Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica
Abstract
:1. Introduction
2. Experimental Section
2.1. In vitro Germination of Seed
2.2. Callus Induction and Somatic Embryo Production
2.3. Histological Observations and Plantlet Regeneration
2.4. Hardening and Transfer of Plant to Soil
2.5. Genetic Fidelity Testing of Somaclones
2.6. Statistical Analysis
3. Results and Discussion
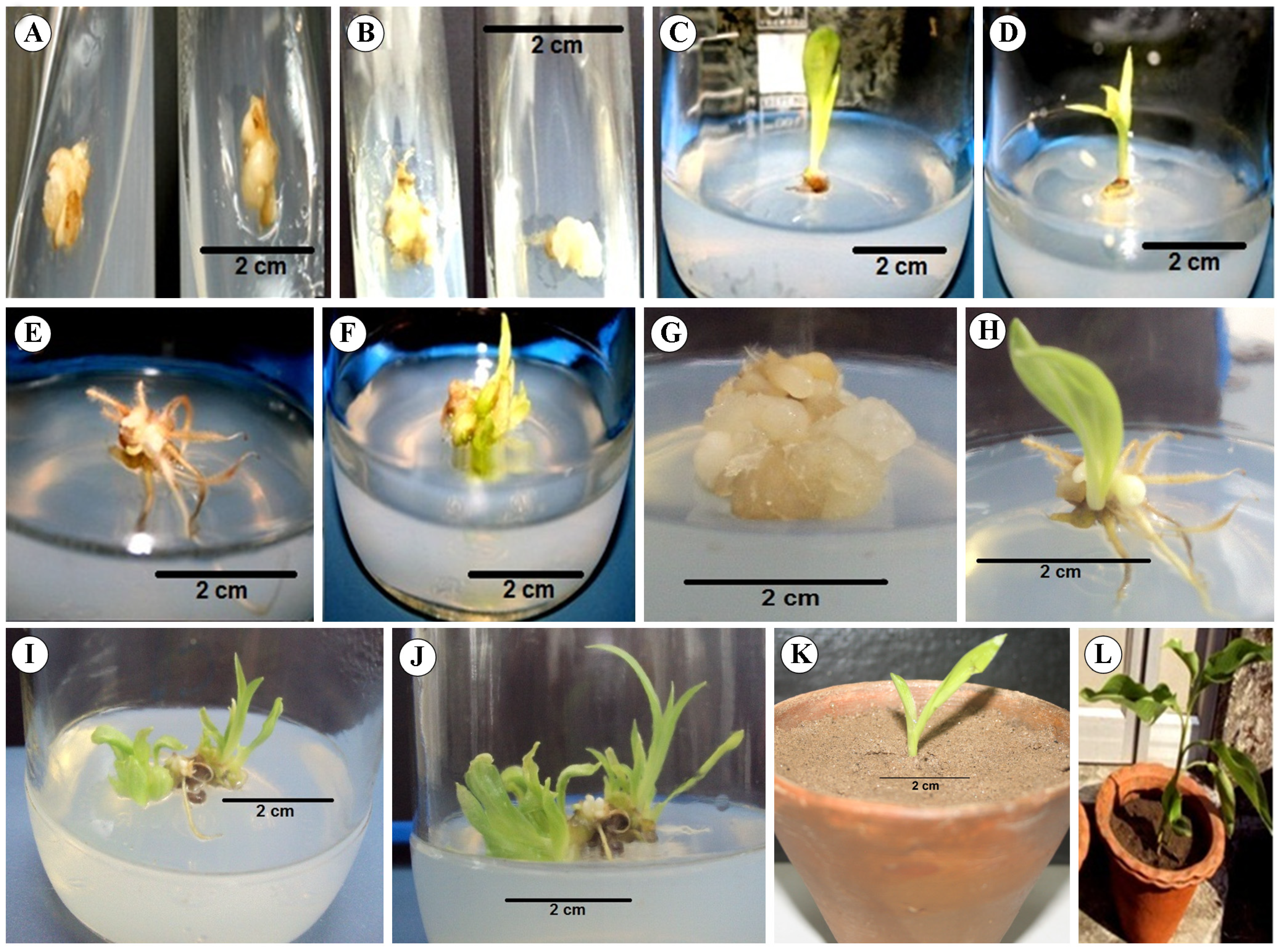
3.1. Establishment of Aseptic Culture
3.2. Callus Induction
3.3. Somatic Embryogenesis and Regeneration
3.3.1. Somatic Embryo Formation
3.3.2. Plantlet Regeneration
