Abstract
Bacillus pumilus is widely used as a biocontrol agent. To further develop the biological control potential of B. pumilus LYMC-3 against pine blight, a statistical experimental design was used to optimize a liquid medium using low-cost substrates to improve its antagonistic activity against Sphaeropsis sapinea. Through the plate antagonism test and greenhouse control effect test, this study determines the antifungal effect of strain LYMC-3 against S. sapinea and pine blight. Moreover, response surface optimization methodologies were used to systematically optimize the medium composition and culture conditions of the LYMC-3 strain. The plate antagonism test showed that the inhibition rate of LYMC-3 fermentation filtrate (diluted 5-fold) was 66.09%. The greenhouse control effect test showed that the control effect of its fermentation filtrate on shoot blight reached 89.99%. The response surface optimization test ultimately determined that a higher inhibition rate can be achieved under these conditions: the optimal medium components were 7.2 g/L glucose, 15 g/L peptone, and 7.1 g/L magnesium sulfate; the optimal culture conditions were 52% liquid volume, 28 °C culture temperature, an initial pH of 7, and 1% bacterial inoculation volume. Under the optimized system, the five-fold diluted LYMC-3 fermentation filtrate inhibition rate against S. sapinea was 81.23%, which was 15.84% higher than that before optimization. Meanwhile, optimize the selection of lower-cost and more commonly used glucose instead of beef paste as the carbon source for the culture medium, and choose cheaper magnesium sulfate instead of sodium chloride as the nitrogen source.
1. Introduction
Pine blight is a host-dominant devastating disease, where needles of pine trees turn yellow followed by reddish brown through the growing season (wilting), accompanied by branches wilt, and stem ulcers and dead tops, ultimately leading to the sudden death of the tree [1]. The causative agent is Sphaeropsis sapinea (Fr.) Dyko and Sutton (formerly Diplodia pinea (Desm.) Kickx, Petrak, and Sydow), which is distributed across 65 countries in Africa, South America, China, the United States, New Zealand, and Australia [2,3]. Due to the warm and dry climate over recent years, pine dieback disease is endemic in European countries and more than 10 provinces in China [4,5]. This has resulted in huge losses of pine plantations in many countries and regions and, therefore, the need to control this disease has received increasing attention.
In recent years, environmental pollution has become an increasingly serious problem and the use of microbial bacterial agents to control plant pests and diseases has gradually increased in order to reduce the environmental problems caused by chemical pesticides. Bacillus is capable of producing endophytic spores that survive under harsh conditions, a property that places it as an ideal biological control agent (BCA) [6]. Meanwhile, Bacillus is one of the most common bacteria among plant endophytes [7] and their endophytic colonization ability plays an important role in the biological control of vascular plant diseases. B. pumiluss is one of the more widely used biocontrol microorganisms within the genus Bacillus [8,9] and has been used against some common plant diseases, such as rice blast [10], strawberry black rot [11], and cucumber wilt [12]. In addition, B. pumilus also plays a powerful role in promoting plant growth and biodegradation [13,14]. Therefore, B. pumilus can be applied to alleviate agriculture and forestry problems instead of using chemical pesticides, with B. pumilus suppressing the occurrence of certain plant diseases and also maintaining the stability of the ecosystem to a certain extent while reducing the adverse effects of chemical pesticides on human health and the environment. Hence, the use of B. pumilus as a biocontrol agent has broad application prospects.
Li et al. isolated a strain of B. pumilus LYMC-3 from the stem of Pinus sylvestris, with highly toxic nematocidal activity [15] and found that the strain was endophytic and could colonize well in P. massoniana Lamb. [16]. Later studies showed that this strain has a broad inhibitory spectrum towards eight plant pathogens, including Sphaeropsis sapinea, Phomopsis macrospora, Pestalotiopsis theae, and Botryosphaeria dothiorella [17]. To further understand the control potential of B. pumilus LYMC-3 against pine blight and to explore the optimal fermentation conditions for antagonizing S. sapinea, this study investigated the greenhouse efficacy of strain LYMC-3 against pine blight by inoculating 2-year-old P. massoniana Lamb. seedlings, and systematically optimized the medium components and fermentation conditions for strain LYMC-3 using the response surface design method to provide a basis for future industrialization of this strain.
2. Materials and Methods
2.1. Preparation of Test Materials
2.1.1. Source of Test Medium and Strain
Bacterial strain activation medium: Nutrient Agar (NA) (components: beef paste 5 g, peptone 10 g, sodium chloride 5 g, agar 20 g, and water to 1 L, pH 7.2).
Bacterial seed solution and single-factor basal medium: Nutrient Broth (NB) medium (components: beef paste 5 g, peptone 10 g, sodium chloride 5 g, and water to 1 L, pH of 7.2).
Fungal medium: Potato Dextrose Agar (PDA) (200 g peeled potato was boiled in water for 20 min and filtered after the addition of glucose 20 g and agar 20 g; water to 1 L).
The B. pumilus strain LYMC-3 was isolated from the stem of P. massoniana Lamb. In Sui Tang Botanical Gardens, Luoyang, China, and is now stored in the China Typical Culture Collection under the catalogue number CCTCC NO:M 2016775.
The S. sapinea strain JX1-2 was isolated from Jiangxi Province, China, and is now stored in the Forest Pathology Laboratory of Nanjing Forestry University.
2.1.2. Strain Activation, Preparation of Seed Solution and Acquisition of Fermentation Broth, Fermentation Filtrate and Bacterial Suspension
B. pumilus was inoculated on NA plates using the plate scribing method [18] and incubated at 28 °C for 24 h to obtain single colonies. A single colony was inoculated into 40% NB and incubated whilst shaking at a constant temperature of 28 °C and 200 rpm for 8 h to obtain the first level seed solution. The primary seed solution (2% inoculum) was transferred into fresh NB medium and incubated in the shaker at 28 °C and 200 rpm for 24 h to obtain the secondary seed solution. The secondary seed solution of B. pumilus LYMC-3 at 2% inoculum was transferred to a 250 mL flask with 100 mL NB medium and incubated at 28 °C, 180 rpm for 72 h to obtain the fermentation broth. The fermentation broth was centrifuged at 4 °C and 12,859 g for 10 min, and the supernatant was collected and filtered through a 0.22 μm microporous membrane to remove bacteria and sterilize the fermentation filtrate. After centrifugation of the fermentation broth, the bacterial precipitate was completely resuspended with 1/2 of the original volume of sterile water to a final bacterial concentration of 3 × 108 cfu/mL, which is the test suspension.
