Abstract
Numerous species of microalgae have been utilized for pigment production. More and more species are gaining popularity due to their ability to accumulate pigments with varying chemical compositions and the fact that some have distinctive byproducts that can be co-produced. Despite the fact that many of the species have unique by-products and traits, they are not being used economically due to high production costs. Utilizing agricultural and industrial wastewater for algae cultivation is one way to lower manufacturing costs. Herbicide-contaminated wastewater can result from agricultural contamination. Norflurazon is a popular pesticide frequently used for weed control. The presence of norflurazon in water renders that water unusable and requires proper treatment. Nanoparticles of ZnO (ZnO NPs), on the other hand, are utilized in a variety of industrial productions of numerous household goods. Water contaminated with ZnO NPs can present potential risks to human health and the environment. In this study, two field isolates of the green microalga Chlamydomonas reinhardtii, a widely used model organism, were examined for their reaction to these two compounds in order to assess the responses of different natural strains to environmental stresses. Norflurazon at 10 µM had a higher inhibitory effect on growth and pigment production than ZnO NPs at 200 mg L−1. Although both norflurazon and ZnO NPs inhibit cell growth and pigmentation, they do so through distinct processes. Norflurazon induces oxidative stress in cells, resulting in photosystem damage. ZnO nanoparticles, on the other hand, did not cause photosystem damage but rather mechanical cell damage and disintegration. In addition, the physiological responses of the two Chlamydomonas strains were distinct, supporting the utilization of natural algal strains for specific types of environmental pollutants.
1. Introduction
Microalgae are photosynthetic unicellular organisms that serve as an essential carbon source, and play crucial roles in balancing the nutrient cycle in aquatic habitats and maintaining the food web in all ecosystems [1,2,3]. Microalgae convert light energy and inorganic materials into useful bioactive substances, including polysaccharides, lipids, pigments, proteins, vitamins, minerals, and antioxidants [4]. These microalgal bioactive chemicals are utilized in a variety of industries, including human and animal nutrition, cosmetics, pharmaceuticals, and other bio-based products [5]. Microalgae are found in aquatic habitats such as lakes, ponds, rivers, and even wastewater [6]. Increasing wastewater from anthropogenic activities such as industrial, agricultural, and household activities has become one of the most pressing environmental concerns and a danger to water security in recent years [7].
Environmental and social consciousness inspires the reuse of wastewater for microalgal production. Norflurazon is a pyridazinone herbicide used for grass and broadleaf weed management. This chemical causes plant bleaching by inhibiting carotenoid synthesis, which results in chlorophyll depletion and photosynthetic suppression in plants [8,9]. Because this herbicide directly inhibits carotenoid synthesis, it has been used in many studies to isolate microalgal strains with high carotenoid levels both via random mutagenesis and genetic engineering [10,11,12]. Nevertheless, studies on differential responses to this herbicide in different strains within the same species are still scarce. In addition to chemicals used in agriculture, which can easily contaminate water bodies, chemicals used in the industrial sector can also contaminate water bodies either from production facilities or from their usage. ZnO nanoparticles (ZnO NPs) are widely used in physical sunscreen and cosmetics, making them one of the most well-known pollutants. Due to their adsorption ability, large surface area, transparency, UV absorption efficiency, and chemical stability, ZnO nanoparticles (ZnO NPs) are some of the most widely used metals in nanotechnology development with industrial applications. They are also used to produce pigments, semiconductors, UV protection films, chemical sensors, and hair care products [13,14,15]. In addition, this particle’s antimicrobial capability has broadened its application in the pharmaceutical and food industries [16,17]. However, ZnO NPs transmit their toxicity across aquatic environments. Consequently, polluted aquatic systems have a significant negative influence on biological ecosystems and pose a threat to human health through the direct absorption of contaminated drinking water or consumption of vegetables and edible microalgae containing ZnO NPs [18,19].
The unicellular green microalga Chlamydomonas reinhardtii is widely used as a model organism for the study of numerous biological processes, including photosynthesis, circadian rhythm, phototaxis, flagella structure, etc., due to its cultivation ease, haploid genome, ability to grow heterotrophically, and the availability of genome sequence and genetic tools [20,21]. This alga produces pigments such as chlorophyll and carotenoids that can be used in the cosmetic, food, and pharmaceutical industries [22]. Numerous investigations have demonstrated that various natural isolates of this alga respond differently to various conditions [23,24]. However, responses to the herbicide norflurazon and the nanoparticles of ZnO NPs remain understudied, particularly in terms of the differential responses of field isolates. In this study, two natural strains of this alga were selected to examine their responses. These strains were examined for alterations in their physiology and pigment production, while they were cultivated in the presence of norflurazon and ZnO NPs. The differences in their sensitivity to the effects of different substances and the effects of different strains and pigment levels are discussed.