3.3.3. Subculturing and Hormone Effect on Shoot Growth
3.3.4. Acclimatization of in vitro Regenerated Plants
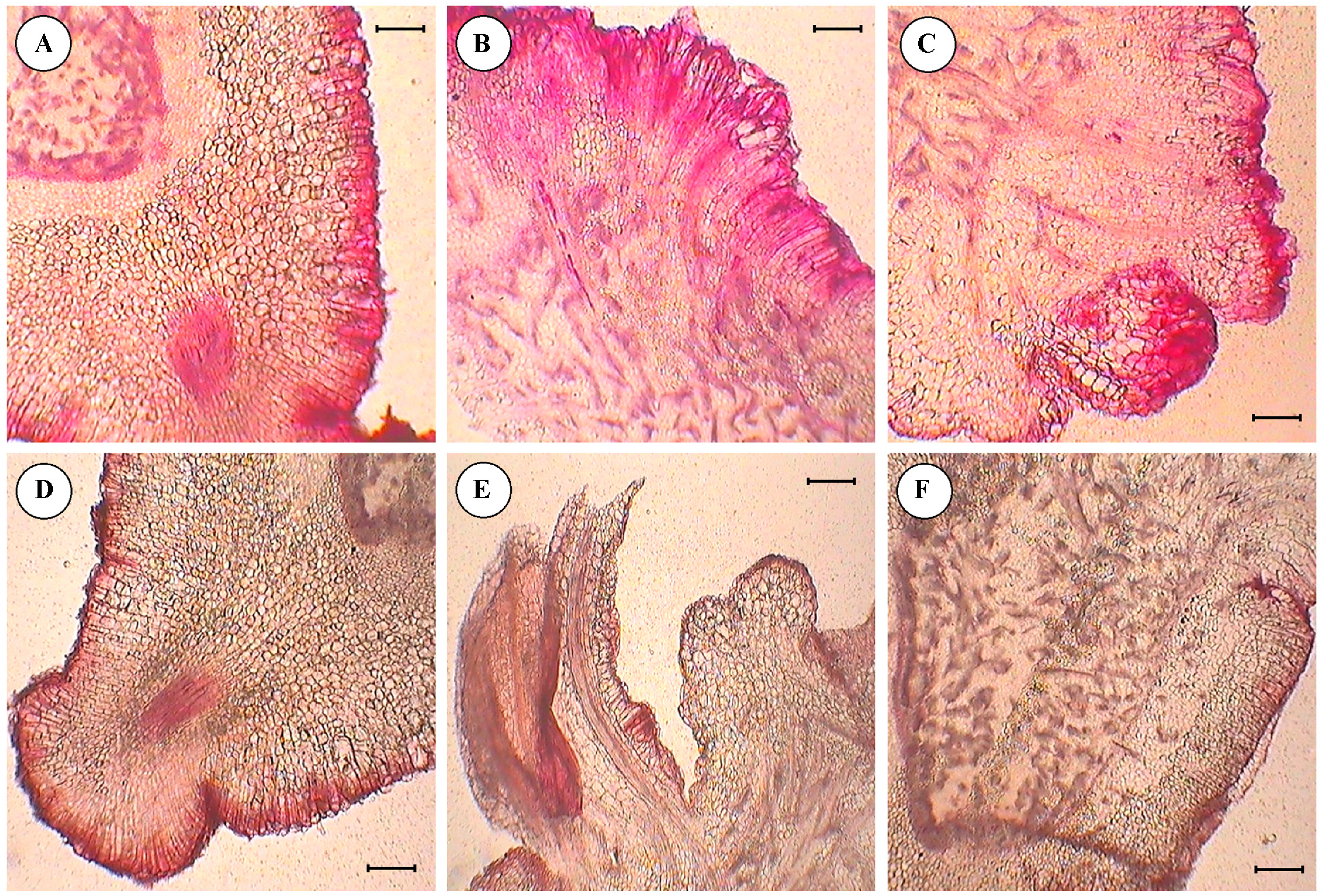
3.4. Histological Studies of Cultured Embryo
- Anthocyanin pigmentation was visualized on the edges of the section in the form of red spots as stained by safranin.
- Primordia of embryo development were observed from the surface of the cotyledon slices, in the form of compact masses of cells arranged sequentially on the edge of section.
- Differentiation and separation of proembryonic globules which were had a well-differentiated epidermis.