2.2. Plate Antagonism Test on Strain LYMC-3 against Sphaeropsis sapinea
The inhibitory effect of B. pumilus LYMC-3 on the pathogenic fungus Sphaeropsis sapinea was determined using the plate standoff method [19] and the mycelial growth rate method [20].
2.2.1. Plate Standoff Method
The pathogenic fungi at 5 d of activation were inoculated in the center of a PDA plate with a 6 mm cake prepared using a puncher, and B. pumilus LYMC-3 was injected in a line at the ends of the cake about 2.5 cm from the center of the circle of pathogenic bacteria. The control was PDA inoculated with only S. sapinea. Each treatment was repeated three times. The treated PDA plates were incubated upside down in a constant temperature incubator at 28 °C for 5 d. The growth radius of the pathogenic colonies facing toward the antagonistic bacteria (measured from the center of the cake circle) was measured.
Inhibition rate = (pathogen control colony radius − treatment colony radius)/(pathogen control colony radius) × 100%.
2.2.2. Mycelial Growth Rate Method
The strain LYMC-3 fermentation filtrate was mixed with PDA medium, heated, and cooled to 45–50 °C and different dilutions (5×, 25×, 50×, 100×, and 250×) were prepared, of which 20 mL was poured onto each plate. The 6 mm diameter pine shoot blight fungal cake was inoculated in the center of the cooled and solidified agar. The control plates had the fermentation filtrate replaced with sterile water and the agar was inoculated with the pathogenic fungus. All treatments were replicated three times. The plates were incubated at a constant temperature of 28 °C and inverted and after 5 days; the diameter of the pathogenic colonies was measured using the crossover method to calculate the inhibition rate [21].
Inhibition rate = (control colony diameter − treatment colony diameter)/(control colony diameter − pathogenic fungus cake diameter) × 100%.
2.3. Greenhouse Efficacy Test of Strain LYMC-3 against Pine Blight Disease
Two-year-old P. massoniana Lamb. seedlings with a similar growth status were selected and sprayed with 2 mL each of fermentation solution, bacterial suspension, and sterile fermentation filtrate of the LYMC-3 strain using the foliar spraying method. The concentration of the fermentation solution and bacterial suspension was 3 × 108 cfu/mL, and sterile water and NB medium were used as controls. Each treatment was repeated six times and, after 4 days, both treatment and control groups were inoculated with equal amounts of S. sapinea cake using the wounded block inoculation method [22]. Firstly, a sterile needle (insect needle with a diameter of 0.71 mm) was used to puncture holes in the middle and upper region of the stem of the Pinus massoniana seedlings. Secondly, a 5 mm long mycelium block of Sphaeropsis sapinea was inoculated on each puncture. Afterwards, the inoculation site was tied with a sealing film (Parafilm, Neenah, WI, USA) and the relative humidity of the inoculation environment was maintained using a humidifier (Bear, Guangzhou, China). The inoculated plants were placed in a controlled greenhouse, and the disease was observed and recorded every 4 days after Inoculation. All treatments were replicated six times. The degree of disease was classified into four levels: 0 for no disease in the whole plant, 1 for ≤25% yellowing in the whole plant, 2 for ≥25.1~50% yellowing in the whole plant, 3 for ≥50.1~75% yellowing in the whole plant, and 4 for >75.1% yellowing in the whole plant.
Disease index formula: disease index = 100 × ∑ (number of diseased plants at each level × representative value of each level)/(total number of plants surveyed × representative value of the highest level).
Relative control effect formula: relative control effect (%) = 100 × (disease index of pathogen only − disease index of different fermentation products of the applied bacterium)/disease index of pathogen only).
2.4. Single-Factor Optimization Test of Strain LYMC-3 Fermentation Conditions
2.4.1. Optimization of Culture Medium Components
Several test carbon sources (glucose, soluble starch, maltose, mannitol, beef paste, and sucrose), nitrogen sources (peptone, yeast powder, urea, soybean peptone, ammonium sulfate, and tryptone), and inorganic salts (magnesium sulfate, calcium chloride, potassium chloride, and zinc sulfate) were selected to replace the corresponding components in the basal medium (NB) in equal amounts. The fermentation filtrate was prepared by shaking B. pumilus LYMC-3 in a 50 mL shake flask for 72 h at 40% loading, 2% inoculum, 200 rpm, and 28 °C. The fermentation filtrate was diluted 5-fold with PDA medium and poured onto plates, and the diameter of pathogenic colonies was measured with reference to the mycelial growth rate method in Section 2.2.2 to determine the best carbon source, nitrogen source, and inorganic salt type. The inhibition effect of each component fermentation filtrate at different concentrations (dilution 5-, 25-, 50-, 100-, and 250-fold) was screened on S. sapinea to determine the subsequent test concentration.
2.4.2. Optimization of Culture Conditions
Using the optimized medium components, the initial culture conditions were 2% inoculum, starting pH at 7.0, loading volume of 40%, temperature at 28 °C, and speed of 200 rpm. The four factors of fermentation temperature, initial pH, inoculum, and loading volume were tested in turn to determine the degree of inhibition of LYMC-3 fermentation filtrate against S. sapinea and to determine the best fermentation conditions for the four factors.
2.5. Response Surface Optimization Test on LYMC-3 Fermentation Conditions
Response Surface Methodology (RSM) is a statistical method to solve multivariate problems by using rational experimental design methods and obtaining clear data through experiments. This is achieved using multiple quadratic regression equations to fit the functional relationship between factors and response values, and seeking optimal process parameters through the analysis of regression equations [23]. The main objective of this study was to examine the effect of test factors (LYMC-3 media components and culture conditions) on the response value (strain LYMC-3 on the rate of inhibition towards S. sapinea), and then optimize the fermentation conditions to obtain the maximum inhibition rate. The response surface optimization test first used the Plackett-Burman (PB) test to screen the test factors that resulted in a significant effect. This was followed by conducting the steepest climb test to determine the optimum concentration range of the important factors, and then the Box–Behnken design was performed to initially fit the quadratic regression equation of response values and test factors to determine the optimal combination of test factors. ANOVA was then used to calculate the statistical significance of the fitted quadratic model. Finally, the Box–Behnken design was used to generate a three-dimensional response surface to visualize the independent interaction of the test factors with the response values, and to evaluate whether the results predicted by the regression model were satisfactory through experimental validation. In this study, Design-Expert 10 software (Stat-Ease, Minneapolis, MN, USA, https://design-expert2.software.informer.com/10.0/ (accessed on 1 July 2022)) was used for experimental design, mathematical modeling, and optimization.