2. Materials and Methods
2.1. Algal Strains and Culture Conditions
Chlamydomonas reinhardtii CC-2344 and CC-4414 strains were provided by the Chlamydomonas Resource Center (University of Minnesota). Strains were maintained at 25 °C on TAP agar plates [25] at 50 µmoles photons m−2 s−1. Strains were inoculated into liquid cultures of TAP medium in Erlenmeyer flasks and cultivated with shaking at 120 rpm under 200 µmoles photons m−2 s−1 illumination using fluorescent lamps. Log phase cultures were diluted to a density of 1 × 106 cells mL−1 for all experiments.
To investigate the effect of norflurazon and ZnO NPs on the growth of Chlamydomonas on TAP agar plates, a 3 µL aliquot of two dilutions (1 × 106 cells mL−1 and 1 × 105 cells mL−1) was spotted onto plates containing various concentrations of norflurazon (Sigma-Aldrich, Poole, UK) and ZnO nanopowder, <100 nm particle size (Sigma-Aldrich, UK). Plates were incubated at 25 °C under 200 µmoles photons m−2 s−1 of illumination for one week. Liquid cultures were prepared with a volume of 50 mL containing various concentrations of chemicals. Cultures were incubated at 25 °C with shaking at 120 rpm under 200 µmoles photons m−2 s−1 of illumination. All experiments were performed with three biological replicates.
2.2. Growth and Cell Morphology
Standard curves of the relationship between cell density and optical density at 750 nm were generated by counting cells under a microscope with a hemocytometer. To estimate cell density, 1 mL of culture was sampled and the optical density at 750 nm was measured. The morphology of cells was examined using a light microscope.
2.3. Cell Viability Assay
Cell viability was assessed using the Evans blue staining method [26]. The percentage of Chlamydomonas cells that did not absorb Evans blue dye (Sigma-Aldrich, UK), which stains nonviable cells, was used to estimate cell viability. A volume of 1 mL of Chlamydomonas cells was incubated with 1% (w/v) Evans blue for 15 min. Dye unbound to live cells was removed by thorough washing with fresh TAP medium, while dye bound to dead cells was solubilized in a solution containing 50% (v/v) methanol and 1% (w/v) sodium dodecyl sulfate (SDS) for 30 min at 50 °C, and quantified via absorbance at 600 nm. The data are expressed as a percentage of total killing calibrated by Evans blue staining of equivalent cells that were heat-treated (~100% mortality) [27,28].
2.4. Photosynthetic Activity and Pigment Content
The fluorescence parameter measuring the quantum yield of PSII (Fv/Fm) was determined using Z985 Cuvette AquaPen (Qubit Systems, Ontario, Canada). Prior to measurement, 1 mL of each culture was kept in darkness for at least 20 min. For the estimation of the chlorophyll a, chlorophyll b, and total carotenoid contents, the pigments were extracted from 1 mL of culture. Cells were collected via centrifugation for 10 min at 7500× g, and resuspended in 1 mL of 80% acetone. Extraction was performed via vortexing until the cells were white. After centrifugation, the supernatant was subjected to spectrophotometer measurement, using the optical density at 470, 646, and 663 nm. The concentrations of pigments were calculated according to the equations previously reported [29]. For all measurements, an absorbance of 720 nm was used to correct for the contaminated color compound [30].
2.5. Statistical Analysis
Statistical analyses were conducted using SPSS Statistics version 22.0. Analysis of variance (ANOVA) was calculated to determine statistical significance (p < 0.05). All experiments were performed with three biological replicates and the results are expressed as mean values ± standard deviation (SD).
