Relative Salt Tolerance of Seven Strawberry Cultivars
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Materials
2.2. Treatments
2.3. Growth, Yield, and Visual Quality
2.4. Gas Exchange and Leaf Greenness
2.5. Mineral Analysis
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
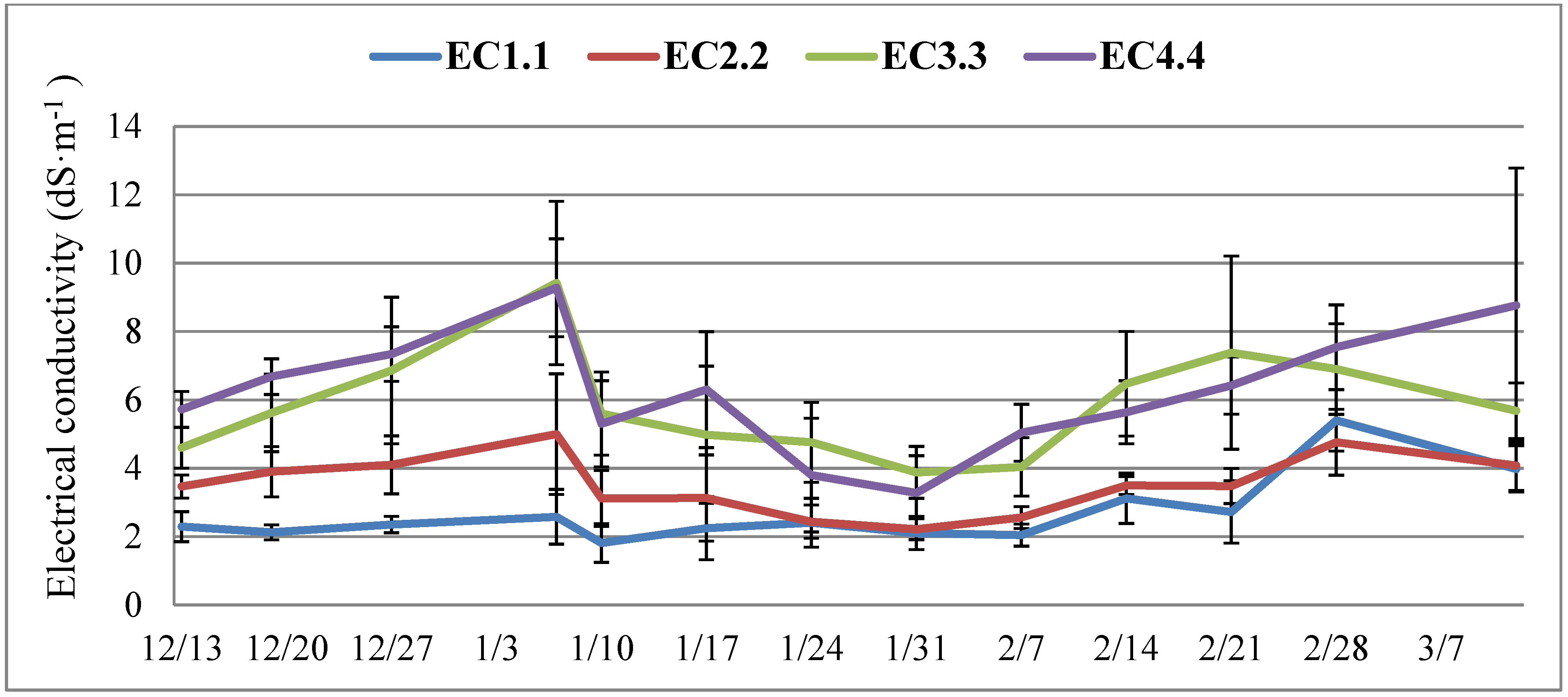
3.1. Leachate EC
3.2. Plant Growth and Visual Quality
| Variety | Treatment (dS·m−1) | Visual Score | Width (cm) | No. of Leaves | Leaf Area (cm2) | Shoot DW (g) |
|---|---|---|---|---|---|---|
| Albion | 1.1 | 4.1 a z | 10.8 a | 9 a | 663 a | 11.9 a |
| 2.2 | 3.9 a | 11.2 a | 6 a | 565 a | 9.7 a | |
| 3.3 | 4.0 a | 11.5 a | 5 a | 526 a | 8.6 a | |
| 4.4 | 3.5 a | 8.3 a | 6 a | 444 a | 6.9 a | |
| Mean | 3.9 A y | 10.5 AB | 7 C | 555 BC | 9.4 BC | |
| Benicia | 1.1 | 3.6 a | 8.6 a | 8 a | 420 a | 6.8 ab |
| 2.2 | 3.3 a | 8.2 a | 9 a | 404 a | 7.5 a | |
| 3.3 | 3.5 a | 9.0 a | 11 a | 357 a | 7.6 a | |
| 4.4 | 2.9 a | 3.0 a | 4 a | 230 a | 4.1 b | |
| Mean | 3.3 ABC | 7.0 B | 8 BC | 349 D | 6.4 CD | |
| Camarosa | 1.1 | 4.2 a | 12.4 a | 17 a | 756 a | 11.9 a |
| 2.2 | 4.2 a | 12.8 a | 12 ab | 704 a | 10.2 a | |
| 3.3 | 3.6 ab | 11.7 a | 8 b | 646 a | 9.3 a | |
| 4.4 | 3.0 b | 10.0 a | 10 b | 608 a | 9.1 a | |
| Mean | 3.8 AB | 11.8 A | 12 AB | 684 AB | 10.2 AB | |
| Camino Real | 1.1 | 3.7 a | 12.9 a | 18 a | 779 a | 14.8 a |
| 2.2 | 3.8 a | 11.1 ab | 11 ab | 673 ab | 11.4 ab | |
| 3.3 | 2.6 b | 12.2a | 6 b | 408 b | 7.5 b | |
| 4.4 | 2.7 b | 6.2 b | 6 b | 443 b | 7.2 b | |
| Mean | 3.2 BC | 10.5 AB | 11 ABC | 580 BC | 10.3 AB | |
| Chandler | 1.1 | 4.0 a | 11.4 a | 16 a | 854 a | 15.2 a |
| 2.2 | 4.1 a | 11.9 a | 15 a | 861 a | 16.0 a | |
| 3.3 | 3.7 a | 9.1 ab | 17 a | 759 a | 11.3 ab | |
| 4.4 | 2.6 b | 3.8 b | 10 a | 558 a | 8.5 b | |
| Mean | 3.6 ABC | 9.2 AB | 15 A | 831 A | 12.8 A | |
| Radiance | 1.1 | 3.3 ab | 7.5 a | 6 a | 433 a | 6.1 a |
| 2.2 | 3.9 a | 11.2 a | 10 a | 507 a | 6.9 a | |