2.5.1. Plackett–Burman (PB) Trial
The PB design is able to estimate the key effects of factors with a minimum number of trials in order to quickly and efficiently select the most significantly influenced factors from the many factors examined for the next step of the test. Referring to Tao et al. [24] and Wu et al. [25], a PB test protocol was developed using Design-Expert 10 software based on the results of the single-factor optimization test, with one low level and one high level for each variable, and three replications were set for each group of tests. The inhibition rate of strain LYMC-3 was used as the response value to screen for factors that had significant effects on the mycelial growth of S. sapinea.
2.5.2. Steepest Climb Test
The steepest climb test can guide the factors of highest significance to approach the maximum response area, so as to determine the optimal level combination center, and further optimize the test scheme so that the Box–Behnken design achieves the optimal response value more quickly. The PB test results were used to filter the more significant impact of the factors on the inhibition rate, and their positive and negative effects (positive effects from low values upward; negative effects from high values downward decrease). The test results also assisted in determining the climbing direction and step length, increasing the power of the test to quickly approach the best region, so as to obtain the Box–Behnken design (BBD) center point.
2.5.3. Box–Behnken Design
The Box–Behnken design (BBD) is one of the most commonly used designs in RSM, and is a class of rotatable or nearly rotatable second-order design based on a three-level incomplete factorial design. The Box–Behnken design is based on the variables and concentrations screened by the PB trial and the steepest climb test, and the trial results were analyzed and processed using the Design-Expert 10 software. By using the Box–Behnken design, the effects of different carbon, nitrogen, and inorganic salt concentrations on fungal inhibition by the LYMC-3 fermentation medium were investigated, and the relationships between the response values and the quadratic regression equations of the test factors were fitted. In addition, an analysis of variance (ANOVA) was used to analyze the interaction between the input parameters and to determine the statistical significance (i.e., P and F values) of the fitted quadratic models.
2.5.4. Response Surface Optimization
Based on the Box–Behnken design, a three-dimensional response surface was generated to visualize the independent interactions of the test factors on the response values (inhibition rate). Maximizing the expectation function over a given response range is the primary goal of optimization. In this study, the maximum inhibition rate was targeted to obtain the expected value of the test factor of interest [26].
2.5.5. Comparison of Fungal Inhibition Ability of Strain LYMC-3 before and after Medium Optimization
The mycelial growth rate method was used to determine the inhibition rate of strain LYMC-3 against S. sapinea before and after optimization of fermentation conditions, and each treatment was repeated five times.
2.6. Data Analysis and Processing
Analysis of variance and Duncan’s multiple comparisons were performed using SPSS Statistics 26 (IBM, Armonk, NY, USA) software (p < 0.05); response surface design was conducted using Design-Expert 10; and translation of the data was achieved using Prism 9.3.1(GraphPad, San Diego, CA, USA).
3. Results and Analysis
3.1. Antagonistic Activity of Strain LYMC-3 against S. sapinea
As shown in Figure 1, strain LYMC-3 displayed significant antagonistic activity against S. sapinea when it was cultured together with S. sapinea. LYMC-3 could effectively limit the growth of pathogenic mycelium so that the pathogenic colonies could not extend around the plate (Figure 1B). After 5 days of incubation, the pathogenic colonies in the control group grew normally (Figure 1A) with an average radius of 4.51 cm, while in the plate inoculated with strain LYMC-3, the average radius of the pathogenic colonies was only 1.23 cm, and the inhibition rate of strain LYMC-3 achieved 72.65%.
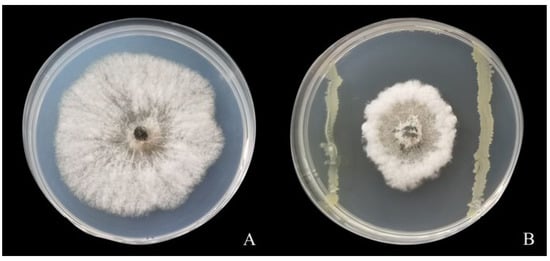
Figure 1.
Inhibitory effect of LYMC-3 strain on S. sapinea. Note: (A) Non-treated S. sapinea; (B) Antagonistic S. sapinea by B. pumilus.
In addition, the effect of different dilutions of the LYMC-3 fermentation filtrate on S. sapinea mycelial growth was different (Figure 2). The strongest inhibitory effect was noted at five-fold dilution with an inhibition rate of 66.09%, after which the inhibition effect gradually decreased with the increase in the dilution factor. At 25-fold dilution, some inhibition was observable (20.14%), while only 4.58% of the pathogen was inhibited when treated with 50-fold dilution fermentation mixture, and at 100-times dilution; the strain LYMC-3 fermentation filtrate had no inhibitory effect.
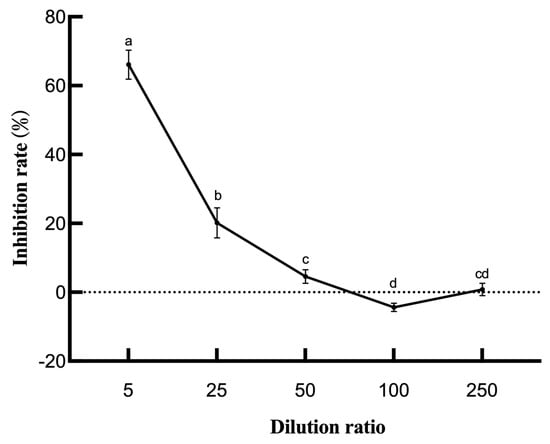
Figure 2.
Inhibition rate of the B. pumilus fermentation broth against S. sapinea. Note: Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with different letters have significant differences (p < 0.05; Duncan test).
