Effects of Inoculation with Newly Isolated Cold-Adapted Bacteria on Winter Cattle Manure Composting in the Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Cold-Adapted Strains
2.2. Preparation of the Compound Microbial Agents
2.3. Large-Scale Compost Settings and Sample Collection
2.4. Analysis of Physical and Chemical Indexes
2.5. Microbial Community Analysis
2.5.1. Total DNA Extraction
2.5.2. High-Throughput Sequencing and Bacterial Diversity Analysis
2.6. Statistical Analysis
3. Results
3.1. Screening, Identification, Antagonistic Activity, and Organic Compound Degradation Characteristics of Cold-Tolerant Bacterial Strains
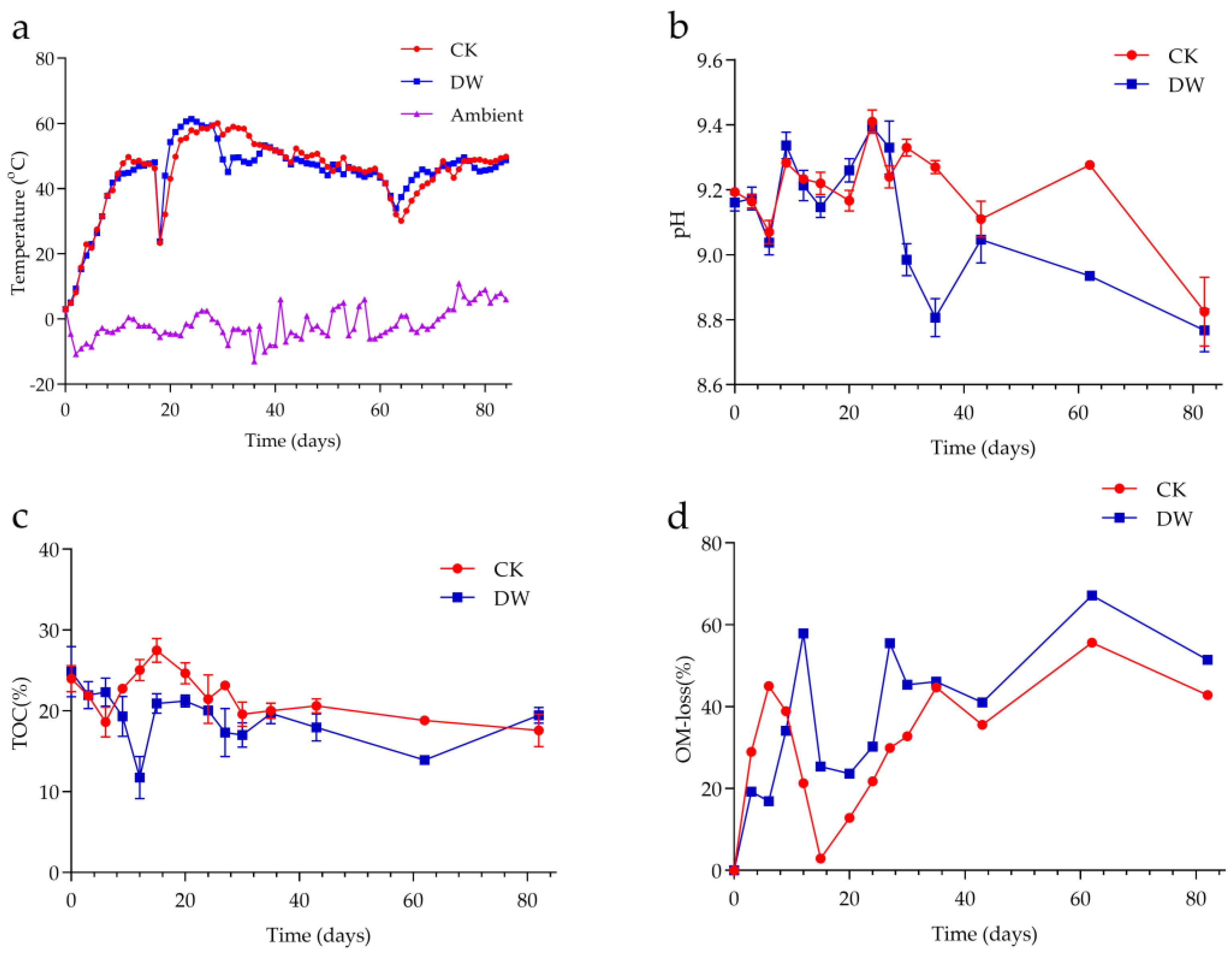
3.2. Effect of Inoculants on Compost Temperature, pH, and OM Degradation
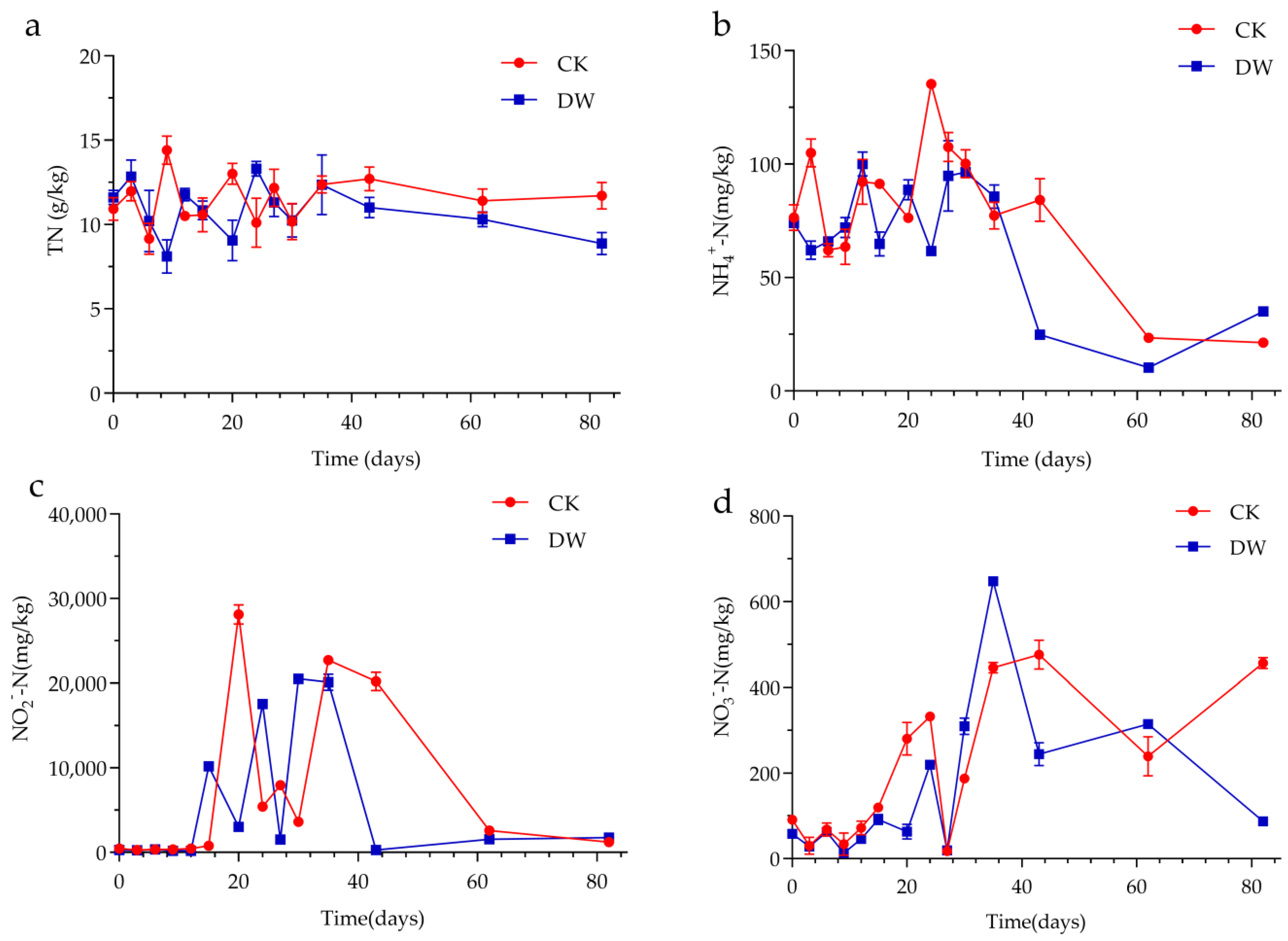
3.3. Conversion of Nitrogen during Composting
3.4. Compost Maturity Analysis
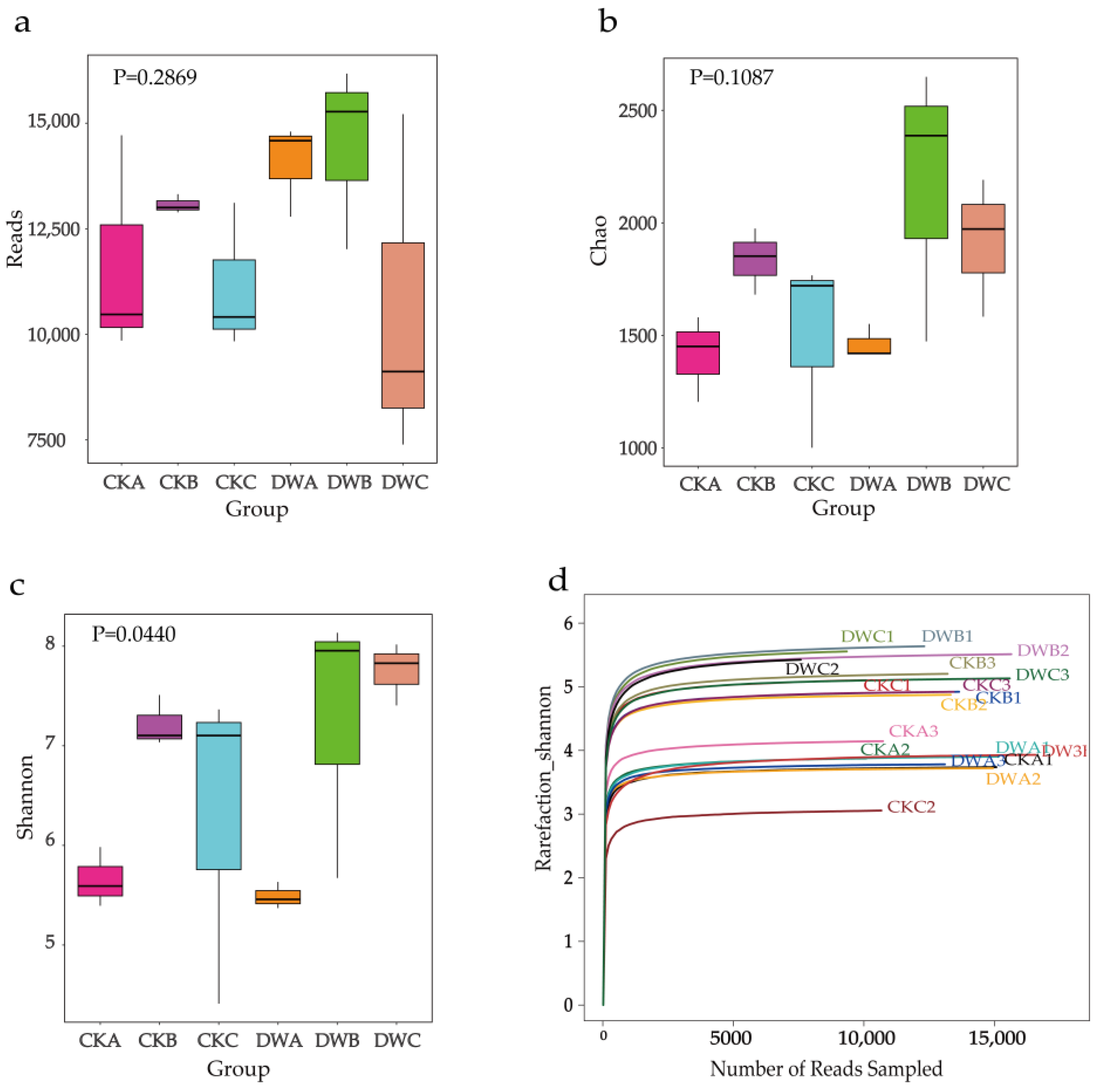
3.5. Changes in Microbial Communities and the Relationship between Environmental Factors and Microbial Communities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Wang, X.; Zhang, X.; Misselbrook, T.H.; Bai, Z.; Wang, H.; Ma, L. The