Reproducible Method for 1-Methylcylopropene (1−MCP) Application and Quantitation for Post-Harvest Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagent Acquisition
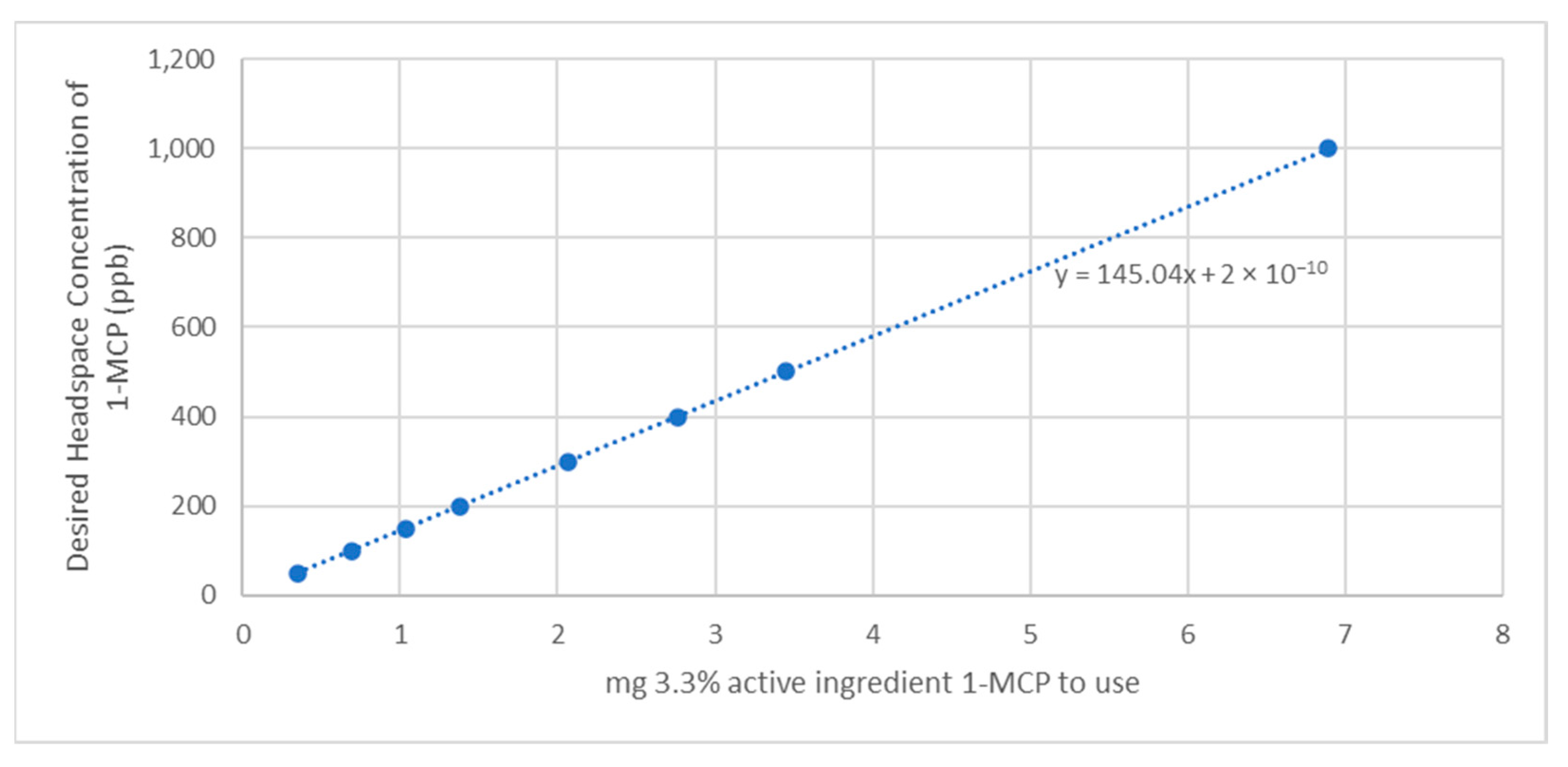
2.2. 1−MCP Theoretical Yield Calculations
2.3. 1−MCP Application and Sampling
2.4. Surrogate Standard Application and Sampling
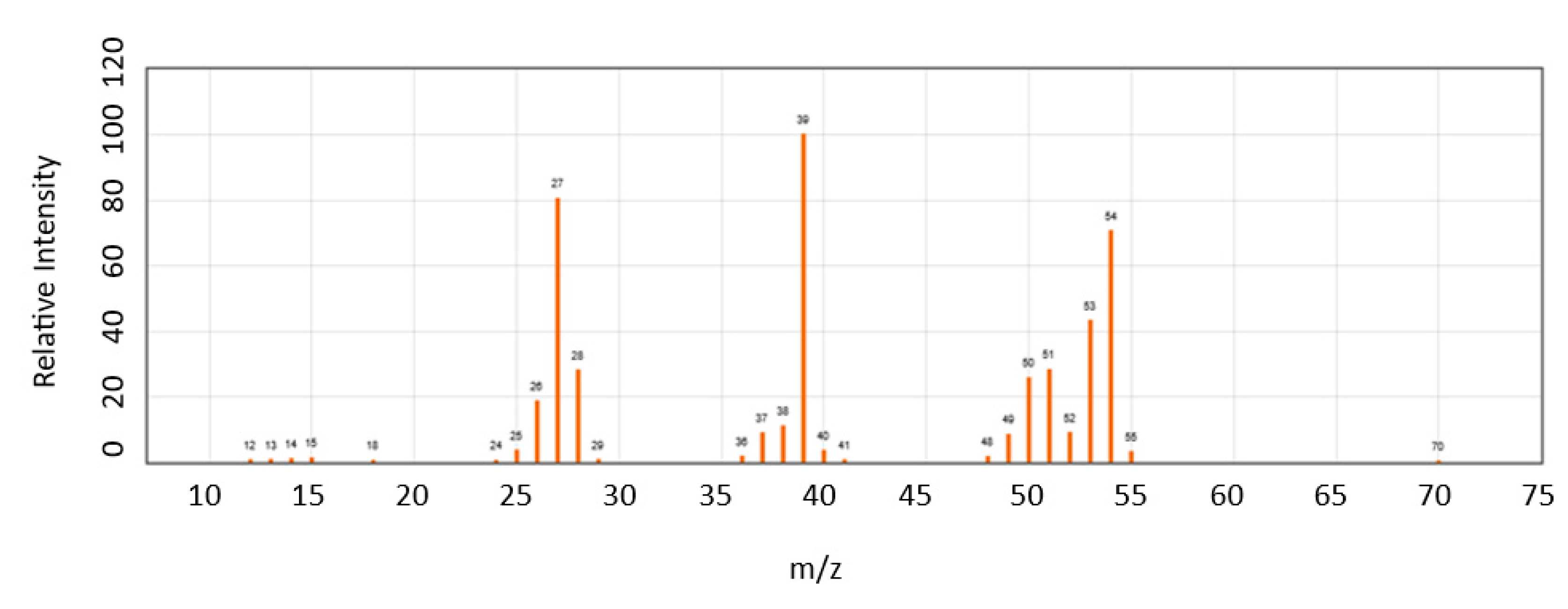
2.5. The 1−MCP Detection and Quantification
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022; FAO: Québec City, QC, Canada, 2022. [Google Scholar]
- Sisler, E.C.; Blankenship, S.M. Method of Counteracting an Ethylene Response in Plants. U.S. Patent 5518988A, 21 May 1996. [Google Scholar]
- Sisler, E.C.; Blankenship, S.M. Diazocyclopentadiene (DACP), a light sensitive reagent for the ethylene receptor in plants. Plant Growth Regul. 1993, 12, 125–132. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Selvarajah, S.; Bauchot, A.D.; John, P. Internal browning in cold-stored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol. Technol. 2001, 23, 167–170. [Google Scholar] [CrossRef]
- Sozzi, G.O.; Abrajan-Villasenor, M.A.; Trinchero, G.D.; Fraschina, A.A. Postharvest response of ‘Brown Turkey’ figs (Ficus carica L.) to the inhibition of ethylene perception. J. Sci. Food Agric. 2005, 85, 2503–2508. [Google Scholar] [CrossRef]
- Zozio, S.; Servent, A.; Hubert, O.; Hiol, A.; Pallet, D.; Mbéguié-A-Mbeguie, D. Physicochemical and biochemical characterization of ripening in jujube (Ziziphus mauritiana Lamk) fruits from two accessions grown in Guadeloupe. Sci. Hortic. 2014, 175, 290–297. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Feng, X.; Lin, L.; Zhao, Y.; Jiang, W. Effects of 1−MCP and exogenous ethylene on fruit ripening and antioxidants in stored mango. Plant Growth Regul. 2009, 57, 185–192. [Google Scholar] [CrossRef]
