Abstract
Fusarium oxysporum, a common fungal pathogen that infects economic crops, causes Fusarium wilt disease to Saposhnikovia divaricata at an annual incidence rate of more than 15%. This study aimed to assess the potential of rhizospheric fungi as antifungal agents against Fusarium wilt of Saposhnikovia divaricata. In this study, 104 fungi were isolated from S. divaricata rhizospheric soil. Twelve rhizospheric strains that showed antagonistic activity against F. oxysporum, MR-16, MR-32, MR-38, etc., were screened out. Biocontrol activities of the twelve strains, especially MR-16, were subsequently characterized and evaluated. Strain MR-16 as potential stock for biocontrol had good antibiotic activity against F. oxysporum in vitro experiment. Based on the analysis of morphological properties and rDNA internal transcribed spacers (ITS), we identified an isolate MR-16 as Penicillium caperatum (GenBank No. OK287146.1), a new record of this species of China. The results of the in vitro antagonistic assay indicated that the conidial germination rate was significantly decreased, and the mycelia morphology of F. oxysporum induced change via the culture filtrate of P. caperatum MR-16, such as deformation and degradation. In an outdoor pot experiment, inoculation of S. divaricata plants with F. oxysporum created severe wilting symptoms; however, in inoculation trials, MR-16 effectively suppressed disease lesions, with a strong control efficacy of 60.76%. In addition, strain MR-16 could successfully colonize and form stable populations in the soil, and it showed a continuous positive growth-promoting effect on S. divaricata plants.
1. Introduction
As a worldwide pathogenic fungus, Fusarium oxysporum is one of the top 10 most important plant-pathogenic fungi that has a wide host range [1]. F. oxysporum infects a wide variety of agricultural, horticultural, and medicinal plants as a ubiquitous soil-borne pathogen that produces severe losses in crops [2]. The dry root of Saposhnikovia divaricata (Turcz.) Schisck., known as Saposhnikoviae Radix, called “Fang-feng” in China, is one of the most famous Chinese traditional herbs, and it has been commonly used in the clinical compound prescription for the treatment of rheumatism, headache, vertigo, and arthralgia in China and other Asian countries [3,4,5].
The main source of Chinese herbal material of Saposhnikoviae Radix is the cultivated S. divaricata in the Chinese market [6,7], with an increasing cultivated area in northern China due to the over-digging of the wild resource, S. divaricata. Due to the geographical environment, Fusarium wilt disease, which is caused by overwintering spores of F. oxysporum, is one of the serious diseases that occur in the cultivated area of S. divaricata. After a disease outbreak, F. oxysporum penetrates the root epidermis, spreads through the vascular bundle, and conquers the xylem vessels of plants, resulting in vessel blockage and severe water stress. The disease symptoms caused by S. divaricate, Fusarium wilt, includes stunting, epinasty, yellowing, and wilting of the leaves; browning, withering, and necrosis of the stems; eventually, progressive wilting, defoliation, metabolic failure of aboveground plants, and, often, plant death [8]. The annual incidence of Fusarium wilt is more than 15%, which seriously affects the yield of S. divaricata [8]. Notwithstanding, chemical fungicides can effectively control Fusarium wilt of S. divaricata and are the main strategy for control of this disease. However, the soil microbiota have been disrupted due to aggressive fungicide use, which reduces numbers of beneficial soil microorganisms, causing resistant pathogens and other agroecological pollution [9,10]. Consequently, the use of alternative approaches is required, which can suppress the development of pathogens to achieve eco-friendly and sustainable management [11].
The probiotic microorganisms used for the control of plant disease are effective and ecologically safe and provide substantial economic and ecological benefits [12]. Biological control is an ideal solution to challenge pesticide resistance. Fungal pathogens are otherwise beneficial to plants and can be used in accordance with organic farming practices because they could be self-propagating, conferring resistance via multiple strategies. For example, Bacillus subtilis [13,14] and Trichoderma harzianum [1,15,16] have strong adaptability to all sorts of environmental conditions and can inhibit pathogens propagation, promote plant growth, and have been developed as biological agents. However, biological control of Fusarium wilt of S. divaricata has not yet been reported. Therefore, in this study, we screened and isolated several biocontrol strains from rhizosphere soils collected from S. divaricata fields, the rhizosphere fungus MR-16, which has an antagonistic effect on Fusarium wilt of S. divaricata, was mainly identified, and its ability of soil colonization, growth promotion, and biological control was explored. Our aim was to evaluate the possibility of using rhizosphere fungi as biocontrol agents to control S. divaricata wilt disease and provide a good source for the development of biological fungi, which could provide a theoretical foundation for the biological control of plant diseases.
2. Materials and Methods
2.1. Rhizospheric Fungi of S. divaricata
Rhizosphere soil samples of healthy S. divaricata were collected from a field of the Jilin Agricultural University (JLAU), Changchun, China; the depth of excavation was 30 cm. We isolated the rhizospheric fungi according to the previously described dilution plate method with some modifications [17]. In brief, ten grams of soil sample was suspended in 90 mL of ddH2O and vortexed thoroughly for 10 min. The suspension of soil was then diluted and spread on a potato dextrose agar (PDA) medium. Various fungal colonies were selected and purified on the PDA medium, numbered as MR, and then stored at −20 °C for later use.