| 3.3 | 3.1 ab | 8.0 a | 7 a | 381 a | 5.1 a | |
| 4.4 | 1.8 b | 7.1 a | 5 a | 369 a | 5.3 a | |
| Mean | 3.0 C | 8.6 AB | 7 BC | 428 CD | 5.9 D | |
| San Andreas | 1.1 | 3.9 a | 11.6 a | 16 a | 814 a | 12.3 a |
| 2.2 | 4.1 a | 12.3 a | 17 a | 774 a | 13.3 a | |
| 3.3 | 3.9 a | 12.2 a | 13 a | 645 a | 10.3 a | |
| 4.4 | 3.8 a | 7.9 a | 11 a | 558 a | 8.5 a | |
| Mean | 3.9 A | 11.0 A | 14 A | 691 AB | 11.0 AB | |
| Salt treatment | 1.1 | 3.8 a x | 10.9 a | 13 a | 706 a | 11.5 a |
| 2.2 | 3.9 a | 11.2 a | 11 ab | 653 ab | 10.6 ab | |
| 3.3 | 3.5 a | 10.5 a | 10 ab | 538 bc | 8.6 bc | |
| 4.4 | 2.9 b | 6.5 b | 8 b | 457 c | 7.1 c | |
| Cultivar | *** w | *** | *** | *** | *** | |
| Treatment | *** | *** | *** | *** | *** | |
| Cultivar × Treatment | * | NS | NS | NS | NS | |
| Variety | Treatment (dS·m−1) | No. of Mature Fruits | FW of Mature Fruits (g) | No. of Immature Fruits | FW of Immature Fruits (g) |
|---|---|---|---|---|---|
| Albion | 1.1 | 7 a z | 103.5 a | 5 a | 14.5 ab |
| 2.2 | 7 a | 105.2 a | 6 a | 23.9 a | |
| 3.3 | 6 a | 89.4 a | 5 a | 12.1 ab | |
| 4.4 | 7 a | 81.8 a | 4 a | 9.8 b | |
| Mean | 7 CD y | 95.2 A | 5.1 C | 14.9 ABC | |
| Benicia | 1.1 | 11 a | 130.4 a | 5 a | 10.3 a |
| 2.2 | 7ab | 82.7 ab | 4 a | 9.4 a | |
| 3.3 | 8 ab | 94.4 ab | 5 a | 14.8 a | |
| 4.4 | 5 b | 56.4 b | 3 a | 8.3 a | |
| Mean | 8 CD | 88.6 AB | 4.1 C | 10.5 C | |
| Camarosa | 1.1 | 11 a | 122.8 a | 7 a | 10.6 a |
| 2.2 | 12 a | 126.3 a | 6 a | 9.0 a | |
| 3.3 | 12 a | 103.1 a | 5 a | 7.0 a | |
| 4.4 | 12 a | 100.9 a | 5 a | 7.3 a | |
| Mean | 12 A | 114.5 A | 5.7 C | 8.6 C | |
| Camino Real | 1.1 | 8 a | 138.3 a | 6 a | 17.6 a |
| 2.2 | 6 ab | 90.7 ab | 8 a | 21.0 a | |
| 3.3 | 7 ab | 112.2 ab | 4 a | 10.5 a | |
| 4.4 | 5 b | 60.6 b | 7 a | 18.2 a | |
| Mean | 7 CD | 100.3 A | 6.2 BC | 16.7 ABC | |
| Chandler | 1.1 | 11 a | 135.0 a | 10 a | 27.5 a |
| 2.2 | 9 a | 127.2 a | 12 a | 28.7 a | |