- Watkins, C.B. The effect of 1−MCP on the development of physiological storage disorders in horticultural crops. Stewart Postharvest Rev. 2007, 3, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M. (Max) Meta-analysis of the effects of 1-methylcyclopropene (1−MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Mackay, A.B.; Kupferman, E.M. Inhibition of PAL, CHS, and ERS1 in ‘Red d’Anjou’ Pear (Pyrus communis L.) by 1−MCP. Postharvest Biol. Technol. 2007, 45, 46–55. [Google Scholar] [CrossRef]
- Ekman, J.H.; Clayton, M.; Biasi, W.V.; Mitcham, E.J. Interactions between 1−MCP concentration, treatment interval and storage time for ‘Bartlett’ pears. Postharvest Biol. Technol. 2004, 31, 127–136. [Google Scholar] [CrossRef]
- Vallejo, F.; Beaudry, R. Depletion of 1−MCP by ‘non-target’ materials from fruit storage facilities. Postharvest Biol. Technol. 2006, 40, 177–182. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO Specifications and Evaluations for Agricultural Pesticides 1-Methylcyclopropene; FAO: Québec City, QC, Canada, 2006. [Google Scholar]
- Lee, Y.S.; Beaudry, R.; Kim, J.N.; Harte, B.R. Development of a 1-Methylcyclopropene (1−MCP) Sachet Release System. J. Food Sci. 2006, 71, C1–C6. [Google Scholar] [CrossRef]
- Gamrasni, D.; Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1−MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Gamrasni, D.; Goldway, M.; Stern, Y.; Breitel, D.; Aharoni, A. 1−MCP (1-methylcyclopropene) Treatment Protocol for Fruit or Vegetables. Bio-Protocol 2017, 7, e2278. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). SmartFresh ProTabs Non-PRIA (Pesticide Registration Improvement Act) Labeling Amendment; 2021. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/071297-00010-20210204.pdf (accessed on 10 November 2023).