2.2. In Vitro Inhibition Activity and Inhibition Spectrum
The antagonistic activities of fungi isolates derived from rhizospheric soils against F. oxysporum were performed by dual-culture and confrontation culture experiments [18,19]. Nine plant pathogens (provided by the Plant Disease Integrated Management Laboratory, Jilin Agricultural University), including Botrytis cinerea, F. solani, Phytophthora cactorum, F. equiseti, Mycocentrospora acerina, Rhizoctonia solani, Alternaria tenuissima, Cylindrocarpon destructans, and A. liriodendron were used to test a broad spectrum of activities in vitro on the MR-16 isolate using dual culture assays [18] on PDA in Petri dishes (φ = 90 mm) at 25 °C for 7 d in the dark. In parallel, we similarly prepared control plates but used the pathogens without MR-16. We prepared three replicates for each assay. We preserved all the fungal pathogens at the Key Laboratory for Ecological Restoration and Ecosystem Management of Jilin Province at Jilin Agricultural University.
2.3. Culture Characteristics and Phylogenetic Analysis of MR-16
The isolate MR-16 was inoculated on Czapek yeast autolysate agar with 5% NaCl (CYAS), Czapek dox agar (CA), Czapek yeast extract agar (CYA), 25% glycerol nitrate agar base (G25N), malt extract agar (MEA), and PDA plates at 25 °C for 10 d in the dark in a three-point manner under aseptic conditions with three replicates for each experiment. The colony texture, the abundance, texture, and color of mycelia, and the presence and colors of soluble pigments and exudates of the strains were observed in each medium plate, and the colony diameter was recorded [20,21]. The colors of the fungal colonies were determined by comparison with the color charts of the International Society Color Council and the National Bureau of Standards.
For micro-morphological identification, the morphological properties of mycelia, conidia, and sclerotia of strain MR-16 were observed using a ZEISS sigma300 field emission scanning electron microscope (Carl Zeiss Jena, Oberkochen, Germany). For molecular identification, the genomic DNA from the mycelia of the isolate MR-16 was extracted using a TaKaRa MiniBEST Universal Genomic DNA Extraction Kit Ver.5.0 (Takara Bio, Shiga, Japan). The strain was incubated on a rotary shaker (150 rpm) at 25 °C for 5 days. PCR of ITS rDNA was performed according to a previous report [22]. The PCR products were subjected to agarose gel electrophoresis and sent to Sangon Biotech (Shanghai, China) for sequencing. The obtained sequences were submitted to the NCBI’s nucleotide database for comparative analysis using the Basic Local Alignment Search Tool (BLAST). Phylogenetic analysis of the isolated strain was conducted by the Maximum Likelihood method using MEGA X [23], and the phylogenetic tree was constructed by the neighbor-joining (NJ) method.
2.4. Effects of MR-16 on Mycelial Growth of F. oxysporum
We evaluated the antagonistic activity of the culture filtrate of MR-16 on a PDA mixture medium using culture filtrate assays (CFA), as described by [24]. In brief, the PDA mixture medium was obtained in sterile conditions when the culture filtrate of MR-16 and PDA were mixed in a ratio of 1:4 (v/v). The agar-mycelium discs (8 mm diameter) of F. oxysporum were taken from the edge of an actively growing fungal colony for CFA. The agar-mycelium discs of F. oxysporum were placed in the center of PDA media containing culture filtrate of MR-16, as well as control of the PDA medium without culture filtrate were prepared, and all assays were prepared three replicates. All treatments were in dark cultures at 25 °C for 5 d, and observed the mycelial morphology of F. oxysporum every day. We determined the inhibition rate (%) of MR-16 culture filtrate using the following formula:
where is the diameter of F. oxysporum colonies growing in PDA, and is the diameter of F. oxysporum colonies growing in PDA with MR-16 culture filtrate.
2.5. Effects of MR-16 on Conidial Germination of F. oxysporum
The spore suspension of the fungal pathogen was prepared as follows: F. oxysporum was incubated on PDA at 25 °C for 10 d. Then, the surface of F. oxysporum conidia on PDA was eluted with sterile distilled water (sdH2O), and fungal spores were collected using a spreader and then were filtered through cheesecloth. The spore concentration was adjusted to 106 CFU·mL−1, which we then stored at 4 °C until later use.
The spore suspension of F. oxysporum and MR-16 culture filtrate were mixed in a ratio of 1:1 (v/v) with a control mixed sdH2O, which then had three replicates prepared. All the treatments were incubated at 25 °C. At 6, 12, 24, and 48 h, we observed conidia of F. oxysporum using the method described by [25].
2.6. Soil Colonization Assays
We obtained a modified variant of the rifampicin-resistant (Rif) mutant, as described by Darma et al. (2020) [26]. For successive cultures, we inoculated the Rif mutant of MR-16 isolate into PDB containing increasing concentrations of rifampicin (Rif, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China): 50, 100, 200, 300, 350, and 400 μg·mL−1. We tested the stability of Rif mutants of the MR-16 isolate by subculturing on PDA with 400 μg·mL−1 of Rif, observing no significant change compared with the MR-16 isolate. We labeled the Rif mutants of MR-16 isolate as MRRif-16 after we tested there was good stability of Rif mutants of the MR-16 isolate and stored them at −20 °C until use.
MRRif-16 strains were used for the colonization of soil from S. divaricata field to verify their ability to colonize plant soil. For this experiment, a one-year-old S. divaricata seedling was grown in a polypropylene pot (d = 200 mm, h = 180 mm) filled with soil previously mixed with the spore suspension of MRRif-16 at a ratio of 1:10 (v/v) under greenhouse conditions (16 h-sunlight at 28 °C and 8 h- darkness at 16 °C). We prepared 20 replicates for each treatment in a randomized complete block design (RCBD). After 7 to 35 days of inoculation, we recovered strain MRRif-16 and isolated it from the soil, and we determined and recorded the amount of soil colonization.