3.2. Greenhouse Efficacy of Strain LYMC-3 against Pine Dieback Disease
The fermentation solution, bacterial suspension, and sterile fermentation filtrate obtained from the LYMC-3 culture were applied individually to 2-year-old Pinus massoniana Lamb. seedlings via foliar spraying, and 4 days later the seedlings were inoculated with S. sapinea. The results showed that on the 6th day after inoculation, control seedlings subjected to treatment with water (CK 1) and NB (CK 2) showed different degrees of wilt symptoms, some of the pine seedling needles began to turn green and yellow at the tips, and some began to wilt. On the 16th day after inoculation, the disease indices of CK1 and CK2 reached 83.33 and 66.67, respectively (Table 1), and the disease indices of fermentation solution, bacterial suspension, and fermentation filtrate treatments reached 23.33, 30, and 6.67. The effect of fermentation solution, bacterial suspension, and fermentation filtrate treatments reached 65.01%, 63.99%, and 89.99%, respectively (Table 1, Figure 3). The experimental results showed that foliar spraying with B. pumilus LYMC-3 suspension, fermentation solution, and fermentation filtrate could prevent pine dieback disease, with the fermentation filtrate providing the best control effect.

Table 1.
Effects of different B. pumilus fermentation treatments on indoor control of pine blight. Note: (CK1) The control group sprayed with sterile water; (CK2) The control group sprayed with NB medium.

Figure 3.
Biocontrol effects of different B. pumilus inoculants on pine dieback. Note: (A) NB culture sprayed at the base on pine dieback; (B) The biocontrol effect of spraying sterile water on pine blight; (C) The biocontrol effect of spraying fermentation filtrate on pine dieback; (D) Spraying fermentation broth on pine dieback reduced the occurrence of pine blight; (E) The biocontrol effect of spraying bacterial suspension on pine blight.
3.3. Optimization of Strain LYMC-3 Medium Components
3.3.1. Effect of Different Carbon Sources on the Growth of S. sapinea
NB medium was used as the base fermentation medium, and the inhibition effect of LYMC-3 fermentation broth on S. sapinea was measured when glucose, soluble starch, maltose, mannitol, beef paste, and sucrose were selected as the individual carbon sources within the medium. As shown in Figure 4A, the inhibition rate of the LYMC-3 fermentation filtrate was the highest when glucose was used as the carbon source, so it is appropriate to select glucose as the most suitable carbon source for strain LYMC-3 to antagonize S. sapinea. Meanwhile, the inhibition rate of strain LYMC-3 against S. sapinea at different glucose concentrations was different (Figure 4B), with the highest inhibition rate achieved at 7 g/L glucose concentration. Therefore, the glucose concentration of 7 g/L was selected as the carbon source for the subsequent fermentation tests.
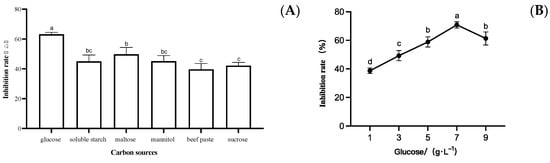
Figure 4.
Effects of different carbon sources on the growth of S. sapinea. Note: (A) Inhibition rate of B. pumilus fermentation filtrate with different carbon source culture media on S. sapinea; (B) Inhibition rate of fermentation filtrate of B. pumilus with different glucose concentrations on S. sapinea. Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with different letters have significant differences (p < 0.05; Duncan test).
3.3.2. Effect of Different Nitrogen Sources on the Growth of S. sapinea
The inhibitory effect of fermentation filtrate on S. sapinea was measured when peptone, yeast powder, urea, soybean peptone, ammonium sulfate, and tryptone were individually assessed as the nitrogen sources of the medium; the optimized NB medium with glucose at a concentration of 7 g/L as the carbon source was used as the base fermentation medium. The strain LYMC-3 fermentation filtrate had the highest inhibition rate when peptone was used as the nitrogen source (Figure 5A), so peptone was selected as the most suitable nitrogen source for strain LYMC-3 to antagonize S. sapinea. As shown in Figure 5B, the inhibitory feature of strain LYMC-3 against S. sapinea varied under different peptone concentrations, and the highest inhibition rate was achieved when the peptone concentration was 15 g/L. Therefore, 15 g/L peptone was selected as the nitrogen source for the subsequent fermentation tests.
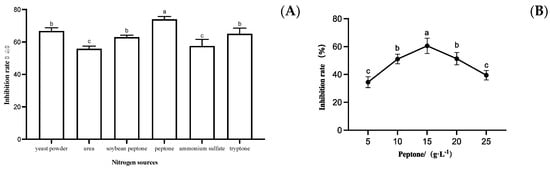
Figure 5.
Effects of different nitrogen sources on the growth of S. sapinea. Note: (A) Inhibition rate of B. pumilus fermentation filtrate with different nitrogen source culture media on S. sapinea; (B) Inhibition rate of fermentation filtrate of B. pumilus with different peptone concentrations on S. sapinea. Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with different letters have significant differences (p < 0.05; Duncan test).
3.3.3. Effect of Different Inorganic Salts on the Growth of S. sapine
The optimized NB medium with a concentration of 7 g/L glucose as the carbon source and 15 g/L peptone as the nitrogen source was used as the base fermentation medium, and the inorganic salts of magnesium sulfate, calcium chloride, sodium chloride, potassium chloride, and zinc sulfate were individually tested to determine the inhibition effect of the fermentation filtrate on S. sapine. From Figure 6A, it can be seen that the inhibition rate of strain LYMC-3 fermentation filtrate was the highest when magnesium sulfate was used as the inorganic salt, so magnesium sulfate was selected as the most suitable inorganic salt for strain LYMC-3 to antagonize S. sapine. As shown in Figure 6B, the inhibition rate of strain LYMC-3 against S. sapine varied under different magnesium sulfate concentrations, and the highest inhibition rate was achieved when the magnesium sulfate concentration was 7 g/L. Therefore, at this point, the optimized fermentation medium for strain LYMC-3 is NB with 7 g/L glucose as the carbon source and 15 g/L peptone as the nitrogen source and 7 g/L magnesium sulfate.