3. Results
3.1. Algal Growth and Responses to Chemicals
A desirable trait for microalgae designed for cultivation in wastewater is resistance to contaminants. Two distinct kinds of pollutants were chosen, with norflurazon representing agricultural pollutants and ZnO NPs representing industrial pollutants. CC-2344 and CC-4414, two Chlamydomonas field isolates, were examined [24]. Cells were spotted directly on plates containing varying concentrations of norflurazon or ZnO NPs. Strains were able to grow similarly under controlled conditions, as seen by growth and color on plates in the absence of chemicals (Figure 1a). The concentrations of norflurazon at 5 µM and 10 µM inhibited the growth of CC-4414, but CC-2344 was able to grow at these concentrations. In the presence of ZnO NPs, dark green cells turned light green as ZnO NP concentrations increased, yet cells were still able to proliferate. At a higher ZnO NP concentration, the cells of CC-2344 were greener than those of CC-4414. Similar outcomes were seen for liquid cultures supplemented with norflurazon and ZnO NPs (Figure 1b).
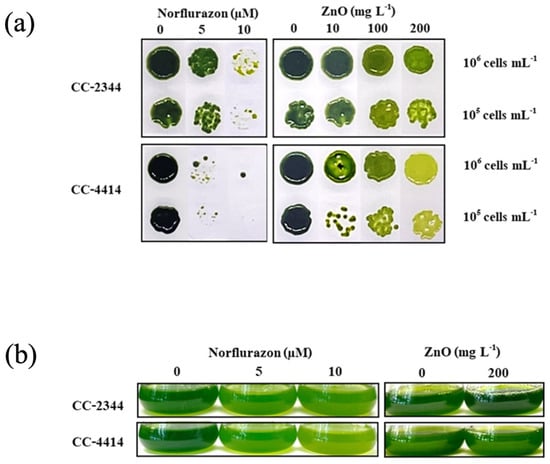
Figure 1.
Growth of C. reiinhardtii CC-2344 and CC-4414 cells with and without norflurazon and ZnO NPs on plates (a) and in liquid cultures (b). Exponential phase cultures were diluted to the indicated cell density, and a 3 µL aliquot was spotted onto plates with the indicated chemical at different concentrations. Liquid cultures were diluted to a density of 1 × 106 cells mL−1 and the chemical at the indicated concentration was added. Photographs were taken after 96 h.
The growth kinetics of CC-2344 and CC-4414 under identical conditions were distinct. Under the control conditions, both strains grew rapidly over the first 48 h and then maintained a constant growth rate between 72 and 96 h (Figure 2a,b). Even though both strains reached the stationary phase after 48 h, the cell density of CC-4414 was higher than that of CC-2344 and remained higher throughout the experiment. The addition of norflurazon hindered the growth of both strains in a dose-dependent manner, as evidenced by the lower initial cell density in cultures containing norflurazon. Similar to norflurazon, ZnO NPs decreased the growth of both strains, although the suppression was not as strong. In all situations, CC-4414 exhibited greater growth compared to CC-2344.

Figure 2.
Growth and percentage of cell survival. Cell density (a,b) and percentage of cell survival (c,d) under norflurazon and ZnO NP conditions, respectively. Blue and red solid lines are CC-2344 and CC-4414 with no chemicals, respectively. Blue and red dashed lines and dotted lines represent CC-2344 and CC-4414 with the indicated concentration of the chemical, respectively. All data are means ± SD of three biological replicates. Significant differences between the control culture and chemical-added culture within the same condition are indicated by asterisks (*) (p < 0.05).
In order to determine the proportion of surviving cells, cell viability assay was performed using the Evans blue staining method. Norflurazon at 5 µM caused a small but significant reduction in cell viability of both strains at 24 h and 48 h (Figure 2c). At 72 h and 96 h, however, the cell viability of cultures with 5 uM of norflurazon was similar to that of the control. The presence of 10 µM norflurazon resulted in a considerable reduction in growth within the first 48 h of treatment, and the effects were stronger toward the end of the experiment. At 96 h, the cell viability of CC-2344 and CC-4414 in the presence of 10 µM norflurazon was at 47.1% ± 10.3 and 53.1% ± 10.4, respectively. The presence of ZnO NPs led to a strong reduction in the percentage of cell viability of CC-4414 at the beginning of the experiment, but the value recovered at later time points (Figure 2d). After 96 h, the percentage of cell viability in the presence of ZnO NPs was the same as that of the control in both strains.