2.7. Biocontrol Activity of MR-16 Isolate on S. divaricata Wilt
We performed a modified version of the antifungal assay described by [27,28]. As previously described, the spore suspension of isolate MR-16 was prepared and adjusted to 1 × 107 CFU·mL−1. We pre-infected the soil substrate (soil from S. divaricata field, vermiculite; 2:1, v/v) with F. oxysporum. The assays were performed based on five different treatments as follows: untreated control (water), carbendazim 50% WP (5.0 g L−1) fungicide treatment, bacterial suspension of B. subtilis (108 CFU·mL−1), spore suspension of T. harzianum (107 CFU·mL−1), and spore suspension of MR-16 (107 CFU·mL−1). One-year-old S. divaricata plants inoculated with F. oxysporum (cultivated in the Medicinal Botanical Garden of JLAU) were transplanted and grown for 70 d with 20 replicates for each treatment in a CRBD. We calculated the disease index of S. divaricata Fusarium wilt and control efficacy of Fusarium wilt disease as previously described by [29].
The disease rating scale (0–9) for root rot was as follows: 0 = asymptomatic healthy plants, healthy; 1 = onset of symptoms, leaves displaying yellowing and wilting, lesions covering < 10% of the leaves; 3 = leaves displaying wilting and yellowing, lesions covering 11% to 25% of the leaves; 5 = indicating wilt symptoms, lesions covering 26% to 50% of the leaves; 7 = leaves displaying wilting, yellowing, and browning lesions, lesions covering 51% to 75% of the leaves; 9 = leaves show infection, complete dying, and drying of the plant, lesions covering 76% to 100% of the leaves.
where to represent the number of plants with each corresponding disease scale, and N represents the total number of plants assessed.
2.8. Plant Growth Promotion of MR-16 Isolate
To evaluate the effects of strain MR-16 on the growth of S. divaricata, we performed virulence assays of the spore suspension of MR-16 by inoculating S. divaricata plants. The plants were dipped in a suspension of 1 × 107 CFU mL−1 fungal spores of MR-16. We maintained the inoculated plants in a phytotron at 25~28 °C with 70% relative humidity, which we then monitored daily for plant growth.
The assays were performed based on four different treatments: nontreated control (water), bacterial suspension of B. subtilis (108 CFU·mL−1), spore suspension of T. harzianum (1 × 107 CFU·mL−1), and spore suspension of MR-16 (1 × 107 CFU·mL−1) in order to evaluate the effect of the spore suspension of MR-16 on the yield of S. divaricata. One seedling of S. divaricata was transplanted into a polypropylene pot (28 cm diameter and 20 cm height) filled with soil and vermiculite (2:1). We then performed 20 replications for each treatment in an RCBD. After 60 d with conventional agricultural management, we randomly selected nine S. divaricata plants. Subsequently, we measured and recorded the S. divaricata growth characteristics, including plant height, root length, the plant’s fresh biomass and dry biomass, and the root’s fresh biomass and dry biomass.
2.9. Statistical Analysis
The results were tested using analysis of variance (ANOVA) with 95% confidence intervals for Duncan’s DMRT in SPSS Statistics 13.0 software and graphed it with OriginPro 9.5.
3. Results
3.1. Antagonistic Activities of Fungal Isolate against F. oxysporum
In this study, we evaluated the antagonistic activities in vitro of 104 fungal isolates using F. oxysporum causing Fusarium wilt of S. divaricata. Among them, 12 fungal isolates (11.54%) acted as antagonists and displayed significant growth inhibition against F. oxysporum on PDA, which showed antagonistic rates of more than 56%. Compared with the other 11 strains, such as MR-82, strain MR-16 showed a significantly antagonistic effect against F. oxysporum (p < 0.05), which the inhibition rate and inhibition zone were 66.67% and 10.05 mm, respectively, and the spread of the pathogenic fungus was effectively controlled (Table 1). According to several experimental verifications, we found that the inhibitory activity of the MR-16 strain was stable, so the strain can be considered as a candidate for further studies.

Table 1.
Antifungal activities of selected rhizospheric fungi against F. oxysporum.
3.2. Antifungal Spectrum of MR-16 Isolate
The fungal isolate MR-16 showed the ability to inhibit the growth of nine plant pathogenic fungi, with inhibition rates ranging from 54.44% to 80.00%. Of the nine inhibited fungi, the growth of six pathogenic fungi, such as P. cactorum, C. destructans, M. acerina, etc., were significantly inhibited (greater than 71% inhibition rate). Moreover, the suppression rates of strain MR-16 against R. solani were as high as 54% (Table 2). The results showed that isolate MR-16 has broad-spectrum inhibition and could be developed as a potential biological control agent for fungal diseases of S. divaricata and other plants.

Table 2.
Antibiotic activities of strain MR-16 against fungal pathogens.