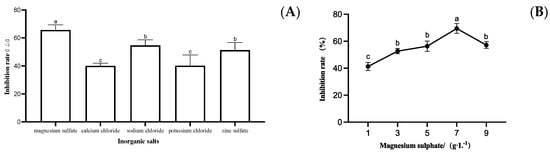
Figure 6.
Effects of different inorganic salts on the growth of S. sapine. Note: (A) Inhibition rate of B. pumilus fermentation filtrate with different inorganic salts culture media on S. sapinea; (B) Inhibition rate of fermentation filtrate of B. pumilus with different magnesium sulfate concentrations on S. sapinea. Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with different letters have significant differences (p < 0.05; Duncan test).
3.4. Optimization Results of Culture Conditions of Strain LYMC-3
As shown in Figure 7, strain LYMC-3 showed the highest inhibition rate against S. sapine when the initial pH was 7, the incubation temperature was 28 °C, the loading volume was 50%, and the inoculum was 1%. The antagonistic effect of strain LYMC-3 on S. sapine was the strongest compared with other culture conditions when the growth rate of S. sapine mycelium was inhibited. Therefore, the subsequent fermentation conditions for strain LYMC-3 were selected as pH 7, incubation temperature 28 °C, loading volume 50%, and inoculum volume 1%.
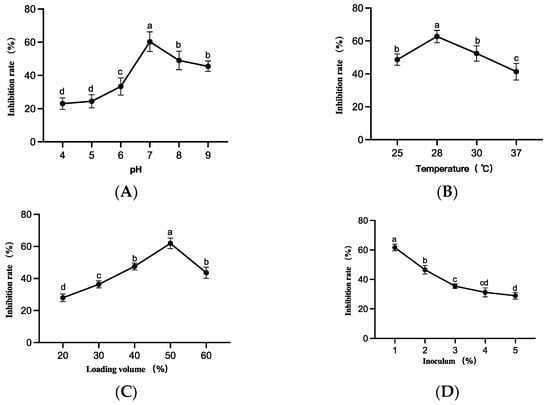
Figure 7.
Effect of different culture conditions on the growth of S. sapine. Note: (A) Inhibition rate of B. pumilus against S. sapine in different pH cultures; (B) Inhibition rate of B. pumilus against S. sapine in different temperature cultures; (C) Inhibition rate of B. pumilus against S. sapine in different loading volume cultures; (D) Inhibition rate of B. pumilus against S. sapine in different inoculum volume cultures. Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with different letters have significant differences (p < 0.05; Duncan test).
3.5. Response Surface Optimization Results of Strain LYMC-3 Culture Conditions
3.5.1. PB Trial
Design-Expert 10 software was used to plan the PB trial (Table 2); from the trial design, the model R2 was 96.84%, R2 adj was 91.31%, and p = 0.0075 (<0.01) (Table 3), indicating that the model is more reliable. According to the standardized positive and negative effect, the standardized effect on the inhibition rate with the increase in initial pH showed a negative effect, and the inhibition rate showed a positive effect when the level of variables such as glucose concentration, peptone concentration, magnesium sulfate concentration, loading volume, temperature, and inoculum volume increased. The three most significant factors, i.e., glucose concentration, magnesium sulfate concentration, and loading volume, were subjected to the next steepest climbing test.

Table 2.
Analysis of variance for each factor in the PB trial.

Table 3.
Analysis of variance for each factor in the PB trial.
3.5.2. The Steepest Climbing Test
From the PB test, it is known that glucose concentration, magnesium sulfate concentration, and loading volume have positive effects, and these were gradually increased in the climbing test. As shown in Table 4, the inhibition rate of strain LYMC-3 was the most pronounced in test 4. Therefore, the conditions of test 4 were selected as the central point of the response surface test factor level, i.e., 7.2 g/L glucose, 7.2 g/L magnesium sulfate, and 54% loading volume.

Table 4.
Steepest climb test.
3.5.3. BBD Experiments
Glucose concentration, magnesium sulfate concentration, and loading volume in the steepest climb test were labeled as A, B, and C. As shown in Table 5, the model’s features were R2 = 0.9649, R2 adj = 0.9199, and p = 0.0003, and the loss of fit term was p > 0.05, indicating that the model is significant and can be used for the analysis and prediction of response values. C, A2, and B2 had a highly significant effect (p < 0.01), while A, B, and C2 had some effect but were not significant (p < 0.10). The test results were analyzed using the Design-Expert10 software, and the quadratic regression equation of response values and test factors was fitted as follows: inhibition rate = 75.81 + 2.10A + 2.97B − 4.92C + 1.53AB + 1.96AC + 4.41BC − 11.53A2 − 8B2 − 2.88C2.

Table 5.
BBD ANOVA results.
Response surface and contour plots were plotted according to the regression equations and the results are shown in Figure 8. The contours for glucose concentration and magnesium sulfate concentration were approximately circular, indicating that the interaction between glucose concentration and magnesium sulfate concentration had no significant effect on the inhibition rate. The contours of glucose concentration and loading volume were approximately elliptical, indicating that the interaction between these two factors had a significant effect on the inhibition rate. The contours of magnesium sulfate concentration and loading volume were approximately elliptical, indicating that the interaction between magnesium sulfate concentration and loading volume had a significant effect on the inhibition rate. The optimal medium components for LYMC-3 to produce inhibitory substances as obtained using the software were 7.2 g/L glucose, 15 g/L peptone, and 7.1 g/L magnesium sulfate, and the culture conditions were 52% loading volume, 28 °C incubation temperature, 1% inoculum, and initial pH 7.
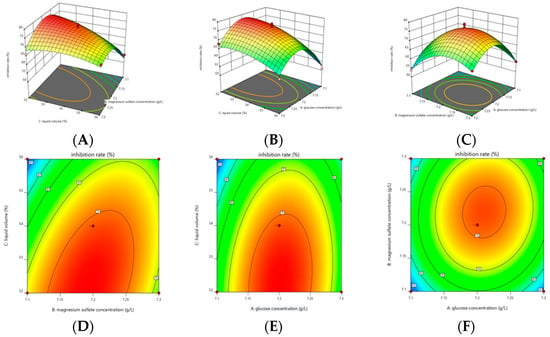
Figure 8.