| 3.3 | 9 a | 112.0 a | 9 a | 21.1 a | |
| 4.4 | 7 a | 69.5 a | 9 a | 16.0 a | |
| Mean | 9 BC | 112.3 A | 9.8 A | 23.1 A | |
| Radiance | 1.1 | 13 a | 128.4 a | 7 a | 12.7 a |
| 2.2 | 11 a | 124.7 a | 7 a | 17.6 a | |
| 3.3 | 12 a | 119.0 a | 6 a | 11.3 a | |
| 4.4 | 9 a | 90.4 a | 6 a | 10.5 a | |
| Mean | 11 AB | 117.8 A | 6.3 BC | 13.3 BC | |
| San Andreas | 1.1 | 6 a | 66.8 a | 10 a | 31.7 a |
| 2.2 | 6 a | 70.1 a | 9 a | 18.7 ab | |
| 3.3 | 5 a | 54.4 a | 10 a | 17.8 ab | |
| 4.4 | 5 a | 41.0 a | 6 a | 13.3 b | |
| Mean | 5 D | 57.6 B | 8.6 AB | 19.8 AB | |
| Salt treatment | 1.1 | 10 a x | 118.8 a | 7 a | 17.8 a |
| 2.2 | 8 ab | 103.5 a | 7 a | 17.3 a | |
| 3.3 | 8 ab | 97.3 a | 6 a | 13.8 a | |
| 4.4 | 7 b | 69.0 b | 6 a | 12.1 a | |
| Cultivar | *** w | *** | * | ** | |
| Treatment | *** | *** | *** | *** | |
| Cultivar × Treatment | NS | NS | NS | NS | |
3.3. Gas Exchange and Leaf Greenness
| Variety | Treatment | E (mmol·m−2·s−1) | gs (mmol·m−2·s−1) | Pn (μmol·m−2·s−1) | SPAD |
|---|---|---|---|---|---|
| Albion | 1.1 | 7.9 a z | 422.6 a | 16.2 a | 46.3 a |
| 2.2 | 7.1 a | 396.3 a | 15.6 a | 46.6 a | |
| 3.3 | 6.3 a | 306.4 a | 13.2 a | 45.5 a | |
| 4.4 | 6.1 a | 323.8 a | 13.9 a | 47.2 a | |
| Mean | 6.9 A y | 360.5 AB | 14.7 A | 46.4 BC | |
| Benicia | 1.1 | 7.8 a | 478.0 a | 16.0 a | 43.1 a |
| 2.2 | 7.1 a | 391.6 a | 15.2 a | 47.4 a | |
| 3.3 | 7.3 a | 424.8 a | 15.6 a | 46.6 a | |
| 4.4 | 6.3 a | 299.4 a | 13.4 a | 47.0 a | |
| Mean | 7.1 A | 392.6 A | 14.9 A | 46.2 BC | |
| Camarosa | 1.1 | 7.3 a | 369.0 a | 14.7 ab | 45.1 a |
| 2.2 | 7.6 a | 366.6 a | 15.8 a | 44.2 a | |
| 3.3 | 6.4 a | 269.8 a | 11.4 b | 46.2 a | |
| 4.4 | 6.2 a | 314.3 a | 12.2 ab | 47.2 a | |
| Mean | 6.9 A | 334.1 AB | 13.7 A | 45.6 C | |
| Camino Real | 1.1 | 7.9 a | 369.6 a | 14.0 ab | 49.4 a |
| 2.2 | 7.3 a | 420.5 a | 17.0 a | 49.4 a | |
| 3.3 | 6.6 a | 304.8 a | 12.4 b | 47.1 a | |