3.3. Identification of Antagonistic Strain MR-16
3.3.1. Identification of Culture Characteristics
The colony morphologies of strain MR-16 on CA, CYA, CYAS, G25N, MEA, and PDA medium are shown in Figure 1. On CA, the colony diameter was 45.3 mm on average; the middle of the colony was fluffy; the mycelia were spread and sparse; the front of the colony was yellowish; exudate was absent, as was soluble pigment absent. For colonies growing on CYA, their diameter was 43.5 mm on average, and the colony surface was flat and velutinous, and the middle of colony was brilliant yellow; the mycelia were tangerine in the margins of the colony; exudate and soluble pigment were absent. On CYAS, the colony diameter was 15.6 mm on average; the mycelium texture was velutinous, slowly growing; the margins of the colony were regular; the colony surface was brilliant yellow; exudate and soluble pigment were absent. For colonies growing on G25N, their diameter was 10.2 mm on average; the colony texture was fluffy; the reverse color of central colonies was red-brown, and the margins were white and irregular, barely growing; mycelia were sparse; exudate and soluble pigment were absent. On MEA, the colony diameter was 46.3 mm on average; colony texture was granular, fluffy, and plain; the middle of the colony was blackish green at the center; the mycelia of margins were white; exudate and soluble pigment were absent. Colonies growing on PDA were, on average, 45.7 mm in diameter; colony texture was flat and fluffy; and colony edge was chartreuse, which was covered with grayish-green to white mycelium and showed heavy sporulation. These morphological characteristics of MR-16 on the media are similar to those of the genus Penicillium, as described by [30,31].
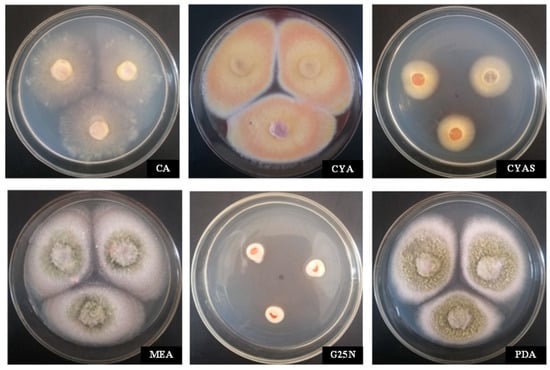
Figure 1.
Morphological characteristics of the cultures of MR-16 on six differential media. The front side of the colony was cultured on CA, CYA, CYAS, MEA, G25N, and PDA at 25 °C in the dark for 10 days.
3.3.2. Identification of Microscopic Features
Our observed microscopic characteristics of isolate MR-16 are similar to those of the genus Penicillium, as described by Kong [32]. The conidiophores of isolate MR-16 were born from hyphae with a smooth wall. Stipe was 30–55 × 2–3.5 μm with no enlargement. Branching patterns of penicillus were terminal and monoverticillate with 2–5 obviously lanceolate phialides (7–12 × 3–3.5 μm). MR-16 had ellipsoidal-to-subspheroidal and 2–3 × 1.5–2 μm conidia with rough walls. The ascus of MR-16 was 110–160 × 90–140 μm, and ascospores were ellipsoidal with rough walls, 2–2.5 × 1.5–2 μm (Figure 2).
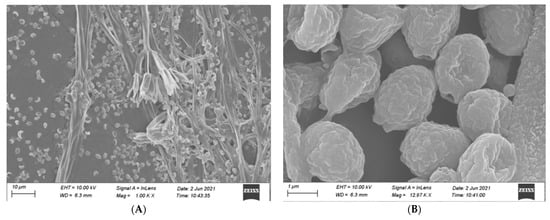
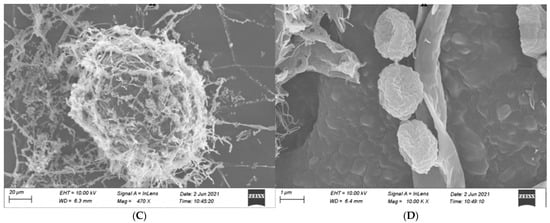
Figure 2.
Morphology of reproductive structures of MR-16. (A): Phialide, metulae and Stipe, (B): Conidia, (C): Ascomata, (D): Ascospores. Scale bars: (A) = 10 μm; (B) = 1 μm; (C) = 20 μm; and (D) = 1 μm.
3.3.3. Molecular Identification
The ITS gene sequence of strain MR-16 was amplified and sequenced to obtain a base sequence of 565 bp with the GenBank accession number OK287146.1. We found that MR-16 showed high sequence homology with Penicillium caperatum (MK450677.1) and P. javanicum (MH865296.1) of over 99%, P. meloforme (MT529271.1), and P. setosum (MK450718.1) by comparison of the 5.8S rRNA gene fragment using NCBI’s BLAST. Moreover, MR-16 showed similarity to P. pulvillorum (MH865335.1), P. janthinellum (KP992936.1), and P. tanzanicum (KT887863.1) of close to 98%. Based on a phylogenetic tree construction, the results indicated that strain MR-16 and P. caperatum (NR_138333.1) have high homology and are in the same clade (Figure 3).
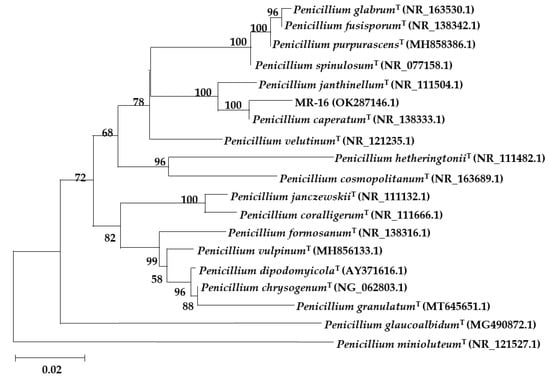
Figure 3.
Phylogenetic analysis of MR-16 and its relatives based on the nucleotide sequences of ITS sequences by using the neighbor-joining method (NJ). The values of significant bootstrap (>58%) and the scale (0.02) of the phylogenetic tree are exhibited.
Based on the above identification results, the MR-16 strain was identified as P. caperatum, which is the first time that the strain MR-16 has been discovered and reported in China [31,32].