3.2. Photosynthesis and Pigment Content
To maximize algal growth, the efficiency of light usage is essential, particularly in outdoor environments where the light intensity varies constantly. The maximum quantum yield of PSII (Fv/Fm), which measures photosynthetic capability, can be used to assess algal health by measuring the efficiency of light utilization via photosystem II (PSII). In the control conditions, the Fv/Fm values of both strains remained steady between 0.60 and 0.75 (Figure 3a,b). At 72 and 96 h, CC-4414 had greater values than CC-2344. At 24 h, norflurazon caused a significant drop in Fv/Fm in both strains, but the value of CC-4414 at 10 µM norflurazon was lower than that of CC-2344 (Figure 3a). The levels of CC-2344 were stable throughout the experiment after 24 h. CC-4414, on the other hand, demonstrated a significant recovery from 24 h to 72 h, with values stabilizing at 96 h. Norflurazon at 10 µM decreased the values greater than 5 µM at all time periods. The presence of ZnO NPs had no influence on Fv/Fm, and the values of both strains were identical (Figure 3b).

Figure 3.
Chlorophyll fluorescent parameter. The maximum quantum yield of PSII (Fv/Fm) under the presence of norflurazon (a) and ZnO NPs (b). Blue and red solid lines are CC-2344 and CC-4414 with no chemicals, respectively. Blue and red dashed lines and dotted lines represent CC-2344 and CC-4414 with the indicated concentration of the chemical, respectively. All data are means ± SD of three biological replicates. All data are means ± SD of three biological replicates. Significant differences between the control culture and the chemical-added culture within the same condition are indicated by asterisks (*) (p < 0.05).
To determine whether the presence of chemicals has any effect on pigment content, the amount of pigment per cell was calculated. In the absence of chemicals, the chlorophyll and carotenoid content of both strains were comparable (Figure 4a–d). Norflurazon dose-dependently decreased both chlorophyll and carotenoid concentrations (Figure 4a,c). At 24 h, the effect was more pronounced in CC-4414 than in CC-2344 (Figure 4a,c). At 96 h, however, CC-4414 was able to recover to have higher levels of both pigments than CC-2344 (Figure 4a,c). The presence of ZnO NPs decreased the levels of both pigments at 24 h and at 48 h (Figure 4b,d). Nevertheless, CC-4414 had higher pigment levels than CC-2344 at the end of the experiment (Figure 4b,d).

Figure 4.
Pigment content per cell unit. Chlorophyll content (a,b) and carotenoid content (c,d) under norflurazon and ZnO NP conditions, respectively. Blue and red solid lines are CC-2344 and CC-4414 with no chemicals, respectively. Blue and red dashed lines and dotted lines represent CC-2344 and CC-4414 with the indicated concentration of the chemical, respectively. All data are means ± SD of three biological replicates. Significant differences between the control culture and the chemical-added culture within the same condition are indicated by asterisks (*) (p < 0.05).
3.3. Cell Morphology
Over the course of 96 h, the shape of algal cells cultured with and without herbicide was examined. The cells of both strains were ellipsoidal or nearly spherical, measuring around 10 µm in length and 8 µm in width, with smooth surfaces (Figure 5a,f). Within cells, chlorophyll pigment is densely distributed. Norflurazon caused both strains to have a somewhat lighter hue (Figure 5b,c,g,h). In the presence of norflurazon, the pyrenoid of CC-2344 became less apparent, whereas that of CC-4414 remained intact (Figure 5b,c,g,h). On the other hand, ZnO NPs caused CC-2344 to shrink but not CC-4414 (Figure 5d,i). The cell surface of CC-2344 was irregular, with rough edges, and pieces of cells could be seen outside of the cells (Figure 5d). The cell was a bit paler in CC-4414, but the corners were not as ragged as in CC-2344 (Figure 5d,i). Interestingly, CC-4414 displayed cell aggregates with a higher degree of aggregation in the beginning of the experiment compared to the end, while CC-2344 did not exhibit cell aggregates (Figure 5e,j).
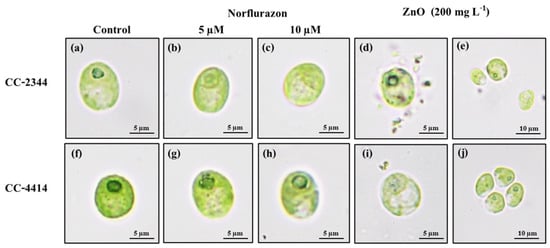
Figure 5.
Morphological changes in cells exposed to norflurazon and ZnO NPs for 96 h. CC-2344 and CC-4414 were cultivated in TAP (a,f), TAP with norflurazon (b,c,g,h), and ZnO NPs (d,e,i,j).