- Details of epidermal cell divisions, like periclinal and anticlinal walls and mitotically-active cells were observed.
- Embryonic axes with shoot meristems, cortical tissues, central cylinders and procambial strands were clearly visible.
3.5. Genetic Fidelity among in vitro Raised Plantlets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakornthap, A. Radiation-induced somatic mutations in the ornamental Canna. In Rome Meeting on the Uses of Induced Mutations in Plant; Breeding Pergamon Press: New York, NY, USA, 1965; pp. 707–721. [Google Scholar]
- Tanaka, N. The utilization of edible Canna plants in southeastern Asia and southern China. Econ. Bot. 2004, 58, 112–114. [Google Scholar] [CrossRef]
- Choudhury, M.D.; Bawari, M.; Singha, L.S. Some antipyretic ethno-medicinal plants of Manipuri Community of Barak Valley, Assam, India. Ethnobot. Leaflets 2010, 1, 21–28. [Google Scholar]
- Singh, P.K.; Kumar, V.; Tiwari, R.K.; Sharma, A.; Rao, C.V.; Singh, R.H. Medico-ethnobotany of Chatara’Block of District Sonebhadra, Uttar Pradesh, India. Advan. Biol. Res. 2010, 4, 65–80. [Google Scholar]
- Mishra, T.; Goyal, A.K.; Middha, S.K.; Sen, A. Antioxidative properties of Canna edulis Ker-Gawl. Ind. J. Nat. Prod. Res. 2011, 2, 315–321. [Google Scholar]
- Mishra, T.; Das, A.P.; Sen, A. Phytochemical screening and in vitro antioxidant profiling of solvent fractions of Canna edulis Ker Gawler. Free Radical. Antioxid. 2012, 2, 13–20. [Google Scholar] [CrossRef]
- Mishra, T.; Goyal, A.K.; Bhattacharya, M.; Kar, P.; Sen, A. Polyethylene glycol mediated protoplast fusion of medicinally important Canna. Res. Plant. Biol. 2015, 5, 20–24. [Google Scholar]
- Sakai, T.; Imai, K. The influences of growth regulators and culture medium composition on shoot-tip cultures of edible Canna. Environ. Control Biol. 2007, 45, 155–163. [Google Scholar] [CrossRef]