3.4. MR-16 Culture Filtrate Inhibition of F. oxysporum Mycelium
The MR-16 culture filtrate substantially inhibited the mycelial growth of F. oxysporum. The healthy mycelia of F. oxysporum had a smooth exterior surface (Figure 4A,B). The inhibited pathogenic colonies thinned, with clear and irregular edges, sparse aerial mycelium, and a slow growth trend, and the mycelia dissolved obviously (Figure 4C).
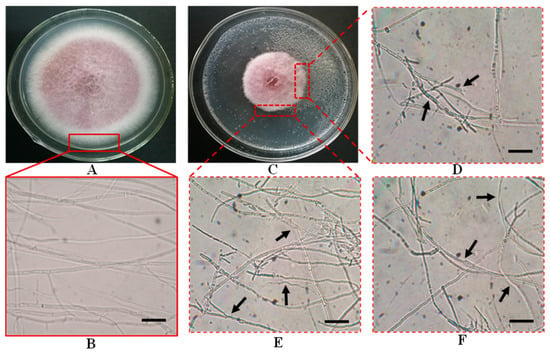
Figure 4.
In vitro inhibition of mycelial growth of F. oxysporum in co-culture with P. caperatum MR-16 on PDA medium. (A,B): F. oxysporum in single culture. (C–F): Mycelia of F. oxysporum in co-culture with culture filtrate of MR-16. Scale bars = 20 μm.
In the co-culture assay, compared to single culture treatments, the mycelia of F. oxysporum at the edge of the colony showed an irregular extension with thickened cell walls; the branching ends were enlarged, mycelia became coarsened, and its surfaces were rough and uneven; including shrunken protoplasts and uneven distribution (Figure 4D). The inhibited mycelia of F. oxysporum were distorted, shortened, constricted, and dilative, among other deformities (Figure 4E,F). The results indicated that P. caperatum MR-16 isolate could inhibit the growth of F. oxysporum by producing certain substances.
3.5. Culture Filtrate of MR-16 on Spore Germination of F. oxysporum in Co-Culture
The effect of the co-culture of MR-16 culture filtrate and spore suspension of F. oxysporum was shown in Table 3. The result demonstrated that the spore germination of the pathogen was significantly inhibited by the strain MR-16 (Figure S1), in which the spore germination rate of F. oxysporum in co-culture for 48 h and the inhibitory rates were 9.04% and 84.88%, respectively (Table 3).

Table 3.
Effects of culture filtrate of MR-16 on the spore germination of F. oxysporum.
3.6. Soil Colonization Ability of MR-16 Isolate
The strain stably grew on PDA plates containing rifampicin (400 μg·mL−1) after 10 generations of subculture, in which the morphology of MRRif-16 had no noticeable change. MR-16 and MRRif-16 had a similar effect against F. oxysporum; the inhibition rate of SWRif-34 was up to 65%. The results indicated that strain MRRif-16 was genetically stable and still maintained high inhibition activity against F. oxysporum. The ability of MRRif-16 to colonize in soil was tested for 35 d. In the soil, the population density of MRRif-16 first increased and then decreased. The MRRif-16 population was 6.05 × 106 CFU per gram of soil at seven days of inoculation. It peaked, and the cell counts increased from 6.05 × 106 to 7.90 × 106 CFU per gram soil at twenty-eight days of inoculation in soil. Subsequently, it began to decrease in the soil. Then, the amount of colonization of the MRRif-16 population was still 5.56 × 106 CFU per gram of soil thirty-five days after inoculation (Figure 5).
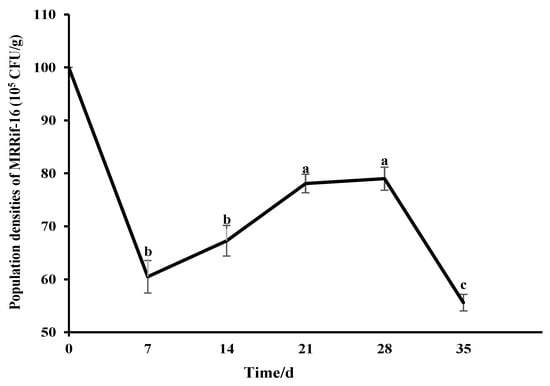
Figure 5.
Colonization of MRRif-16 in the soil in which S. divaricata was grown. Soil samples were treated with a spore suspension of MRRif-16 at a final density of 107 CFU per gram of soil. After 7 d, 14 d, 21 d, 28 d, and 35 d of inoculation, the population densities of the MRRif-16 were determined. The different letters indicate significant differences in Duncan’s DMRT (p < 0.05).
3.7. Control Efficiency of MR-16 against Fusarium wilt of S. divaricata
There was a reduced number of disease symptoms in plants of S. divaricata treated with a spore suspension of MR-16 (Table 4). S. divaricata plants in the nontreated control treatment inoculated with F. oxysporum showed mass disease spots on their leaves, which was up to 50.59 on the disease severity index, whereas Fusarium wilt of S. divaricata was strongly inhibited in the treatment with the spore suspension of MR-16 and the antifungal efficacy was 60.76% ten weeks after the treatment. Moreover, there was no significant difference in antifungal efficacy of the spore suspension of MR-16 compared with that of carbendazim 50% WP (68.38%), a bacterial suspension of Bacillus subtilis (56.15%), or spore suspension of Trichoderma harzianum (61.28%).

Table 4.
Effect of the MR-16 isolate on Fusarium wilt of S. divaricate.