- Ariyanto, H.D.; Yoshii, H. Effect of stepwise humidity change on the release rate constant of 1-methylcyclopropene (1−MCP) in a cyclodextrin inclusion complex powder. Food Packag. Shelf Life 2019, 21, 100322. [Google Scholar] [CrossRef]
- NIST Office of Data and Informatics. 2-Butene. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=107-01-7 (accessed on 10 November 2023).
- NIST Office of Data and Informatics. 1-Butene. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=106-98-9 (accessed on 10 November 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stowe, E.; Mattinson, D.S.; Blauer, J.M.; Dhingra, A. Reproducible Method for 1-Methylcylopropene (1−MCP) Application and Quantitation for Post-Harvest Research. Horticulturae 2024, 10, 5. https://doi.org/10.3390/horticulturae10010005
Stowe E, Mattinson DS, Blauer JM, Dhingra A. Reproducible Method for 1-Methylcylopropene (1−MCP) Application and Quantitation for Post-Harvest Research. Horticulturae. 2024; 10(1):5. https://doi.org/10.3390/horticulturae10010005
Chicago/Turabian StyleStowe, Evan, Dennis Scott Mattinson, Jacob Michael Blauer, and Amit Dhingra. 2024. "Reproducible Method for 1-Methylcylopropene (1−MCP) Application and Quantitation for Post-Harvest Research" Horticulturae 10, no. 1: 5. https://doi.org/10.3390/horticulturae10010005
APA StyleStowe, E., Mattinson, D. S., Blauer, J. M., & Dhingra, A. (2024). Reproducible Method for 1-Methylcylopropene (1−MCP) Application and Quantitation for Post-Harvest Research. Horticulturae, 10(1), 5. https://doi.org/10.3390/horticulturae10010005