| 4.4 | 7.4 a | 393.8 a | 15.8 ab | 47.3 a | |
| Mean | 7.3 A | 373.2 AB | 14.8 A | 48.3 AB | |
| Chandler | 1.1 | 7.1 a | 394.0 a | 15.8 ab | 43.2 a |
| 2.2 | 7.1 a | 407.8 a | 16.5 a | 44.1 a | |
| 3.3 | 6.1 a | 306.8 a | 11.3 b | 40.5 a | |
| 4.4 | 6.2 a | 359.0 a | 15.4 ab | 40.0 a | |
| Mean | 6.6 A | 365.1 AB | 14.6 A | 41.9 D | |
| Radiance | 1.1 | 7.5 ab | 331.8 ab | 14.1 ab | 42.6 a |
| 2.2 | 8.1 a | 375.0 a | 14.9 a | 48.9 a | |
| 3.3 | 5.7 b | 272.8 ab | 11.9 ab | 43.6 a | |
| 4.4 | 5.9 ab | 234.3 b | 9.4 b | 45.6 a | |
| Mean | 6.8 A | 305.7 AB | 12.7 A | 45.8 BC | |
| San Andreas | 1.1 | 7.1 a | 325.0 a | 13.7 ab | 48.5 ab |
| 2.2 | 7.3 a | 356.6 a | 15.2 a | 52.2 a | |
| 3.3 | 6.2 a | 284.8 a | 13.0 ab | 50.0 ab | |
| 4.4 | 6.0 a | 245.2 a | 11.1 b | 47.2 b | |
| Mean | 6.6 A | 301.7 B | 13.2 A | 49.2 A | |
| Salt treatment | 1.1 | 7.5 a x | 384.8 a | 14.9 a | 45.8 a |
| 2.2 | 7.4 a | 385.8 a | 15.7 a | 47.4 a | |
| 3.3 | 6.3 b | 307.8 b | 12.6 b | 45.5 a | |
| 4.4 | 6.3 b | 310.7 b | 13.1 b | 46.1 a | |
| Cultivar | NS w | *** | * | *** | |
| Treatment | *** | *** | *** | NS | |
| Cultivar × Treatment | NS | NS | NS | * | |
3.4. Ion Analysis
| Variety | Treatment | Ion Concentration (mg·g−1) | |||
|---|---|---|---|---|---|
| Na | Ca | Cl | K | ||
| Albion | 1.1 | 0.56 a z | 17.29 a | 3.63 c | 27.03 a |
| 2.2 | 0.82 a | 22.47 a | 8.58 b | 23.46 ab | |
| 3.3 | 0.66 a | 22.08 a | 10.58 ab | 23.34 ab | |
| 4.4 | 0.92 a | 21.37 a | 13.80 a | 21.16 b | |
| Mean | 0.75 A y | 20.80 A | 9.14 A | 23.75 A | |
| Benicia | 1.1 | 0.73 a | 18.52 a | 4.73 d | 23.79 a |
| 2.2 | 0.94 a | 18.49 a | 8.60 c | 21.95 ab | |
| 3.3 | 1.41 a | 18.64 a | 11.58 b | 21.28 ab | |
| 4.4 | 1.55 a | 21.58 a | 16.08 a | 19.46 b | |
| Mean | 1.16 A | 19.31 AB | 10.24 A | 21.62 AB | |
| Camarosa | 1.1 | 0.43 b | 16.09 a | 3.10 c | 22.36 a |
| 2.2 | 0.86 ab | 18.39 a | 9.20 b | 21.49 ab | |
| 3.3 | 1.06 ab | 21.98 a | 14.30 a | 19.29 b | |