3.8. The Growth-Promoting Effect of MR-16 on S. divaricata
The spore suspension of MR-16 showed no pathogenicity on S. divaricata plants at 10 days post-inoculation, and we observed no disease symptoms in S. divaricata. The physiological traits of S. divaricata plants were noticeably affected by the MR-16 isolate, T. harzianum, and B. subtilis (Table 5). The plant height, fresh biomass, total dry plant biomass, fresh root biomass, and dry root biomass of the specie treated with strain MR-16 were significantly higher than those of plants in other treatment groups (p < 0.05). The average increase of plant height, fresh biomass, etc., of S. divaricata in the MR-16 treatment group was 42.09%, compared with those of plants in the CK treatment. The growth of specie, including fresh plant biomass, dry plant biomass, fresh root biomass, and dry root biomass, was promoted by the application of the spore suspension of MR-16, which increased by more than 43% compared with those of the CK. The results demonstrated that the MR-16 isolate could stimulate the growth of S. divaricata as a plant-growth-promoting fungus. MR-16 isolate has the potential to be used as a beneficial microorganism for yield increase.

Table 5.
Effects of MR-16 on promoting the growth of S. divaricate.
4. Discussion
The genus Penicillium is a member of the soil saprophytic group of fungi that are environmentally adaptable, comprising approximately 480 accepted species to date [33]. Although some members of the Penicillium can cause food-borne contamination and fungal diseases in plants and animals [34,35], Penicillium, being one of the first fungal species, has important research and economic value in the field of biotechnology [36]. In addition, some Penicillium strains have strong ecological competitiveness and have shown outstanding applications, including insecticides [37,38], plant growth promotion [39], heavy metal biosorption [40], industrial wastewater treatment [41], etc. They have received considerable attention, have been widely studied by countries worldwide, and are considered to be a suitable source of fungi biocontrol with development and application value [42].
A rhizospheric fungus, strain MR-16 was screened in this study, which showed a strong antagonistic effect on F. oxysporum, which causes Fusarium wilt disease of S. divaricata. This strain has obvious antagonistic effects on nine common pathogenic fungi. Based on our findings of culture characteristics, microscopic features, and molecular identification, strain MR-16 was identified as Penicillium caperatum. MR-16, tested in this study, was first discovered and reported in China, and we isolated this fungal species. P. caperatum is a species in the Lanata-Divaricata section of Penicillium. As a first report, this species was isolated from the soil of Murrumbidgee Irrigation Area in NSW, Australia [33,43].
The antifungal mechanisms of antagonistic fungi mainly include competition, lysis, induced plant resistance, and hyperparasitism [44]. Fungi of the Penicillium genus could effectively antagonistically affect the growth of pathogenic fungi by penicierythritols, calbistrins, and other active substances [45,46]. Moreover, the Penicillium genus can induce resistance in plant hosts, thus protecting them from plant pathogens [47]. The mycelium and spore germination of F. oxysporum was inhibited by P. caperatum aseptic fermentation filtrate, which caused the mycelium to be malformed and the spore germination rate was decreased. It was speculated that P. caperatum could destroy the cell wall or membrane structure of F. oxysporum mycelium by secreting extracellular antibacterial substances. Meanwhile, the respiration of pathogen spores was disturbed, which resulted in the inhibition of the growth of pathogenic fungi, suggesting that the extracellular secondary metabolites of P. caperatum could be developed as potential biocontrol agents for plant diseases. However, the specific substances responsible for the inhibitory effect of P. caperatum remain unknown, which is worth further study.
The effectiveness and stability of biocontrol effects could be directly affected by the ability of antagonistic microorganisms to colonize the soil, which is an important indicator for assessing potential biocontrol sources [48]. In our study, we found that P. caperatum MR-16 possesses soil colonization capacity through antibiotic labeling, which could grow and stably propagate in the soil. We will evaluate the colonial effect of P. caperatum on S. divaricata in future research.
The Penicillium genus can prevent and control plant diseases [49]. In this present study, we isolated Penicillium caperatum from the rhizospheric soil from S. divaricata field, and it was first discovered in soil in China, suggesting that P. caperatum may be a soil saprophytic group of fungi. In addition, we found that Fusarium wilt disease of S. divaricata was effectively prevented and controlled by P. caperatum MR-16, and the effect of MR -16 on the growth-promoting nature of S. divaricata plants was stronger than B. subtilis and T. harzianum. To the best of our knowledge, this is the first report on the bio-control activities of P. caperatum against plant fungal pathogens of a medicinal plant and its plant growth promotion effects. The dominant microorganisms in the habitat or other environmental factors limit the growth and reproduction resulting from the competition of many exogenous microorganisms introduced into the soil, resulting in unsatisfactory biocontrol effects. In addition, the original microecological balance of the soil is affected by non-indigenous microorganisms that may have a substitution effect on the dominant microorganisms in the soil microenvironment [50]. The diversity and structure of indigenous communities play an important role in the functioning of indigenous communities. Excessive interference by exogenous microorganisms may directly or indirectly affect the interaction between host plants and soil microorganisms [51]. T. harzianum and B. subtilis, as exogenous microorganisms not originating from inherent soil from S. divaricata field, may compete with indigenous microorganisms for ecological niches when they are introduced into the soil, which the biological activity weakened, and disease control and plant-growth-promotion capacity decreased [52]. In conclusion, the P. caperatum MR-16, which was isolated and screened from the rhizospheric soil from the S. divaricata field, may be used for eco-friendly biological control of Fusarium wilt disease on S. divaricata and other plants.
