Probiotic-Bacteria (Lactobacillus fermentum)-Wrapped Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory Culture of Lactobacillus Fermentum
2.3. Synthesis of Nanoparticles
2.4. Characterization of Nanoparticles
2.5. Antibacterial Activity Evaluation
3. Results and Discussion
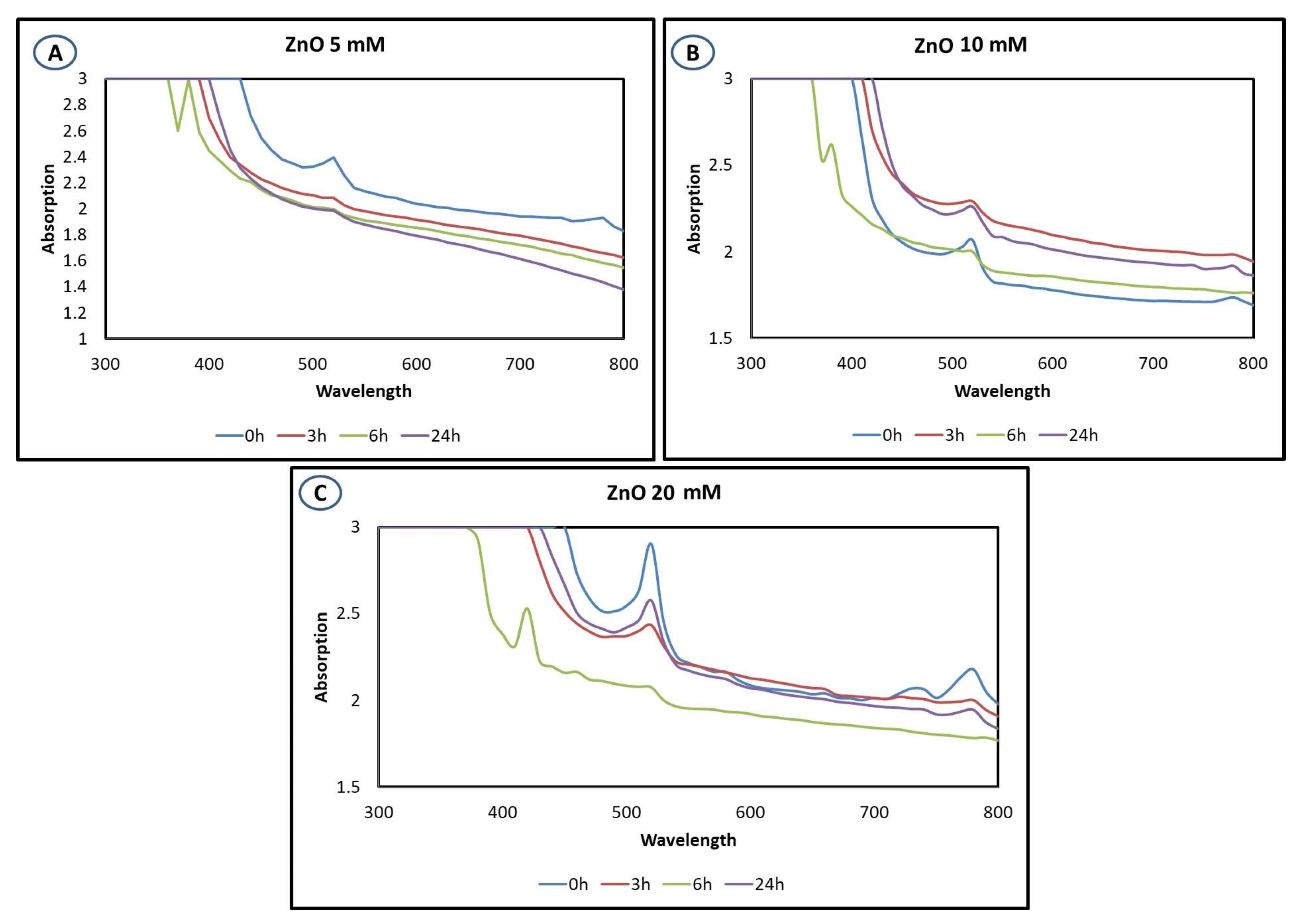
3.1. UV–Visible Spectrophotometer Analysis
3.2. Powdered X-ray Diffraction Analysis
3.3. Identification of Compound Interaction
3.4. SEM Analysis
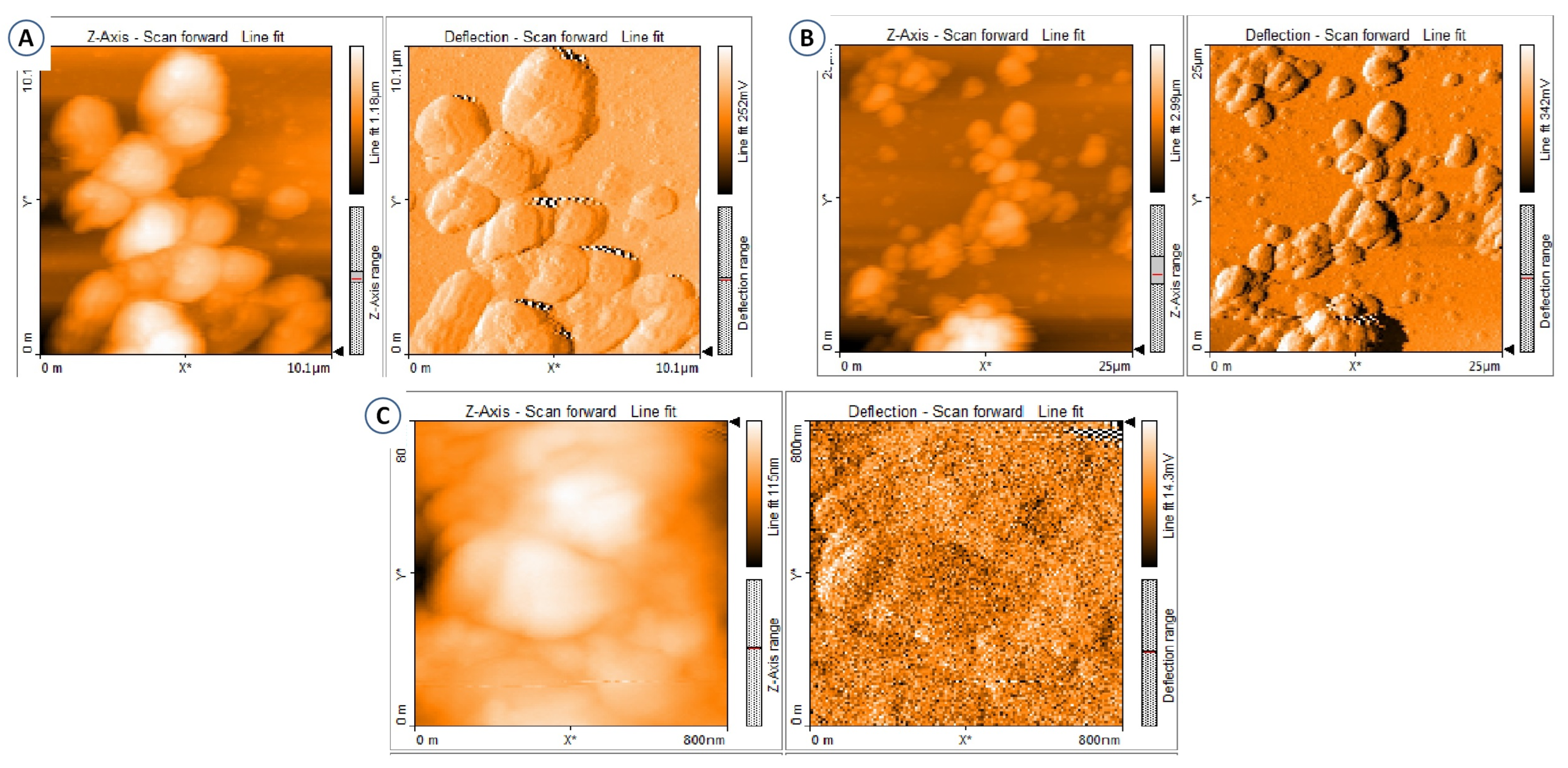
3.5. AFM Analysis