effects of electric field assisted composting on ammonia and nitrous oxide emissions varied with different electrolytes. Bioresour. Technol. 2022, 344, 126194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Y.; Zhang, J.; Zeng, G.; Dong, H.; Cao, W.; Fang, W.; Cheng, Y.; Wang, Y.; Ning, Q. Impacts of iron oxide nanoparticles on organic matter degradation and microbial enzyme activities during agricultural waste composting. Waste Manag. 2019, 95, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, D.; Zhang, Z.; Zhao, Y.; Xie, X.; Wu, J.; Lu, Q.; Wei, Z. Effect of cold-adapted microbial agent inoculation on enzyme activities during composting start-up at low temperature. Bioresour. Technol. 2017, 244, 635–640. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, K.; Xu, P.; Huang, X.; Gu, W.; Zhang, F.; Tang, S. Evolution of microbial community diversity and enzymatic activity during composting. Res. Microbiol. 2013, 164, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, X.; Huang, Y.; Huang, H. Inoculation with nitrogen turnover bacterial agent appropriately increasing nitrogen and promoting maturity in pig manure composting. Waste Manag. 2015, 39, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, S.; Du, S.; Zhang, M.; Cheng, R.; Wu, D. Strategy to Strengthen Rural Domestic Waste Composting at Low Temperature: Choice of Ventilation Condition. Waste Biomass Valorization 2020, 11, 6649–6665. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, H.; Wu, M. Energy performance and consumption for biogas heat pump air conditioner. Energy 2010, 35, 5497–5502. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, J.; Zhao, Q.; Wang, K.; Zhang, Y.; Zheng, Z.; Hao, X. Bioelectrochemically-assisted anaerobic composting process enhancing compost maturity of dewatered sludge with synchronous electricity generation. Bioresour. Technol. 2015, 193, 1–7. [Google Scholar] [CrossRef]
- Xie, X.Y.; Zhao, Y.; Sun, Q.H.; Wang, X.Q.; Cui, H.Y.; Zhang, X.; Li, Y.J.; Wei, Z.M. A novel method for contributing to composting start-up at low temperature by inoculating cold-adapted microbial consortium. Bioresour. Technol. 2017, 238, 39–47. [Google Scholar] [CrossRef]
- Pieter, D.M.; Dominique, A.; Craig, C.; A, C.D. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Chen, H.; Wang, Q.; Liu, T.; Duan, Y.; Awasthi, S.K.; Ren, X.; Tu, Z.; Li, J.; Zhao, J.; et al. Succession of bacteria diversity in the poultry manure composted mixed with clay: Studies upon its dynamics and associations with physicochemical and gaseous parameters. Bioresour. Technol. 2018, 267, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Mendoza-Hernandez, D.; Garcia-de-la-Fuente, R.; Abad, M.; Belda, R.M. Composting versus vermicomposting: A comparative study of organic matter evolution through straight and combined processes. Bioresour. Technol. 2012, 118, 296–305. [Google Scholar] [CrossRef] [PubMed]
- GB/T 32737-2016; The Determination of Soil Ammonium Nitrogen Ultraviolet Spectrophotometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2016.
- HJ 634-2012; Determination of Ammonium Nitrogen, Nitrite Nitrogen, and Nitrate Nitrogen in Soil—Ammonia Alkaline Solution Extraction Spectrophotometry. China Environmental Science Press: Beijing, China, 2012.