| 4.4 | 1.35 a | 21.86 a | 13.43 a | 18.64 b | |
| Mean | 0.89 A | 19.25 AB | 9.78 A | 20.44 B | |
| Camino Real | 1.1 | 0.61 b | 17.08 b | 4.00 c | 26.36 a |
| 2.2 | 0.93 b | 17.66 ab | 9.85 b | 23.95 ab | |
| 3.3 | 1.83 a | 22.30 ab | 16.38 a | 21.26 b | |
| 4.4 | 1.13 b | 22.42 a | 17.55 a | 20.54 b | |
| Mean | 1.13 A | 19.86 A | 11.94 A | 23.03 AB | |
| Chandler | 1.1 | 0.40 c | 16.65 b | 3.63 d | 24.33 a |
| 2.2 | 0.62 bc | 18.42 ab | 9.48 c | 21.91 b | |
| 3.3 | 1.02 ab | 20.74 a | 13.00 b | 20.88 b | |
| 4.4 | 1.31 a | 21.05 a | 19.77 a | 21.82 b | |
| Mean | 0.80 A | 19.10 AB | 10.91 A | 22.24 AB | |
| Radiance | 1.1 | 0.83 a | 21.27 b | 5.38 b | 25.67 a |
| 2.2 | 1.11 a | 19.53 b | 9.70 b | 24.53 a | |
| 3.3 | 1.43 a | 25.84 a | 9.37 b | 21.09 a | |
| 4.4 | 2.21 a | 23.96 ab | 17.23 a | 21.29 a | |
| Mean | 1.27 A | 22.20 A | 10.00 A | 23.41 A | |
| San Andreas | 1.1 | 0.58 b | 13.85 b | 2.98 c | 25.80 a |
| 2.2 | 0.80 ab | 15.31 ab | 7.83 b | 24.75 a | |
| 3.3 | 0.82 ab | 17.94 a | 11.33 a | 22.80 ab | |
| 4.4 | 1.11 a | 18.37 a | 13.78 a | 20.13 b | |
| Mean | 0.83 A | 16.37 B | 8.98 A | 23.37 A | |
| Salt treatment | 1.1 | 0.59 b x | 17.25 b | 3.92 d | 25.05 a |
| 2.2 | 0.87 b | 18.61 b | 9.03 c | 23.15 b | |
| 3.3 | 1.18 a | 21.16 a | 12.47 b | 21.42 c | |
| 4.4 | 1.30 a | 21.32 a | 15.84 a | 20.37 c | |
| Cultivar | *** w | *** | *** | *** | |
| Treatment | *** | *** | *** | *** | |
| Cultivar × Treatment | NS | NS | * | NS | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA, National Agricultural Statistics Service. Noncitrus Fruits and Nuts 2013 Summary; U.S. Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Wallace, R.W.; Stein, L. Strawberry production in Texas. In Production Guide for Texas-Grown Strawberries; Wallace, R.W., Anciso, J., Eds.; Texas A&M AgriLife Extension: College Station, TX, USA; Available online: http://www.agrilifebookstore.org/ (accessed on 25 May 2015).