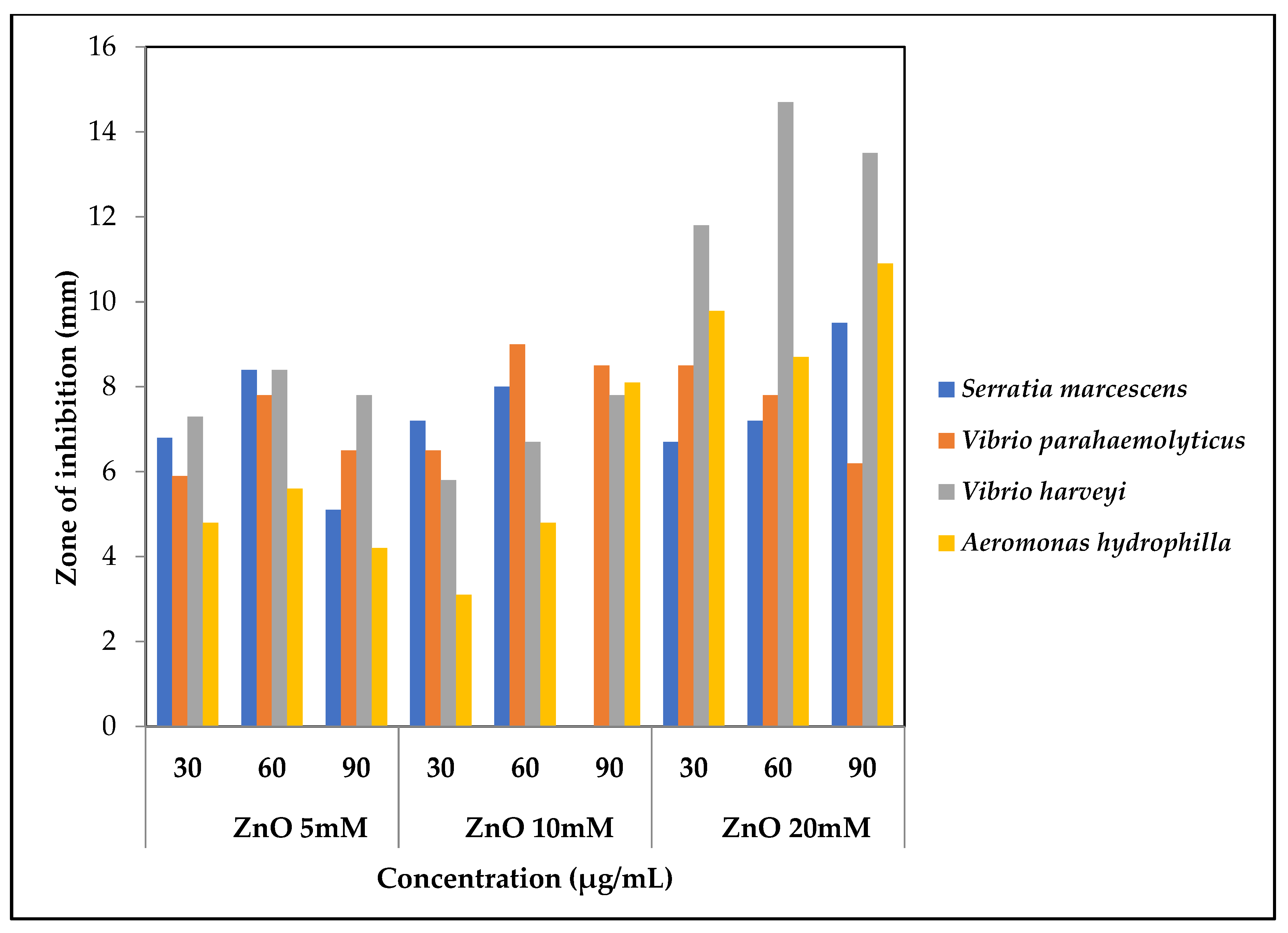
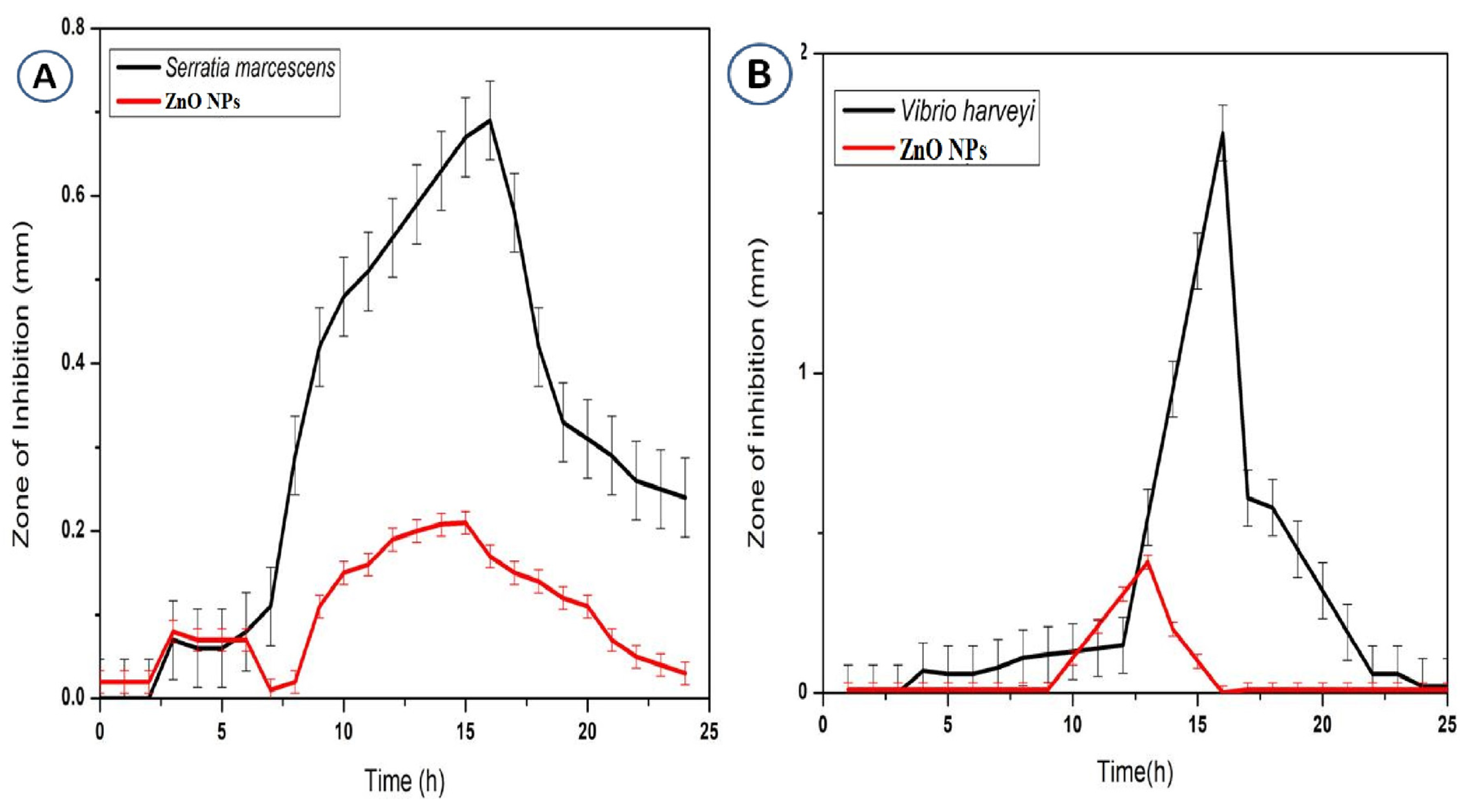
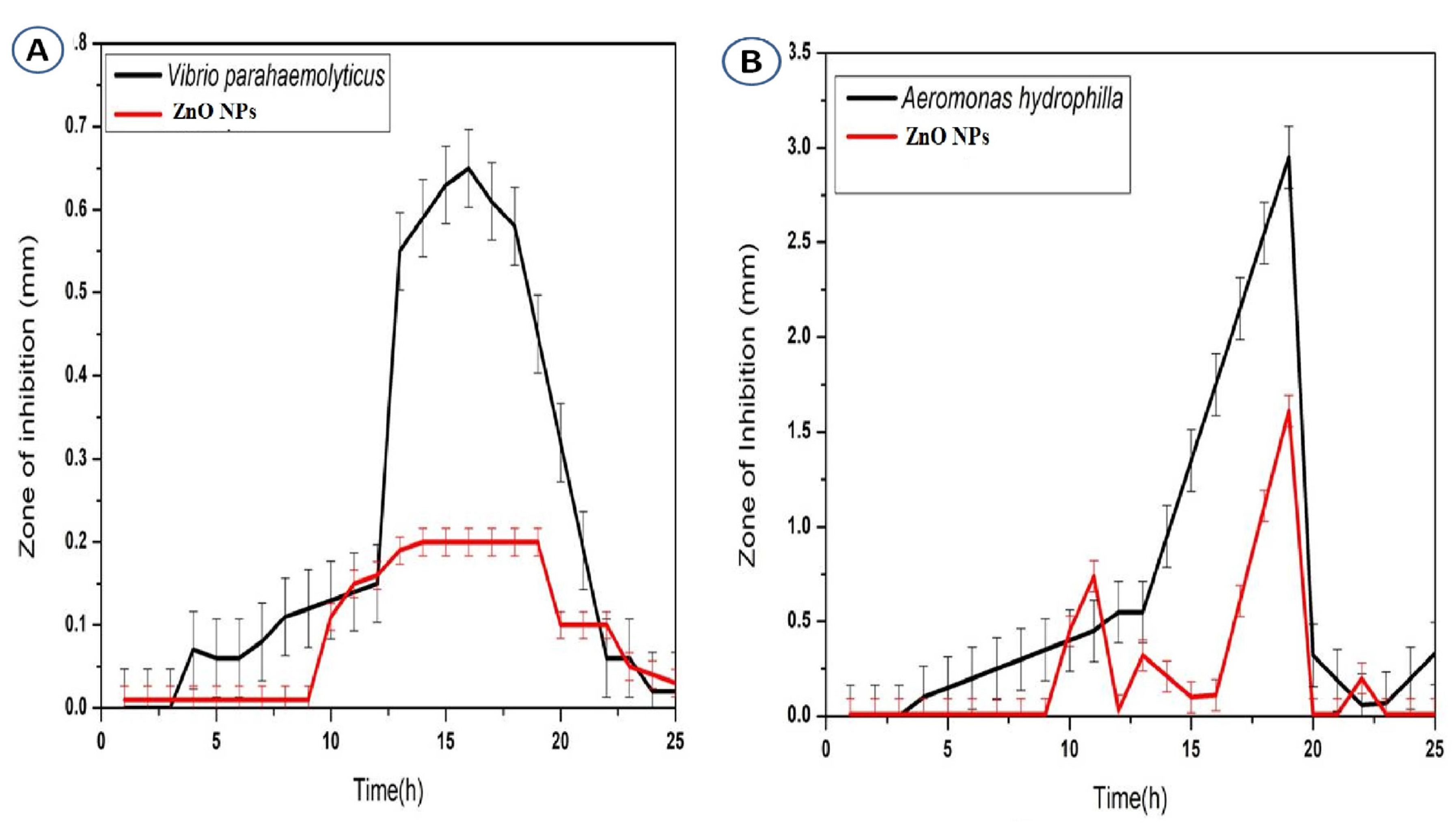
3.6. Antibacterial Activity of Zinc Oxide Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emmett, R.; Akkersdyk, S.; Yeatman, H.; Meyer, B.J. Expanding awareness of docosahexaenoic acid during pregnancy. Nutrients 2013, 5, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Cillero, I.H.; Gil, J.; Irazusta, A.; Torres-Unda, J.; Zarrazquin, I.; Ruiz, F.; Irazusta, J.; Kortajarena, M. Longitudinal study: Lifestyle and cardiovascular health in health science students. Nutr. Hosp. 2014, 30, 1144–1151. [Google Scholar]
- Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work; Centers for Disease Control: Atlanta, GA, USA, 2010. Available online: www.cdc.gov/Features/GetSmart (accessed on 23 March 2023).