- Li, S.; Li, J.; Yuan, J.; Li, G.; Zang, B.; Li, Y. The influences of inoculants from municipal sludge and solid waste on compost stability, maturity and enzyme activities during chicken manure composting. Environ. Technol. 2017, 38, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, R.; Fiamma, M.; Deligios, M.; Pintus, G.; Pellizzaro, G.; Canu, A.; Duce, P.; Squartini, A.; Muresu, R.; Cappuccinelli, P. Microbial immigration across the Mediterranean via airborne dust. Sci. Rep. 2015, 5, 16306. [Google Scholar] [CrossRef]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sánchez-Monedero, M.A. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2009, 101, 1239–1246. [Google Scholar] [CrossRef]
- Sundberg, C.; Smars, S.; Jonsson, H. Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour. Technol. 2004, 95, 145–150. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef]
- Vargas-García, M.C.; Suárez-Estrella, F.; López, M.J.; Moreno, J. Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag. 2009, 30, 771–778. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong, J.W.C. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure-straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Zhang, Y.; Awasthi, M.K.; He, Y.; Li, R.; Zhang, Z. Improvement of humification and mechanism of nitrogen transformation during pig manure composting with Black Tourmaline. Bioresour. Technol. 2020, 307, 123236. [Google Scholar] [CrossRef]
- Wang, S.P.; Wang, L.; Sun, Z.Y.; Wang, S.T.; Shen, C.H.; Tang, Y.Q.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zeng, G.; Dong, H.; Chen, Y.; Huang, C.; Zhu, Y.; Xu, R.; Cheng, Y.; Hou, K.; et al. Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 2018, 261, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Wang, X.; Wang, S.; Ma, L.; Ma, W. Transformation of nitrogen and carbon during composting of manure litter with different methods. Bioresour. Technol. 2019, 293, 122046. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.J.; Sanchez-Arias, V.; Rodriguez, L.; Villasenor, J. Feasibility of composting combinations of sewage sludge, olive mill waste and winery waste in a rotary drum reactor. Waste Manag. 2010, 30, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.K.; Barrington, S.; Martinez, J.; King, S. Effectiveness of three bulking agents for food waste composting. Waste Manag. 2009, 29, 197–203. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.; Ren, X.; Zhang, Z.; Awasthi, M.K. Positive impact of biochar alone and combined with bacterial consortium amendment on improvement of bacterial community during cow manure composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, H.; Zhang, J.; Chen, Y.; Zeng, G.; Yuan, Y.; Cao, W.; Fang, W.; Hou, K.; Wang, B.; et al. Influence of FeONPs amendment on nitrogen conservation and microbial community succession during composting of agricultural waste: Relative contributions of ammonia-oxidizing bacteria and archaea to nitrogen conservation. Bioresour. Technol. 2019, 287, 121463. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, R.; Liu, T.; Zhang, G.; Wu, S.; Xu, K.; Zhang, Y.; Wang, Q.; Kang, J.; Zhang, Z.; et al. Effect of inoculation with newly isolated thermotolerant ammonia-oxidizing bacteria on nitrogen conversion and microbial community during cattle manure composting. J. Environ. Manag. 2022, 317, 115474. [Google Scholar] [CrossRef]
- JUNI, E.; HEYM, G.A. Psychrobacter immobilis gen. nov., sp. nov.: Genospecies Composed of Gram-Negative, Aerobic, Oxidase-Positive Coccobacilli. Int. J. Syst. Bacteriol. 1986, 36, 388–391. [Google Scholar] [CrossRef]
- Raquel, H.-O.; Anissa, N.; Alexis, C.; Perrine, H.; Damien, M.; Sylvain, B.; Chantal, B.; Dominique, C. Psychrobacter pasteurii and Psychrobacter piechaudii sp. nov., two novel species within the genus Psychrobacter. Int. J. Syst. Evol. Microbiol. 2017, 67, 3192–3197. [Google Scholar] [CrossRef]
- Abdellah, Y.A.Y.; Li, C. Livestock Manure Composting in Cold Regions: Challenges and Solutions. Agric. (Pol’nohospodárstvo) 2020, 66, 1–14. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H. Effects of inoculation with lignocellulose-degrading microorganisms on antibiotic resistance genes and the bacterial community during co-composting of swine manure with spent mushroom substrate. Environ. Pollut. 2019, 252, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, G.; Castellano-Hinojosa, A.; Correa-Galeote, D.; Bedmar, E.J. Evolution of bacterial diversity during two-phase olive mill waste (“alperujo”) composting by 16S rRNA gene pyrosequencing. Bioresour. Technol. 2017, 224, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Akunna, J.C.; Clark, M. Performance of a granular-bed anaerobic baffled reactor (GRABBR) treating whisky distillery wastewater. Bioresour. Technol. 2000, 74, 257–261. [Google Scholar] [CrossRef]