3.4. Pigment Production
To compare variations in pigment production, the chlorophyll and carotenoid productions per volume of culture were calculated. The presence of norflurazon lowered the production of pigments in both strains by a significant amount at 96 h (Figure 6a,b). However, in CC-4414, the level of chlorophyll continued to increase with time, whereas the production of carotenoid was stabilized at later time points. At the end of the experiment, both pigments in CC-2344 were stabilized or decreased at a higher concentration of norflurazon. At the same norflurazon concentration at 96 h, CC-4414 produced more of both pigments than CC-2344. Intriguingly, CC-4414 exhibited a lowered accumulation of both pigments at 24 h, but by 96 h, CC-4414 pigment production was higher than that of CC-2344. The addition of ZnO NPs decreased the accumulation of both pigments (Figure 6b,d). The production of both pigments by the two strains was equivalent after 24 h. However, CC-4414 pigment production was substantially greater than CC-2344 pigment production after 96 h.

Figure 6.
Pigment production. Chlorophyll content (a,b) and carotenoid content (c,d) under norflurazon and ZnO NP conditions, respectively. Blue and red solid lines are CC-2344 and CC-4414 with no chemicals, respectively. Blue and red dashed lines and dotted lines represent CC-2344 and CC-4414 with the indicated concentration of the chemical, respectively. All data are means ± SD of three biological replicates. Significant differences between the control culture and the chemical-added culture within the same condition are indicated by asterisks (*) (p < 0.05).
Over the course of 96 h, cell growth and pigment production were highest at the last time point. Therefore, the pigment production at this time point is summarized in Table 1 and Table 2. At 5 and 10 µM, norflurazon decreased chlorophyll production in CC-2344 by approximately 50 and 70 percent, respectively (Table 1). A greater proportion of chlorophyll b was inhibited compared to chlorophyll a. At 10 µM of norflurazon, approximately 50% of chl a production was inhibited, whereas 70% of chl b accumulation was inhibited. The production of carotenoids decreased at the same rate as total chlorophyll. In contrast, ZnO NPs inhibited around 35% of the initial total chlorophyll and carotenoids production. The chl a level was reduced by 30% while the chl b level was reduced by 40%.

Table 1.
Total chlorophyll, chlorophyll a, chlorophyll b, and carotenoids of CC-2344. All data are means ± SD of three biological replicates. Significant differences between the control and each condition are indicated by asterisks (*) (p < 0.05).

Table 2.
Total chlorophyll, chlorophyll a, chlorophyll b, and carotenoids of CC-4414. All data are means ± SD of three biological replicates. Significant differences between the control and each condition are indicated by asterisks (*) (p < 0.05).
Norflurazon reduced 45% and 55% of chlorophyll production in CC-4414 at concentrations of 5 µM and 10 µM, respectively (Table 2). At 5 µM, chl a was inhibited by 30% and chl b was inhibited by 45%. At 10 µM, chl a was inhibited by 53% and chl b was inhibited by 63%. The production of carotenoid was reduced by 37% and 64% as a result of 5 µM and 10 µM of norflurazon, respectively. The presence of ZnO NPs reduced 25% of total chlorophyll production and 35% of carotenoid production. Chl a was suppressed by 30% and chl b was suppressed by approximately 10%.
4. Discussion
4.1. Effects of Norflurazon
Norflurazon is a pesticide employed for weed control in agricultural settings. Norflurazon can potentially pollute ground and surface water sources through soil leaching. This can be detrimental to aquatic life; hence, water polluted with norflurazon must be decontaminated before being released into the environment. Norflurazon directly inhibits phytoene desaturase, the enzyme that catalyzes the early steps of carotenoid production, resulting in a decrease in carotenoids. Carotenoids are accessory pigments for photosynthesis and antioxidants that scavenge photooxidative stress-causing free radicals. Thus, a decrease in carotenoids might result in chloroplast bleaching in the presence of light, ultimately leading to cell death. Many studies have shown that microalgae also showed similar trends in pigment reduction by norflurazon. For example, the growth of the diatom Phaeodactylum tricornutum was found to be sensitive to 10 µM norflurazon resulting in reduced cell density and chlorophyll production [31]. The treatment of 0.02 µM norflurazon combined with high light reduced carotenoids in Haematoccus pluvialis by half [32]. Water tainted with norflurazon could be utilized to cultivate microalgae for high-value products. However, adequate species and strain selection are required to maximize growth and product yield, as various species and strains of the same species may have varying tolerances to norflurazon.
