0.001 *** | |
| Shoots (fresh weight) | Cd0B0 | 0 a | 0.01 a |
| Cd0B1 | 0.018 ab | 0 a | |
| Cd1B0 | 0.015 ab | 0.87 b | |
| Cd1B1 | 0.052 b | 0.95 b | |
| Significance | p = 0.046 * | p < 0.001 *** | |
| Roots | Cd0B0 | 0 a | 0 a |
| Cd0B1 | 0 a | 0 a | |
| Cd1B0 | 27.48 b | 3.43 b | |
| Cd1B1 | 31.11 b | 2.74 b | |
| Significance | p < 0.001 *** | p < 0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grammenou, A.; Petropoulos, S.A.; Antoniadis, V. Bioavailability of Cd in Plantago weldenii and Sonchus oleraceus Plants: The Effects of a Humic and Fulvic Acids-Based Biostimulant. Horticulturae 2024, 10, 74. https://doi.org/10.3390/horticulturae10010074
Grammenou A, Petropoulos SA, Antoniadis V. Bioavailability of Cd in Plantago weldenii and Sonchus oleraceus Plants: The Effects of a Humic and Fulvic Acids-Based Biostimulant. Horticulturae. 2024; 10(1):74. https://doi.org/10.3390/horticulturae10010074
Chicago/Turabian StyleGrammenou, Aspasia, Spyridon A. Petropoulos, and Vasileios Antoniadis. 2024. "Bioavailability of Cd in Plantago weldenii and Sonchus oleraceus Plants: The Effects of a Humic and Fulvic Acids-Based Biostimulant" Horticulturae 10, no. 1: 74. https://doi.org/10.3390/horticulturae10010074
APA StyleGrammenou, A., Petropoulos, S. A., & Antoniadis, V. (2024). Bioavailability of Cd in Plantago weldenii and Sonchus oleraceus Plants: The Effects of a Humic and Fulvic Acids-Based Biostimulant. Horticulturae, 10(1), 74. https://doi.org/10.3390/horticulturae10010074