- Hosoki, T.; Sasaki, H. In vitro propagation of Canna edulis Ker by longitudinal shoot-split method. Plant Tissue Cult. Lett. 1991, 8, 175–178. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Goyal, A.K.; Pradhan, S.; Basistha, B.C.; Sen, A. Micropropagation and assessment of genetic fidelity of Dendrocalamus strictus (Roxb.) nees using RAPD and ISSR markers. Biotech 2014. [Google Scholar] [CrossRef]
- Santra, S.C.; Chatterjee, T.P.; Das, A.P. College Botany Practical, 3rd ed.; New Central Book Agency Ltd.: Kolkata, India, 2006; pp. 321–322. [Google Scholar]
- Sit, A.K.; Bhattacharya, M.; Chenchaiah, K.C. Effect of benzyl amino purine on in vitro shoot multiplication of ginger (Zingiber officinale Rosc. cv. Garubathan). J. Plant. Crop. 2005, 33, 184–186. [Google Scholar]
- Bhattacharya, M.; Sen, A. Rapid in vitro multiplication of disease-free Zingiber officinale Rosc. Ind. J. Plant. Physiol. 2006, 11, 379–384. [Google Scholar]
- Mondal, M.; Gupta, S.; Mukherjee, B.B. In vitro propagation of shoot buds of Carica papaya L. (Caricaceae) var. Honey Dew. Plant Cell Rep. 1990, 8, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Kambaska, K.B.; Santilata, S. Effect of plant growth regulator on micropropagtion of ginger (Zingiber officinale Rosc.) cv. Suprava and Suruchi. J. Agr. Tech. 2009, 5, 271–280. [Google Scholar]
- Tyagi, R.K.; Agrawal, A.; Mahalakshmi, C.; Hussain, Z.; Tyagi, H. Low-cost media for in vitro conservation of turmeric (Curcuma longa L.) and genetic stability assessment using RAPD markers. In Vitro Cell. Develop. Biol.-Plant 2007, 43, 51–58. [Google Scholar] [CrossRef]