- Zhongjun, J.; Ralf, C. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Chodak, M.; Gołębiewski, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Hörnström, E. Phytoplankton in 63 limed lakes in comparison with the distribution in 500 untreated lakes with varying pH. Hydrobiologia 2002, 470, 115–126. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Tkachuk, V.L.; Krause, D.O.; Knox, N.C.; Hamm, A.C.; Zvomuya, F.; Ominski, K.H.; McAllister, T.A. Targeted 16S rRNA high-throughput sequencing to characterize microbial communities during composting of livestock mortalities. J. Appl. Microbiol. 2014, 116, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and diversity of microorganisms and their association with physicochemical properties during green waste thermophilic composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Qu, J.; Zhu, L.; Wang, T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J Microbiol Biotechnol 2016, 32, 101. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.K.; Pope, P.B.; Pedersen, H.L.; Gupta, R.; Morrison, M.; Willats, W.G.T.; Eijsink, V.G.H. Two SusD-like proteins encoded within a polysaccharide utilization locus of an uncultured ruminant Bacteroidetes phylotype bind strongly to cellulose. Appl. Environ. Microbiol. 2012, 78, 5935–5937. [Google Scholar] [CrossRef]
- Xu, S.; Lu, W.; Liu, Y.; Ming, Z.; Liu, Y.; Meng, R.; Wang, H. Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag. 2016, 63, 41–48. [Google Scholar] [CrossRef]
- Preem, J.-K.; Truu, J.; Truu, M.; Mander, Ü.; Oopkaup, K.; Lõhmus, K.; Helmisaari, H.-S.; Uri, V.; Zobel, M. Bacterial community structure and its relationship to soil physico-chemical characteristics in alder stands with different management histories. Ecol. Eng. 2012, 49, 10–17. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, H.U.; Park, K.Y.; Park, H.; Cho, K.H. Identification of the bacterial community of a pilot-scale thermophilic aerobic bioreactor treating sewage sludge. Int. Biodeterior. Biodegrad. 2014, 92, 66–70. [Google Scholar] [CrossRef]
- Guttigoli, A.; Zaman, M.M. Bacteremia and possible endocarditis caused by Moraxella phenylpyruvica. South. Med. J. 2000, 93, 708–709. [Google Scholar] [CrossRef]
- Lloyd-Puryear, M.; Wallace, D.; Baldwin, T.; Hollis, D.G. Meningitis caused by Psychrobacter immobilis in an infant. J. Clin. Microbiol. 1991, 29, 2041–2042. [Google Scholar] [CrossRef]
- Pieter, D.; Michèle, J.; Mario, V.; Georges, W. Psychrobacter isolates of human origin, other than Psychrobacter phenylpyruvicus, are predominantly Psychrobacter faecalis and Psychrobacter pulmonis, with emended description of P. faecalis. Int. J. Syst. Evol. Microbiol. 2012, 62, 671–674. [Google Scholar] [CrossRef]



| Material | Moisture | TOC | TN | C/N |
|---|---|---|---|---|
| Cow dung | 70.21% | 24.79% | 1.25% | 19.82 |
| Straw | 5.96% | 49.86% | 0.60% | 83.34 |
| Strain | Degrades Casein | Degradation of Cellulose | Nitrogen Fixation | Phosphorus Solubilizing |
|---|---|---|---|---|
| Chryseobacterium sp. | + | + | + | − |
| Providencia alcalifaciens | + | + | + | + |
| Brevibacterium frigoritolerans | + | + | + | − |
| Bacillus thuringiensis | + | + | + | − |
| Bacillus mycoides | + | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, D.; Mei, Y.; Zhang, K.; Xu, L.; Zhang, X.; Wang, H. Effects of Inoculation with Newly Isolated Cold-Adapted Bacteria on Winter Cattle Manure Composting in the Tibetan Plateau. Fermentation 2023, 9, 857. https://doi.org/10.3390/fermentation9090857
Huang Y, Wu D, Mei Y, Zhang K, Xu L, Zhang X, Wang H. Effects of Inoculation with Newly Isolated Cold-Adapted Bacteria on Winter Cattle Manure Composting in the Tibetan Plateau. Fermentation. 2023; 9(9):857. https://doi.org/10.3390/fermentation9090857
Chicago/Turabian StyleHuang, Yichen, Diao Wu, Yan Mei, Kun Zhang, Liping Xu, Xin Zhang, and Haiying Wang. 2023. "Effects of Inoculation with Newly Isolated Cold-Adapted Bacteria on Winter Cattle Manure Composting in the Tibetan Plateau" Fermentation 9, no. 9: 857. https://doi.org/10.3390/fermentation9090857
APA StyleHuang, Y., Wu, D., Mei, Y., Zhang, K., Xu, L., Zhang, X., & Wang, H. (2023). Effects of Inoculation with Newly Isolated Cold-Adapted Bacteria on Winter Cattle Manure Composting in the Tibetan Plateau. Fermentation, 9(9), 857. https://doi.org/10.3390/fermentation9090857