5. Conclusions
In the present study, we isolated and identified a potential biocontrol agent for S. divaricata, Penicillium caperatum MR-16. A new record of this species of China, it exhibited biocontrol activity against the Fusarium wilt disease of S. divaricata. This is the first report of MR-16 showing broad-spectrum antibiotic capacities, which displayed good antifungal efficacy in vitro as a beneficial microorganism. In addition, P. caperatum MR-16 could successfully colonize and form a stable population in the soil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9040361/s1, Figure S1: Effects of culture filtrate of MR-16 on the spore germination of F. oxysporum.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, writing—original draft preparation, Y.D., Z.H. and J.W.; investigation, J.W., Y.W. (Yuyi Wang) and Y.W. (Yan Wang); resources, L.Y.; data curation, writing—review and editing, Z.S.; supervision, project administration, Z.S. and Y.W. (Yunhe Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Key Research and Development Project of China (2019YFC1710700), the Jilin Science and Technology Development Project (No: 20200404010YY, 20230508121RC, 20210204011YY), and the Project of National Modern Industrial Technology System (No: CARS-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request to the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex 2022, 49, 100978. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume I, p. 156. ISBN 978-7-5067-7337-9. [Google Scholar]
- Kreiner, J.; Pang, E.; Lenon, G.B.; Yang, A.W.H. Saposhnikoviae divaricata: A phytochemical, pharmacological, and pharmacokinetic review. Chin. J. Nat. Med. 2017, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Jiang, H.; Dai, H.L.; Wang, Z.W.; Jia, G.Z.; Meng, X.C. Feeble antipyretic, analgesic, and anti-inflammatory activities were found with tegular dose 4′-O-beta-D-glucosyl-5-O-methylvisamminol, one of the conventional marker compounds for quality evaluation of Radix Saposhnikoviae. Pharmacogn. Mag. 2017, 13, 168–174. [Google Scholar]
- Ma, J.; Tian, Y.; Wang, Z.; Liu, G.; Wang, Z.; Zhang, M. Isolation and identification of endophytic antagonistic bacteria from Saposhnikovia divaricata. J. Jilin Agric. Univ. 2022, 33, 323–328. [Google Scholar]
- Guo, X.; Tian, X.; Hao, J.; Wang, Y.; Yang, L.; Han, M.; Han, Z. Relationships between Saposhnikovia divaricata chromone content and soil factors in different regions. J. S. China Agric. Univ. 2020, 41, 31–37. [Google Scholar]
- Zeng, L. Diseases and Insect Pests of Medicinal Plants; Guizhou Science and Technology Press: Guiyang, China, 2017; p. 222. [Google Scholar]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.; Stepanek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wang, Y.; Li, J.; Shang, S.B.; Song, Z.Q. Improved application of natural forest product terpene for discovery of potential botanical fungicide. Ind. Crop. Prod. 2018, 126, 103–112. [Google Scholar] [CrossRef]
- Sare, A.R.; Jijakli, M.H.; Massart, S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021, 179, 111572. [Google Scholar] [CrossRef]
- Bollmann-Giolai, A.; Malone, J.G.; Arora, S. Diversity, detection and exploitation: Linking soil fungi and plant disease. Curr. Opin. Microbiol. 2022, 70, 102199. [Google Scholar] [CrossRef]
- Samaras, A.; Karaoglanidis, G.S.; Tzelepis, G. Insights into the multitrophic interactions between the biocontrol agent Bacillus subtilis MBI 600, the pathogen Botrytis cinerea and their plant host. Microbiol. Res. 2021, 248, 126752. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of soil bacterial isolates suppressing different phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yan, Y.; Wang, Y.; Wu, M.; Mao, Q.; Chen, Y.; Ren, J.; Liu, A.; Lin, X.; Ahammed, G.J. Trichoderma asperellum reduces phoxim residue in roots by promoting plant detoxification potential in Solanum lycopersicum L. Environ. Pollut. 2020, 259, 113893. [Google Scholar] [CrossRef] [PubMed]
- Wonglom, P.; Daengsuwan, W.; Ito, S.I.; Sunpapao, A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- Choi, H.W.; Ahsan, S.M. Biocontrol activity of Aspergillus terreus anu-301 against two distinct plant diseases, tomato fusarium wilt and potato soft rot. Plant Pathol. J. 2022, 38, 33–45. [Google Scholar] [CrossRef]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork oak endophytic fungi as potential biocontrol agents against Biscogniauxia mediterranea and Diplodia corticola. J. Fungi 2020, 6, 287. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Visagie, C.M.; Renaud, J.B.; Burgess, K.M.; Malloch, D.W.; Clark, D.; Ketch, L.; Urb, M.; Louis-Seize, G.; Assabgui, R.; Sumarah, M.W.; et al. Fifteen new species of Penicillium. Persoonia 2016, 36, 247–280. [Google Scholar] [CrossRef]
- Han, Z.; Cui, Y.; Wang, Y.; Wang, Y.; Sun, Z.; Han, M.; Yang, L. Effect of rhizospheric fungus on biological control of root rot (Fusarium equiseti) disease of Saposhnikovia divaricata. Agronomy 2022, 12, 2906. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Millan, A.F.S.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control 2021, 160, 104683. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Li, Z.; Li, C.; Li, B.; Gu, X.; Zhang, X.; Zhang, H. Screening and identification of an antagonistic yeast controlling postharvest blue mold decay of pears and the possible mechanisms involved. Biol. Control 2019, 133, 26–33. [Google Scholar] [CrossRef]