- Hussey, G. In vitro propagation of Gladiolus by precocious axillary shoot formation. Sci. Hort. 1977, 6, 287–296. [Google Scholar] [CrossRef]
- Murashige, T.; Nakano, R. Morphogenetic behavior of tobacco tissue cultures and implication of plant senescence. Amer. J. Bot. 1965, 52, 819–827. [Google Scholar] [CrossRef]
- Rout, G.R.; Senapati, S.K.; Aparajita, S.; Palai, S.K. Studies on genetic identification and genetic fidelity of cultivated banana using ISSR markers. Plant Omics 2009, 2, 250–258. [Google Scholar]
- Lakshmanan, V.; Reddampalli Venkataramareddy, S.; Neelwarne, B. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotechnol. 2007, 10, 106–113. [Google Scholar] [CrossRef]
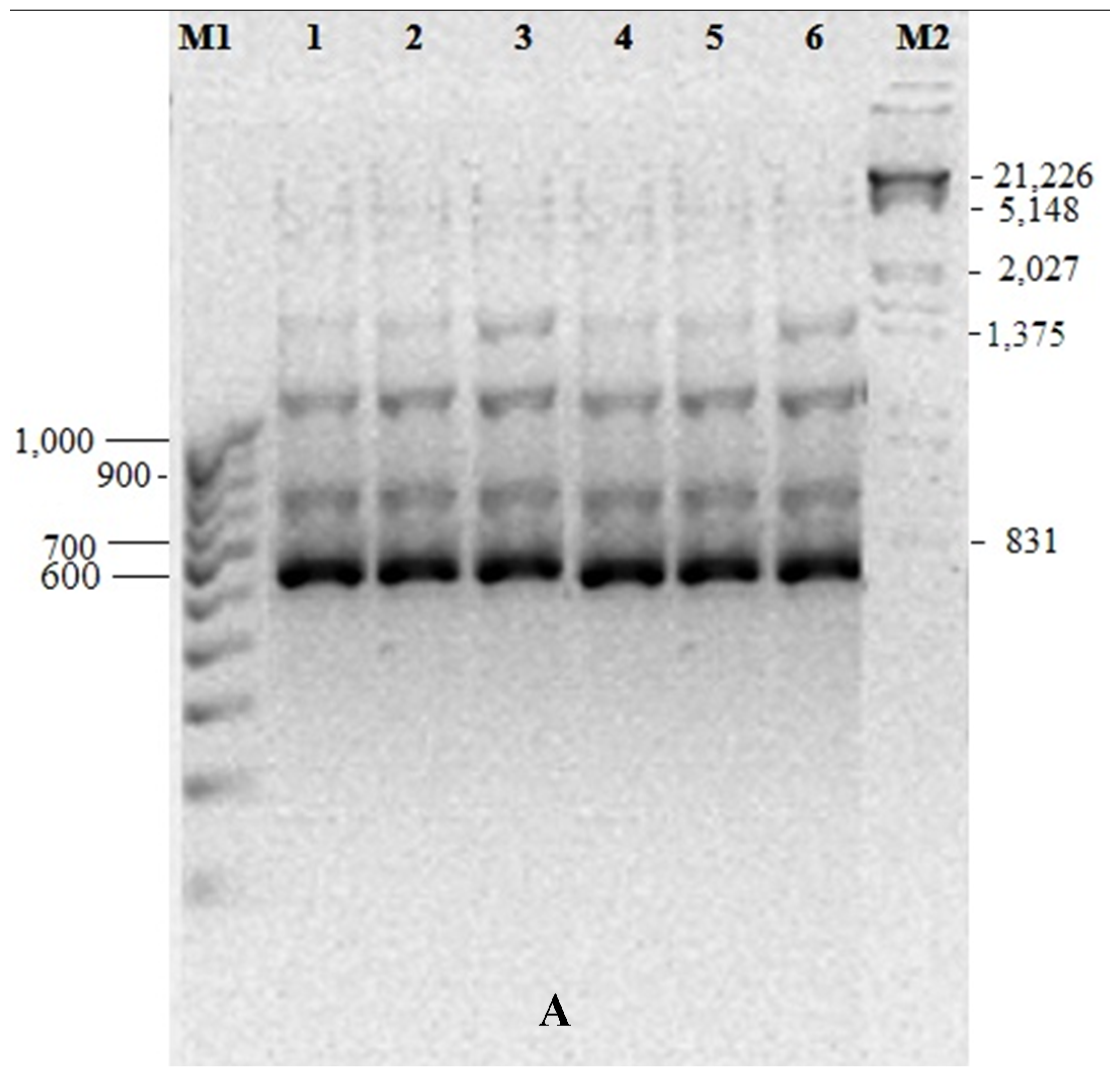
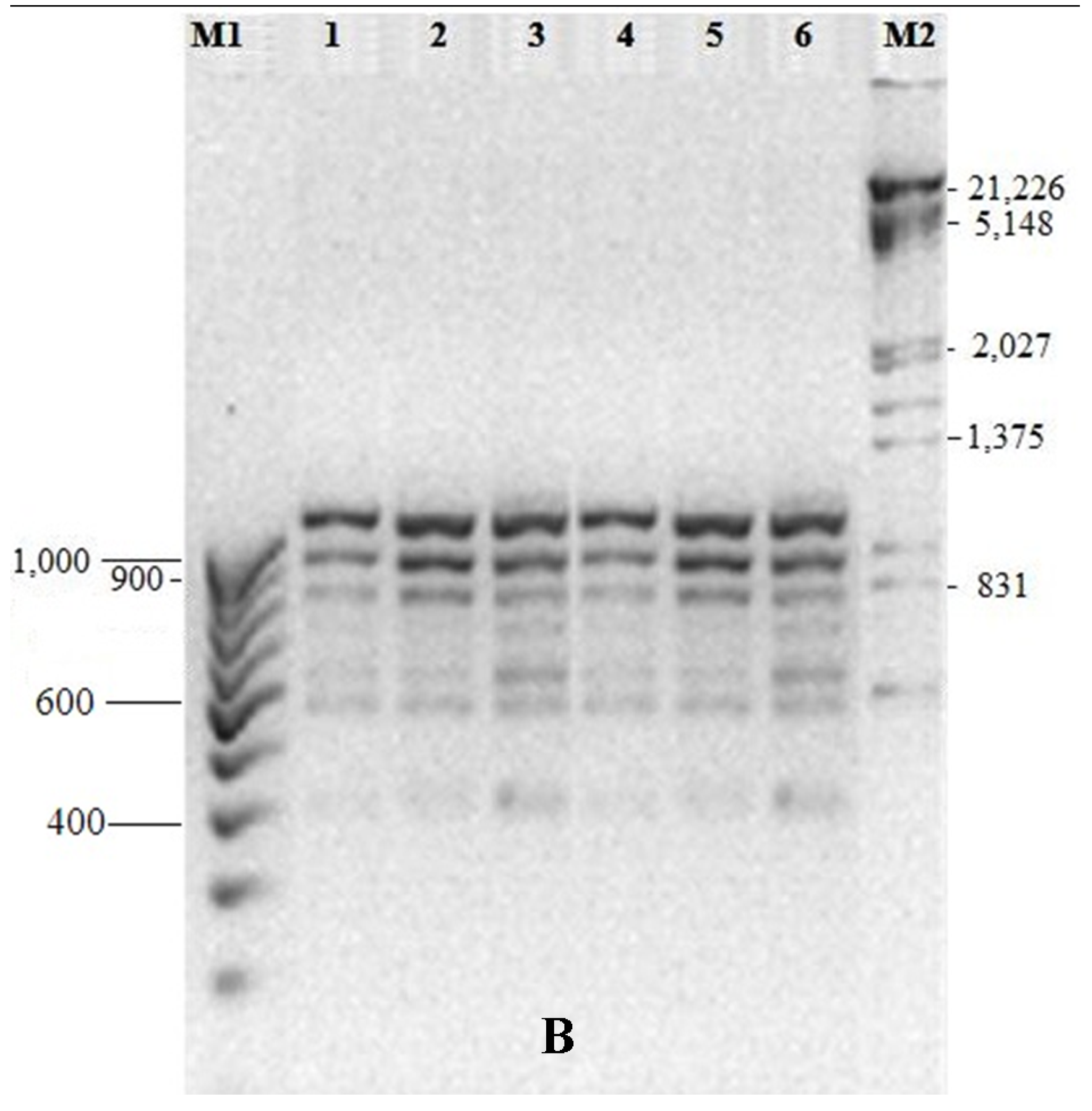
| Total Bands | Sl. No. | Primer ID | Primer Type | Sequence (5’-3’) | Band Size (bp) |
|---|---|---|---|---|---|
| 7 | 1 | OPA01 | RAPD | CAGGCCCTTC | 330–1120 |
| 6 | 2 | 0PA03 | RAPD | AGTCAGCCAC | 400–1260 |
| 6 | 3 | OPA07 | RAPD | GAAACGGGTG | 500–1550 |
| 8 | 4 | 0PA11 | RAPD | CAATCGCCGT | 420–1380 |
| 7 | 5 | OPA17 | RAPD | GACCGCTTGT | 760–1440 |
| 5 | 6 | OPB01 | RAPD | GTTTCGCTCC | 410–1350 |
| 6 | 7 | OPF09 | RAPD | CCAAGCTTCC | 610–1500 |
| 7 | 8 | OPG19 | RAPD | GTCAGGGCAA | 500–1280 |
| 4 | 9 | OPH04 | RAPD | GGAAGTCGCC | 300–1050 |
| 4 | 10 | OPN04 | RAPD | GACCGACCCA | 450–1300 |
| 3 | 11 | UBC810 | ISSR | (GA)8T | 550–800 |
| 6 | 12 | UBC815 | ISSR | (CT)8G | 375–1100 |
| 8 | 13 | UBC822 | ISSR | (TC)8A | 390–1280 |
| 6 | 14 | UBC824 | ISSR | (TC)8G | 450–1110 |
| 8 | 15 | UBC825 | ISSR | (AC)8T | 540–920 |
| 5 | 16 | UBC856 | ISSR | (AC)8YA | 410–980 |
| 9 | 17 | UBC873 | ISSR | (GACA)4 | 390–1240 |
| Media | BAP(mg∙L−1) | Shoot Length (cm) | ||||
|---|---|---|---|---|---|---|
| 15 D # | 30 D | 45 D | 60 D | 75 D | ||