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in fish and seafood. J. Food Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Lenchenko, E.; Lenchenko, S.; Sachivkina, N.; Kuznetsova, O.; Ibragimova, A. Interaction of Cyprinus carpio Linnaeus with the biofilm-forming Aeromonas hydrophila. Vet. World 2022, 15, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Korrapati, P.S. Biosynthesis of titanium dioxide nanoparticles using a probiotic from coal fly ash effluent. Mater. Res. Bull. 2013, 48, 4738–4742. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 504–516. [Google Scholar] [CrossRef]
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Złoch, M.; Buszewski, B. Mechanism study of intracellular zinc oxide nanocomposites formation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 349–358. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Pavithra Marthandam, R.; Jayachandiran, J.; Khatiwada, C.; Kaviyarasu, K.; Ganapathi Raman, R.; Swaminathan, M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Rajiv, P.; Vanathi, P. Effect of Parthenium based vermicompost and zinc oxide nanoparticles on growth and yield of Arachis hypogaea L. in the zinc-deficient soil. Biocatal. Agric. Biotechnol. 2018, 13, 251–257. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Matei, C.; Radu, M.D.; Gheorghe, E. Characterisation and applications of a new composite material obtained by green synthesis, through deposition of zinc oxide onto calcium carbonate precipitated in green seaweeds extract. Ceram. Int. 2018, 44, 4931–4936. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. leaf mediated synthesis of silver and zinc oxide nanoparticles and their antibacterial activities. Microb. Pathog. 2018, 115, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Ultrasound irradiation based in-situ synthesis of star-like Tragacanth gum/zinc oxide nanoparticles on cotton fabric. Ultrason. Sonochem. 2017, 34, 458–465. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterisation and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Amith Yadav, H.; Eraiah, B.; Nagabhushana, H.; Daruka Prasad, B.; Basavaraj, R.B.; Sateesh, M.K.; Shabaaz Begum, J.P.; Darshan, G.P.; Vijayakumar, G.R. Broad spectral inhibitory effects of pale green zinc oxide nanophosphor on bacterial and fungal pathogens. Arab. J. Chem. 2018, 11, 324–342. [Google Scholar] [CrossRef]
- Menon, S.; Devi Ks, S.; Santhiya, R.; Rajeshkumar, S.; Venkat Kumar, S. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Heli, H.; Sharifi, I. Rectangular shaped zinc oxide nanoparticles: Green synthesis by Stevia and its biomedical efficiency. Ceram. Int. 2018, 44, 15596–15602. [Google Scholar] [CrossRef]
- Passos, M.L.; Saraiva, M.L. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Adeeyinwo, C.E.; Okorie, N.N.; Idowu, G.O. Basic calibration of UV/visible spectrophotometer. Int. J. Sci. Technol. 2013, 2, 247–251. [Google Scholar]
- Zare, E.; Pourseyedi, S.; Khatami, M.; Darezereshki, E. Simple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activity. J. Mol. Struct. 2017, 1146, 96–103. [Google Scholar] [CrossRef]
- Archana, B.; Manjunath, K.; Nagaraju, G.; Chandra Sekhar, K.B.; Kottam, N. Enhanced photocatalytic hydrogen generation and photostability of ZnO nanoparticles obtained via green synthesis. Int. J. Hydrogen Energy 2018, 42, 5125–5131. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Gupta, V.; Yadav, T.P.; Gupta, V.; Singh, S.P. Green synthesis, characterisation and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater. Chem. Phys. 2018, 203, 40–48. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, anti-biofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, A.A.; UdriŞTioiu, E.G.; Aboul-Enein, H.Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- Padrela, L.; de Azevedo, E.G.; Velaga, S.P. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev. Ind. Pharm. 2012, 38, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Thakral, N.K.; Zanon, R.L.; Kelly, R.C.; Thakral, S. Applications of powder X-ray diffraction in small molecule pharmaceuticals: Achievements and aspirations. J. Pharm. Sci. 2018, 107, 2969–2982. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Micova, J.; Buryi, M.; Simek, D.; Drahokoupil, J.; Neykova, N.; Chang, Y.Y.; Remes, Z.; Pop-Georgievski, O.; Svoboda, J.; Im, C. Synthesis of zinc oxide nanostructures and comparison of their crystal quality. Appl. Surf. Sci. 2018, 461, 190–195. [Google Scholar] [CrossRef]
- Bhutiya, P.L.; Mahajan, M.S.; Abdul Rasheed, M.; Pandey, M.; Zaheer Hasan, S.; Misra, N. Zinc oxide nanorod clusters deposited seaweed cellulose sheet for antimicrobial activity. Int. J. Biol. Macromol. 2018, 112, 1264–1271. [Google Scholar] [CrossRef]