- Wallace, R.W.; Webb, C.J. Strawberries grown under protective cultivation on the Texas High Plains. J. Amer. Pomol. Soc. 2013, 67, 7–10. [Google Scholar]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. “Elsanta” and “Korona”. Sci. Hortic. 2005, 103, 289–303. [Google Scholar] [CrossRef]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2009, 107, 1413–1420. [Google Scholar] [CrossRef]
- Martinez Barroso, M.C.; Alvarez, C.E. Toxicity symptoms and tolerance of strawberry to salinity in the irrigation water. Sci. Hortic. 1997, 71, 177–188. [Google Scholar] [CrossRef]
- Kepenek, K.; Koyuncu, F. Studies on the salt tolerance of some strawberry cultivars under glasshouse. Acta Hortic. 2002, 573, 297–304. [Google Scholar] [CrossRef]
- Turhan, E.; Eris, A. Changes of growth, amino acids, and ionic composition in strawberry plants under salt stress conditions. Commun. Soil Sci. Plant Anal. 2009, 40, 3308–3322. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Salinity tolerance of five commercial cultivars of strawberry (Fragaria × ananassa Duch.). In Proceedings of the 3rd International Salinity Forum, Riverside Convention Center, CA, USA, 16–18 June 2014; pp. 170–171.
- Wright, R.D. The pour-through nutrient extraction procedure. Hortscience 1986, 21, 227–229. [Google Scholar]
- Harrill, R. Using a Refractometer to Test the Quality of Fruits & Vegetables; Pineknoll Publishing: Keedysville, MD, USA, 1998. [Google Scholar]
- Gavlak, R.G.; Horneck, D.A.; Miller, R.O. Plant, Soil, and Water Reference Methods for the Western Region; Western Regional Extension Publication (WREP): Corvallis, OR, USA, 1994; Volume 125. [Google Scholar]
- Zeng, L.; Shannon, M.C.; Grieve, C.M. Evaluation of salt tolerance in rice genotypes by multiples agronomic parameters. Euphytica 2002, 127, 235–245. [Google Scholar] [CrossRef]
- Niu, G.; Cabrera, R. Growth and physiological responses of landscape plants to saline water irrigation: A review. HortScience 2010, 45, 1605–1609. [Google Scholar]
- Kaya, C.; Higgs, D.; Saltali, K.; Gezerel, O. Response of strawberry growth at high salinity and alkalinity to supplementary potassium. J. Plant Nutr. 2002, 25, 1415–1427. [Google Scholar] [CrossRef]
- Gulen, H.; Turhan, E.; Eris, A. Changes in peroxidase activities and soluble proteins in strawberry varieties under salt stress. Acta Physiolagiae Plant. 2006, 28, 109–116. [Google Scholar] [CrossRef]
- Turhan, E.; Eris, A. Changes of micronutrients, dry weight, and chlorophyll contents in strawberry plants under salt stress conditions. Commun. Soil Sci. Plant Anal. 2005, 36, 1021–1028. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.; Mostafa, M.A.E.; Allen, W.W. Strawberry Deficiency Symptoms: A Visual and Plant Analysis Guide to Fertilization; Agricultural Experiment Station, Division of Agriculture and Natural Resources, University of California: Berkeley, CA, USA, 1980. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Niu, G.; Wallace, R.; Masabni, J.; Gu, M. Relative Salt Tolerance of Seven Strawberry Cultivars. Horticulturae 2015, 1, 27-43. https://doi.org/10.3390/horticulturae1010027
Sun Y, Niu G, Wallace R, Masabni J, Gu M. Relative Salt Tolerance of Seven Strawberry Cultivars. Horticulturae. 2015; 1(1):27-43. https://doi.org/10.3390/horticulturae1010027
Chicago/Turabian StyleSun, Youping, Genhua Niu, Russ Wallace, Joseph Masabni, and Mengmeng Gu. 2015. "Relative Salt Tolerance of Seven Strawberry Cultivars" Horticulturae 1, no. 1: 27-43. https://doi.org/10.3390/horticulturae1010027
APA StyleSun, Y., Niu, G., Wallace, R., Masabni, J., & Gu, M. (2015). Relative Salt Tolerance of Seven Strawberry Cultivars. Horticulturae, 1(1), 27-43. https://doi.org/10.3390/horticulturae1010027