| BM | Control | 0.20 ± 0.32 | 0.48 ± 0.21 | 1.23 ± 0.58 | 1.62 ± 0.82 | 1.81 ± 0.76 |
| 1 | 0.66 ± 0.38 ns | 1.80 ± 1.22 ns | 3.50 ± 1.61 ** | 5.10 ± 0.91 ** | 5.90 ± 1.02 ** | |
| 2 | 1.04 ± 1.07 ns | 3.22 ± 1.81 ** | 4.96 ± 0.92 ** | 6.50 ± 1.12 ** | 7.35 ± 0.84 ** | |
| 3 | 0.51 ± 0.77 ns | 2.40 ± 1.54 ** | 3.20 ± 1.43 ** | 4.32 ± 0.98 ** | 4.88 ± 0.67 ** | |
| 4 | 0.42 ± 0.26 ns | 1.70 ± 0.93 * | 2.61 ± 0.78 * | 3.80 ± 1.31 ** | 4.64 ± 1.95 ** | |
| Shoot No. | ||||||
| Control | 1.24 ± 0.88 | 2.31 ± 0.75 | 3.47 ± 0.92 | 4.84 ± 0.89 | 5.16 ± 0.80 | |
| 1 | 3.29 ± 1.12 ** | 8.96 ± 0.89 ** | 13.44 ± 1.15 ** | 18.88 ± 1.26 ** | 19.56 ± 0.49 ** | |
| 2 | 4.38 ± 1.79 ** | 11.98 ± 1.93 ** | 16.56 ± 0.95 ** | 22.24 ± 1.84 ** | 24.32 ± 1.77 ** | |
| 3 | 3.43 ± 1.56 ** | 9.01 ± 0.99 ** | 12.88 ± 1.77 ** | 17.02 ± 0.97 ** | 19.42 ± 0.88 ** | |
| 4 | 2.27 ± 0.44 ns | 7.99 ± 0.77 ** | 10.42 ± 1.31 ** | 13.62 ± 0.55 ** | 15.31 ± 0.81 ** | |
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, T.; Goyal, A.K.; Sen, A. Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica. Horticulturae 2015, 1, 3-13. https://doi.org/10.3390/horticulturae1010003
Mishra T, Goyal AK, Sen A. Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica. Horticulturae. 2015; 1(1):3-13. https://doi.org/10.3390/horticulturae1010003
Chicago/Turabian StyleMishra, Tanmayee, Arvind Kumar Goyal, and Arnab Sen. 2015. "Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica" Horticulturae 1, no. 1: 3-13. https://doi.org/10.3390/horticulturae1010003
APA StyleMishra, T., Goyal, A. K., & Sen, A. (2015). Somatic Embryogenesis and Genetic Fidelity Study of the Micropropagated Medicinal Species, Canna indica. Horticulturae, 1(1), 3-13. https://doi.org/10.3390/horticulturae1010003