- Darma, S.; Ambara, A.; Aman, A.T.; Annisa, L.; Nurrokhman; Nuryastuti, T.; Wibawa, T. High frequency of azole resistant Candida spp. colonization among presumptive multidrug resistant tuberculosis (MDR-TB) patients. PLoS ONE 2020, 15, e242542. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Amini, J.; Abdollahzadeh, J.; Ashengroph, M. Basidiomycetes fungi as biocontrol agents against take-all disease of wheat. Biol. Control 2019, 130, 34–43. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y.; He, J.; Tang, X.; Wu, X.; Xu, C. Identification of a new Talaromyces strain DYM25 isolated from the Yap Trench as a biocontrol agent against Fusarium wilt of cucumber. Microbiol. Res. 2021, 251, 126841. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, Á.; López-Escudero, F.J.; Trapero, A. A non-pathogenic strain of Fusarium oxysporum as a potential biocontrol agent against Verticillium wilt of olive. Biol. Control 2019, 139, 104045. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Houbraken, J.; Samson, R.A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 2011, 70, 1–51. [Google Scholar] [CrossRef]
- Kong, H. Flora fungorum sinicorum. In Penicillium et Teleomorphi Cognati; Science Press: Beijing, China, 2007; Volume 35, pp. 40–199. ISBN 978-7-0301-9262-2. [Google Scholar]
- Houbraken, J.; de Vries, R.P.; Samson, R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv. Appl. Microbiol. 2014, 86, 199–249. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Radulesco, T.; Varoquaux, A.; Ranque, S.; Dessi, P.; Michel, J.; Cassagne, C. A Case of fungus ball-type maxillary sinusitis due to Penicillium roqueforti. Mycopathologia 2018, 183, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sens. Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Mareze, J.; Ramos-Pereira, J.; Santos, J.A.; Beloti, V.; López-Díaz, T.M. Identification and characterisation of lactobacilli isolated from an artisanal cheese with antifungal and antibacterial activity against cheese spoilage and mycotoxigenic Penicillium spp. Int. Dairy J. 2022, 130, 105367. [Google Scholar] [CrossRef]
- Patil, N.S.; Jadhav, J.P. Significance of Penicillium ochrochloron chitinase as a biocontrol agent against pest Helicoverpa armigera. Chemosphere 2015, 128, 231–235. [Google Scholar] [CrossRef]
- Murali, M.; Amruthesh, K.N. Plant growth-promoting fungus Penicillium oxalicum enhances plant growth and induces resistance in pearl millet against downy mildew disease. J. Phytopathol. 2015, 163, 743–754. [Google Scholar] [CrossRef]
- Din, G.; Hassan, A.; Dunlap, J.; Ripp, S.; Shah, A.A. Cadmium tolerance and bioremediation potential of filamentous fungus Penicillium chrysogenum FMS2 isolated from soil. Int. J. Environ. Sci. Technol. 2022, 19, 2761–2770. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Bisht, N.; Tewari, L. Eco-friendly bioprocess using agar plug immobilized Penicillium crustosum PWWS-6 biomass for treatment of wastewater contaminated with toxic Congo red dye for use in agriculture. Ind. Crop. Prod. 2021, 170, 113755. [Google Scholar] [CrossRef]
- Urooj, F.; Farhat, H.; Tariq, A.; Moin, S.; Sohail, N.; Sultana, V.; Hameedi, S.F.; Shams, Z.I.; Ehteshamul-Haque, S. Role of endophytic Penicillium species and Pseudomonas monteilii in inducing the systemic resistance in okra against root rotting fungi and their effect on some physiochemical properties of okra fruit. J. Appl. Microbiol. 2021, 130, 604–616. [Google Scholar] [CrossRef]
- Udagawa, S.; Horie, Y. Some Eupenicillium from soils of New Guinea. Trans. Mycol. Soc. Jpn. 1973, 14, 370–387. [Google Scholar]
- Kepler, R.M.; Maul, J.E.; Rehner, S.A. Managing the plant microbiome for biocontrol fungi: Examples from Hypocreales. Curr. Opin. Microbiol. 2017, 37, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wei, X.L.; Xue, L.; Zhang, Z.F.; Zhang, P. Antimicrobial meroterpenoids and erythritol derivatives isolated from the marine-algal-derived endophytic fungus Penicillium chrysogenum XNM-12. Mar. Drugs 2020, 18, 578. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, M.; Chang, Z.; Zhang, J.; Xu, Y. Purification, identification and bioactivity of antagonistic chemicals produced by biocontrol agents of Penicillium striatisporum Pst10. Jiangsu J. Agric. Sci. 2013, 5, 1011–1018. [Google Scholar]
- Ting, A.S.Y.; Mah, S.W.; Tee, C.S. Evaluating the feasibility of induced host resistance by endophytic isolate Penicillium citrinum BTF08 as a control mechanism for Fusarium wilt in banana plantlets. Biol. Control 2012, 61, 155–159. [Google Scholar] [CrossRef]
- Xian, H.Q.; Liu, L.; Li, Y.H.; Yang, Y.N.; Yang, S. Molecular tagging of biocontrol fungus Trichoderma asperellum and its colonization in soil. J. Appl. Microbiol. 2020, 128, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chang, Z.; Zhao, J.; Zhou, M. Antifungal activity of Penicillium striatisporum Pst10 and its biocontrol effect on Phytophthora root rot of chilli pepper. Biol. Control 2008, 44, 24–31. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, J. Hazard analysis of the impact of biocontrolling microbes on soil micro-ecosystem. J. Microbiol. 2006, 26, 85–88. [Google Scholar] [CrossRef]
- Ma, C.; Gong, X.; Gao, H.; Wu, J.; Li, D.; Chen, X.; Li, H.; Liu, M. Legacy impacts on the relationships between soil microbial community and the invasion potential of non-indigenous bacteria. Acta Ecol. Sin. 2019, 39, 1–9. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; Tuinen, D.V.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).