3D response surfaces and 2D contour plots for inhibition rate by B. pumilus. Note: 3D response surfaces (A) and 2D contour plots (D) show the effects of magnesium sulfate concentration, loading volume, and their mutual interaction on the inhibition rate; 3D response surfaces (B) and 2D contour plots (E) show the effects of glucose concentration, loading volume, and their mutual interaction on inhibition rate; and 3D response surfaces (C) and 2D contour plots (F) show the effects of glucose concentration, magnesium sulfate concentration, and their mutual interaction on inhibition rate.
3.6. Comparison of Strain LYMC-3 Fungal Inhibition Ability before and after Medium Optimization
We noted that after optimization of the fermentation conditions for strain LYMC-3 (medium components were 7.2 g/L glucose, 15 g/L peptone, and 7.1 g/L magnesium sulfate; culture conditions were 52% loading volume, 28 °C incubation temperature, 1% inoculum, and initial pH 7), the LYMC-3 inhibition effect on the growth of S. sapinea mycelium was significantly stronger than before optimization (medium components were 5 g/L beef paste, 10 g/L peptone, and 5 g/L sodium chloride; culture conditions were 40% loading volume, 28°C incubation temperature, and 2% inoculum) (Figure 9). The inhibition rate of the five-fold diluted fermentation filtrate of strain LYMC-3 against S. sapinea was determined using the mycelial growth rate method. The pathogenic colonies in the control group grew normally (Figure 9C) with an average radius of 7.52 mm, while in the plate inoculated with strain LYMC-3 before optimization the average radius of the pathogenic colonies was 2.60 cm, and the average radius of the pathogenic colonies with strain LYMC-3 after optimization was 1.41cm. The rate was 65.39% before optimization and increased to 81.23% after optimization, an increase of 15.84% compared with that before optimization.
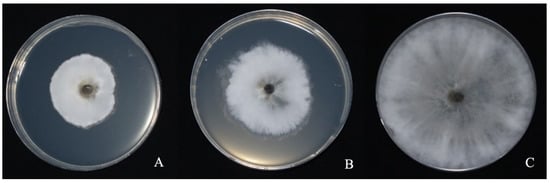
Figure 9.
The antifungal effect of LYMC-3 strain fermentation filtrate on S. sapinea before and after optimization. Note: (A) Fermentation filtrate diluted 5 times after optimization for the treatment of S. sapinea; (B) The fermentation filtrate diluted 5 times before optimization for the treatment of S. sapinea; (C) The blank control without fermentation filtrate treatment.
4. Discussion
Biocontrol bacteria have the advantages of a short life cycle, easy colonization, strong fecundity, rich metabolites, and so on. They are a kind of very important biological control resources. The ND11 strain of B. pumilus screened by Zhang et al. can significantly inhibit the growth of Rhizoctonia solani AG-1A, and has a greenhouse control effect of 70.69% against rice sheath blight [27]. Cao [28] found that B. velezensis and B. methylotrophic have inhibitory effects on Dothiorella gregaria, with antibacterial rates of 57.50% and 56.67%, respectively. Zhang et al. [29] reported that the five-fold dilution of the fermentation filtrate of Stenotrophomonas maltohilia YCYM-04 and B. velezensis YCYM-09 had antibacterial rates of 71.8% and 50.6% against Phytophthora nicotianae, respectively.
The production of inhibitory substances by microorganisms can vary under different culture conditions. The present experiment showed that the fermentation products of B. pumilus LYMC-3 had an improved ability to inhibit fungi when the carbon source of the medium was glucose, which may be related to the fact that monosaccharides are more favorable for the uptake and production of inhibitory substances by the organism compared to disaccharides and polysaccharides. This is consistent with the results Santra et al. [30] obtained when optimizing the carbon source for fermentation of the endophytic fungus Colletotrichum alatae LCS1 to improve the production of inhibitory substances. He et al. [31] reported that B. amyloliquefaciens ESB-2 produced the best concentration of the inhibitory substance Macrolactin A in the presence of 14.8 g/L peptone, which was similar to the type and concentration of nitrogen source in this study. In addition, the growth and metabolism of microorganisms require a suitable ratio of carbon to nitrogen, and a low carbon to nitrogen ratio will cause overgrowth and premature senescence of the bacteria and autolysis, while a high carbon to nitrogen ratio is not conducive to the reproduction of the bacteria resulting in a low fermentation density [32]. In this study, we noted that both low and high concentrations of carbon and nitrogen sources reduced the inhibition rate of B. pumilus LYMC-3, which indicates that a suitable carbon to nitrogen ratio is beneficial for the production of inhibitory substances by strain LYMC-3. Inorganic salts provide essential mineral elements for microbial growth, and Li et al. [33] reported that magnesium sulfate played an important role in improving the production of antagonistic B. subtilis BS501a metabolites. We also observed higher inhibition by strain LYMC-3 when magnesium sulfate was used as an inorganic salt component of the fermentation medium.
In addition to the medium components, suitable culture conditions are also necessary for the production of bacterial inhibitory substances. The lower loading volume required for increased inhibition by strain LYMC-3 suggests that oxygen favors the production of LYMC-3 inhibitory substances and that the metabolic rate of the strain can be increased by employing higher aeration during the logarithmic growth phase of the organism [34]. Xin et al. [35] showed that the optimal initial pH of antifungal substances produced by B. amylolyticus HRH317 was 7. Zhang et al. [36] found that B. amyloliquefaciens-9 had high inhibition activity when cultured at 37 °C. He et al. [37] reported that the optimized culture conditions for protease production by B. subtilis SCK6 were 35 °C and pH 7.0. From our findings, it is noted that for the production of inhibitory substances, the initial pH of strain LYMC-3 was similar to that of Bacillus sp. in most studies, but the low incubation temperature may be related to the large temperature span designed in the previous single-factor test, and the temperature range between 28 °C and 37 °C could be refined later to obtain the optimal incubation temperature. Serrano et al. [38] reported that Paenibacillus polymyxa RNC-D produced antimicrobial metabolites at an optimal fermentation inoculum of 5%. In the present study, the optimum inoculum for strain LYMC-3 was less than 1% and the optimum inoculum interval below this value remains to be investigated.