- Paul, B.; Vadivel, S.; Dhar, S.S.; Debbarma, S.; Kumaravel, M. One-pot green synthesis of zinc oxide nano rice and its application as sonocatalyst for degradation of organic dye and synthesis of 2-benzimidazole derivatives. J. Phys. Chem. Solids 2017, 104, 152–159. [Google Scholar] [CrossRef]
- Zarnowiec, P.; Lechowicz, L.; Czerwonka, G.; Kaca, W. Fourier transform infrared spectroscopy (FTIR) as a tool for the identification and differentiation of pathogenic bacteria. Curr. Med. Chem. 2015, 22, 1710–1718. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; Volume 7, pp. 7–9. [Google Scholar]
- Lemine, O.M. Microstructural characterisation of α-Fe2O3 nanoparticles using, XRD line profiles analysis, FE-SEM and FT-IR. Superlattices Microstruct. 2009, 45, 576–582. [Google Scholar] [CrossRef]
- Simonet, B.M.; Valcárcel, M. Monitoring nanoparticles in the environment. Anal. Bioanal. Chem. 2009, 393, 17–21. [Google Scholar] [CrossRef]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef]
- Akbari, A.; Khammar, M.; Taherzadeh, D.; Rajabian, A.; Khorsand Zak, A.; Darroudi, M. Zinc-doped cerium oxide nanoparticles: Sol-gel synthesis, characterisation, and investigation of their in vitro cytotoxicity effects. J. Mol. Struct. 2017, 1149, 771–776. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S.C. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesised using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Sajja, S.; Chandler, M.; Fedorov, D.; Kasprzak, W.K.; Lushnikov, A.; Viard, M.; Shah, A.; Dang, D.; Dahl, J.; Worku, B.; et al. Dynamic behavior of RNA nanoparticles analyzed by AFM on a mica/air interface. Langmuir 2018, 34, 15099–15108. [Google Scholar] [CrossRef]
- Omidi, E.; Korayem, A.H.; Korayem, M.H. Sensitivity analysis of nanoparticles pushing manipulation by AFM in a robust controlled process. Precis. Eng. 2013, 37, 658–670. [Google Scholar] [CrossRef]
- Baer, D.R.; Gaspar, D.J.; Nachimuthu, P.; Techane, S.D.; Castner, D.G. Application of surface chemical analysis tools for characterization of nanoparticles. Anal. Bioanal. Chem. 2010, 396, 983–1002. [Google Scholar] [CrossRef]
- Aswathy, R.; Gabylis, B.; Anwesha, S.; Bhaskara Rao, K. Green synthesis and characterization of marine yeast-mediated silver and zinc oxide nanoparticles and assessment of their antioxidant activity. Asian J. Pharm. Clin. Res. 2017, 10, 235–240. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Hńfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.; Ann, L.C.; Bakhori, S.K.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Sumitha, S.; Ning, C.Y.; Xian, O.Y.; Kiew Yu, U.; Paliwal, N.; Shah, S.A.; Tripathy, M. Durian waste mediated green synthesis of zinc oxide nanoparticles and evaluation of their antibacterial, antioxidant, cytotoxicity and photocatalytic activity. Green Chem. Lett. Rev. 2020, 13, 102–116. [Google Scholar] [CrossRef]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, R. Zinc oxide nanoleaves: A scalable disperser-assisted sonochemical approach for synthesis and an antibacterial application. Ultrason. Sonochem. 2018, 41, 47–58. [Google Scholar] [CrossRef]
- Saravanan, M.; Gopinath, V.; Chaurasia, M.; Syed, A.; Ameen, F.; Purushothaman, N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb. Pathog. 2018, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.L. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugam, R.; Munusamy, T.; Jayakodi, S.; Al-Ghanim, K.A.; Nicoletti, M.; Sachivkina, N.; Govindarajan, M. Probiotic-Bacteria (Lactobacillus fermentum)-Wrapped Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Antibacterial Activity. Fermentation 2023, 9, 413. https://doi.org/10.3390/fermentation9050413
Shanmugam R, Munusamy T, Jayakodi S, Al-Ghanim KA, Nicoletti M, Sachivkina N, Govindarajan M. Probiotic-Bacteria (Lactobacillus fermentum)-Wrapped Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Antibacterial Activity. Fermentation. 2023; 9(5):413. https://doi.org/10.3390/fermentation9050413
Chicago/Turabian StyleShanmugam, Rajeshkumar, Tharani Munusamy, Santhoshkumar Jayakodi, Khalid A. Al-Ghanim, Marcello Nicoletti, Nadezhda Sachivkina, and Marimuthu Govindarajan. 2023. "Probiotic-Bacteria (Lactobacillus fermentum)-Wrapped Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Antibacterial Activity" Fermentation 9, no. 5: 413. https://doi.org/10.3390/fermentation9050413
APA StyleShanmugam, R., Munusamy, T., Jayakodi, S., Al-Ghanim, K. A., Nicoletti, M., Sachivkina, N., & Govindarajan, M. (2023). Probiotic-Bacteria (Lactobacillus fermentum)-Wrapped Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Antibacterial Activity. Fermentation, 9(5), 413. https://doi.org/10.3390/fermentation9050413