Dai et al. [39] used response surface testing and noted that the optimal formulation of fermentation medium for B. pumilus HR10 to produce antagonistic constituents towards S. sapinea was 12 g/L corn flour, 15 g/L beef extract, and 13 g/L magnesium sulfate. In our experiment, based on the optimization of the fermentation medium components, the overall fermentation conditions were also enhanced and the optimal formulation of the fermentation medium was 7.2 g/L glucose, 15 g/L peptone, and 7.1 g/L magnesium sulfate, and the culture conditions were 52% loading volume, 28 °C incubation temperature, 1% inoculum, and initial pH 7. Both this study and Dai et al. (2022) showed that when magnesium sulfate was used as the inorganic salt component of the fermentation medium the inhibition rate of B. pumilus was higher. However, the composition and concentration of the optimal carbon and nitrogen sources of the optimized fermentation medium for the production of B. pumilus inhibitory substances differed, as did the concentration of inorganic salts. The inhibition rate of B. pumilus HR10 was 87.04 ± 3.2% after optimization by Dai et al. (2022). The inhibition rate for LYMC-3 was 65.39% before optimization and reached 81.23% after optimization, which was 15.84% higher than prior to optimization. At the same time, selected lower-cost glucose and magnesium sulfate as carbon sources and inorganic salts for fermentation culture medium provide important technical support for the future industrial production and promotion of strain LYMC-3 for controlling pine shoot blight.
5. Conclusions
This study obtained the antibacterial rate of the fermentation filtrate of strain LYMC-3 against S. sapinea when diluted five times through plate antagonistic experiments, which was 66.09%. The greenhouse control effect test showed that the fermentation filtrate of strain LYMC-3 had a control effect of 89.99% on pine shoot blight. Using statistical experimental design, we explored the fermentation medium and culture conditions with the strongest antibacterial effect of B. pumilus LYMC-3 on S. sapinea through single-factor experiments, Plackett-Burman trial, steepest climbing experiment, Box–Behnken experimental design, and Response Surface Methodology. The optimal medium components to produce antifungal substances by strain LYMC-3 were obtained, including 7.2 g/L glucose, 15 g/L peptone, and 7.1 g/L magnesium sulfate. The culture conditions were 52% liquid content, 28 °C culture temperature, 1% inoculation amount, and initial pH 7. The antifungal rate of strain LYMC-3 against S. sapinea aeruginosa increased by 15.84% compared to before optimization.
Author Contributions
Conceptualization, M.P. and J.T.; methodology, M.P. and J.T.; formal analysis, M.P. and Y.W.; investigation, M.P., F.L. and J.H.; resources, Y.W.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P. and J.T.; visualization, M.P.; supervision M.P.; project administration, M.P. and J.T.; funding acquisition, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2021YFD1400300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swart, W.J.; Knox-Davies, P.S.; Wingfield, M.J. Sphaeropsis sapinea, with special reference to its occurrence on Pinus spp. in South Africa. J. S. Afr. For. Assoc. 1986, 135, 1–8. [Google Scholar]
- Wu, X.Q. Occurence and control countermeasure of Sphaeropsis sapinea shoot blight on conifer in the world. World For. Res. 1999, 12, 16–21. [Google Scholar]
- Xie, X.; Lliang, J.; Zhu, Y.P.; Hu, R.R.; Chen, Y.Y.; Zhang, X.Y. Diversity and community structure of endophytic fungi in the pure forest of pinus densiflorainfected by different incidences of Sphaeropsis sapinea. Sci. Silvae Sin. 2020, 56, 51–57. [Google Scholar]
- Blumenstein, K.; Bukamp, J.; Langer, G.J.; Schler, R.; Terhonen, E. Sphaeropsis sapinea and associated endophytes in Scots pine: Interactions and effect on the host under variable water content. Front. For. Glob. Chang. 2021, 4, 655769. [Google Scholar] [CrossRef]
- Bukamp, J.; Blumenstein, K.; Terhonen, E.; Langer, G.J. Differences in the virulence of Sphaeropsis sapinea strains originating from Scots pine and non-pine hosts. For. Pathol. 2021, 51, e12712. [Google Scholar]
- Xinyi, C.; Yuanyuan, Z.; Xuechi, F.; Yan, L.; Qi, W. Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol. Technol. 2016, 115, 113–121. [Google Scholar]
- Mahaffee, W.F.; Kloepper, J.W. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb. Ecol. 1997, 34, 210–223. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Yu, J.; Guo, Z.; Wan, S. Biological control of peanut southern blight (Sclerotium rolfsii) by the strain Bacillus pumilus LX11. Biocontrol Sci. Technol. 2020, 30, 485–489. [Google Scholar] [CrossRef]
- Mohit, A.; Shrivardhan, D.; Ramesh, C.; Dubey, P.; Kumar, D. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017, 205, 40–47. [Google Scholar]
- Sha, Y.; Zeng, Q.; Sui, S. Screening and application of Bacillus strains isolated from nonrhizospheric rice soil for the biocontrol of rice blast. Plant Pathol. J. 2020, 36, 231–243. [Google Scholar] [CrossRef]
- Abd-El-Kareem, F.; Elshahawy, I.E.; Abd-Elgawad, M.M.M. Application of Bacillus pumilus isolates for management of black rot disease in strawberry. Egypt. J. Biol. Pest Control 2021, 31, 25. [Google Scholar] [CrossRef]
- Huang, X.; Nan, Z.; Yong, X.; Yang, X.; Shen, Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbio. Res. 2012, 167, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ma, X.; Lang, D.; Zhang, X.; Zhou, L.; Wang, L.; Zhang, X. Encapsulation of Bacillus pumilus G5 from polyvinyl alcoholsodium alginate (PVA-SA) and its implications in improving plant growth and soil fertility under drought and salt soil conditions. Int. J. Biol. Macromol. 2022, 209, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Bonifer, K.S.; Wen, X.; Hasim, S.; Phillips, E.K.; Reynolds, T.B. Bacillus pumilus B12 degrades polylactic acid and degradation is affected by changing nutrient conditions. Front. Microbiol. 2019, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Chen, F. Bacillus pumilus strain LYMC-3 shows nematicidal activity against Bursaphelenchus xylophilus via the production of a guanidine compound. Biocontrol Sci. Technol. 2018, 28, 1128–1139. [Google Scholar] [CrossRef]
- Li, L.L.; Tan, J.J.; Chen, F.M. Colonization of GFP-tagged Bacillus pumilus strain LYMC-3 in masson pine. J. Huazhong Agric. Univ. 2016, 35, 68–73. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, M.L.; Tan, J.J.; Li, L.L.; Hao, D.J. The antagonism of Bacillus pumilus LYMC⁃3 strain on Phomopsis macrospora. J. Nanjing For. Univ. 2022, 46, 151–156. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Tu, Q.; Chen, J.; Guo, J. Screening and identification of antagonistic bacteria with potential for biological control of Penicillium italicum of citrus fruits. Sci. Hortic. 2013, 150, 125–129. [Google Scholar] [CrossRef]
- Xian, Z.A.; Hl, A.; Xk, A.; Sl, B.; Fl, A. Isolation, identification and optimization of fermentation conditions against Sclerotinia sclerotiorum strains in high salt Doenjang. Food Sci. Hum. Wellness 2021, 10, 205–213. [Google Scholar]
- Xue, Y.Y.; Fan, W.Y.; Zhang, S.W.; Xu, B.L. Screening, identification and biocontrol effect of antagonistic Actinomycetes against the pathogen of Cytospora sp. for apple tree. J. Appl. Ecol. 2016, 27, 3379–3386. [Google Scholar]
- Zhang, M.J.; Zheng, X.R.; Li, H.; Chen, F.M. Alternaria alternata, the causal agent of a new needle blight disease on Pinus bungeana. J. Fungi 2023, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Yang, Y.; Wei, H.; Xue, L.; Min, Z.; Jinxiu, C. Optimization of combined microwave and hot air drying technology for purple cabbage by Response Surface Methodology (RSM). Food Sci. Nutr. 2021, 87, 2980–2998. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Feng, X.; Sheng, Y.; Song, Z. Optimization of the artemisia polysaccharide fermentation process by Aspergillus niger. Front. Nutr. 2022, 9, 842766. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Miao, W.; Yang, Y.; Fan, G.; Wu, C.; Li, T.; Xie, C.; Shen, D. Preparation of monascus-fermented ginkgo seeds: Optimization of fermentation parameters and evaluation of bioactivity. Food Sci. Biotechnol. 2022, 31, 721–730. [Google Scholar] [CrossRef]
- Daghaghele, S.; Kiasat, A.R.; Safieddin Ardebili, S.M.; Mirzajani, R. Intensification of extraction of antioxidant compounds from moringa oleifera leaves using ultrasound-assisted approach: BBD-RSM design. Int. J. Fruit Sci. 2021, 21, 693–705. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zheng, L.Y.; Wang, J.C.; Zhu, F.; Niu, D.D. Screening of biocontrol bacteria for rice sheath blight and their relations with the formation of infection cushion of the pathogen. J. Plant Prot. 2021, 48, 289–297. [Google Scholar] [CrossRef]
- Cao, N.; Zhu, W.B.; Hhuang, Q.; Liu, X.F. Antibacterial activity study of two antagonistic bacteria of Dothiorella gregaria. For. Eng. 2021, 37, 72–78. [Google Scholar] [CrossRef]
- Zhang, M.M.; Liang, J.Y.; Wang, Q.F.; Du, Y.F.; Zhang, J.; Liu, Q.J.; Peng, J.F.; Fu, B. Identification and control efficiency of biocontrol bacteria against tobacco black shank. J. Henan Agric. Univ. 2022, 56, 219–227. [Google Scholar]
- Santra, H.K.; Banerjee, D. Bioactivity study and metabolic profiling of Colletotrichum alatae LCS1, an endophyte of club moss Lycopodium clavatum L. PLoS ONE 2022, 17, e0267302. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Wu, B.; Zhou, H.; Zhu, P.; Yang, R.; Yan, X. Response surface methodology optimization of fermentation conditions for rapid and efficient accumulation of macrolactin A by marine Bacillus amyloliquefaciens ESB-2. Molecules 2012, 18, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Touratier, F.; Legendre, L.; Vézina, A. Model of bacterial growth influenced by substrate C:N ratio and concentration. Aquat. Microb. Ecol. 1999, 19, 105–118. [Google Scholar] [CrossRef]
- Li, R.F.; Xu, Y. Fermentation optimization to improve production of antagonistic metabolites by Bacillus subtilis strain BS501a. J. Cent. South Univ. Technol. 2011, 18, 1047–1053. [Google Scholar] [CrossRef]
- Yousten, A.A.; Wallis, D.A.; Singer, S. Effect of oxygen on growth, sporulation, and mosquito larval toxin formation by Bacillus sphaericus 1593. Curr. Microbiol. 1984, 11, 175–178. [Google Scholar] [CrossRef]
- Xin, L.; Hao, L.; Ling, M. Optimization of fermentation conditions of antibacterial substances produced by Bacillus amyloliquefaciens HRH_(317). China Brew. 2017, 36, 25–29. [Google Scholar]
- Zhang, W.; Wei, L.; Xu, R.; Lin, G.; Xin, H.; Lv, Z.; Qian, H.; Shi, H. Evaluation of the antibacterial material production in the fermentation of Bacillus amyloliquefaciens-9 from whitespotted bamboo shark (Chiloscyllium plagiosum). Mar. Drugs 2020, 18, 119. [Google Scholar] [CrossRef]
- He, F.; Chao, J.; Yang, D.; Zhang, X.; Yang, C.; Xu, Z.; Jiewei, T.; Yongqiang, T. Optimization of fermentation conditions for production of neutral metalloprotease by Bacillus subtilis SCK6 and its application in goatskin-dehairing. Prep. Biochem. Biotechnol. 2021, 52, 789–799. [Google Scholar] [CrossRef]
- Serrano, N.; Rodrigues, L.; Hokka, C.; Sousa, C.; Teixeira, J.; Mussatto, S. Optimal glucose and inoculum concentrations for production of bioactive molecules by Paenibacillus polymyxa RNC-D. Chem. Pap. 2012, 66, 1111–1117. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.-H.; Li, M.; Zhu, M.-L.; Wen, T.-Y.; Wu, X.-Q. Medium optimization to analyze the protein composition of Bacillus pumilus HR10 antagonizing Sphaeropsis sapinea. AMB Expr. 2022, 12, 61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).