On plates and in liquid culture, CC-2344 and CC-4414 norflurazon phenotypes differed from one another. On plates, cells are unable to swim away or aggregate to alleviate the impacts of reactive oxygen species (ROS) production that is amplified by light, which may be the survival mechanisms essential for CC-4414. The initial cells of this strain were extremely sensitive to norflurazon in liquid culture. If these first cells perished, the culture would be incapable of proliferating. Even though the initial cells of CC-4414 were highly sensitive to norflurazon, if they were given time to activate cellular responses to protect themselves from the effects of norflurazon, they could exhibit better survival. Therefore, the screening procedure for strains used for cultivation is essential because phenotypes on a plate may not correspond to the same phenotypic variations in liquid culture. In this instance, CC-4414 was more sensitive to norflurazon when grown on plates, but it was more resistant than CC-2344 when grown in liquid culture.
In both strains, a greater percentage of chl b than chl a was inhibited. The reason for this is that chl b is mostly localized in the light harvesting chlorophyll-protein complex (LHCII) [33]. Under circumstances that promoted ROS production, the LHCII levels decreased to reduce the antenna size to lower than the amount of light that would enter the photosystems and minimize ROS production [34]. Comparing the two strains, CC-4414 exhibited superior recovery after norflurazon inhibition. Because PSII is the most sensitive component of photosynthesis, the maximum photosynthetic quantum yield, Fv/Fm, is the most sensitive measurement that can be used to assess algal health. CC-4414 reacts rapidly to the presence of norflurazon, as evidenced by a significant decrease in Fv/Fm. Nonetheless, this strain is better able to recover from stress, demonstrating the capacity to restore PSII function. CC-2344, on the other hand, exhibited a decrease in Fv/Fm, but not as severe as CC-4414, but was unable to recover as well as CC-4414. The remarkable recovery of CC-4414, specifically the Fv/Fm value, suggests that Fv/Fm can be utilized to identify strains that will thrive in different stresses because of its sensitivity to environmental changes. Changes in this value can be used to predict how cells would behave and adapt prior to the emergence of other phenotypes. Nonetheless, the length of cultivation is significant since different time periods provide distinct effects. When initially exposed to norflurazon, CC-4414 demonstrated increased sensitivity. If screening by Fv/Fm at 24 h, strains that would actually be superior for long-term growth would be overlooked, as this strain provided superior growth and pigmentation at 96 h.
CC-4414 demonstrated a stronger physiological reaction to norflurazon than CC-2344, as evidenced by a greater decrease in Fv/Fm and a reduction in chlorophyll and carotenoid levels. The effect was at its peak at 24 h, and all values returned close to initial values at 96 h. However, the contrary was observed with CC-2344, which was not significantly influenced at first but exhibited a stronger inhibition over time. With the addition of norflurazon in the medium, this high sensitivity and dramatic response may be more effective at sending signals to the cells to adjust to this alteration. This may then allow CC-4414 in adjusting its physiology and gene expression in order to recuperate more effectively in this new environment. In this strain, mechanisms that aid in lowering ROS, such as decreasing antenna size, initiating the non-photochemical quenching process, and activating antioxidant systems, may be greatly stimulated. The recovery of Fv/Fm indicated that photosynthesis had resumed its functioning, which implies that PSII had been protected from ROS and photosynthesis had been restored. This allowed cell division to continue despite the presence of norflurazon. The absence of a comparable recovery in CC-2344 suggests that systems that protect PSII or manage ROS may not be as effective. The difference in ROS management between these two strains is currently under investigation.
4.2. Effects of ZnO NPs
There have been inconsistent findings, with some indicating that ZnO NPs can be hazardous to algae and others finding no substantial influence. Studies conducted on Ostreococcus tauri and Nannochloris sp. demonstrated that ZnO NPs had no influence on cell growth and morphology [35]. A study conducted on Chlorella vulgaris revealed that ZnO NPs at concentrations greater than 1 mg L−1 reduced growth and photosynthetic efficiency [36]. In Scenedesmus obliquus, ZnO NPs at concentrations of more than 50 mg mL−1 altered the cell wall structure and harmed the photosynthetic pigments [37]. After 96 h of exposure to ZnO NPs, Spirulina (Arthrospira) platensis cells exhibited decreased cell viability, decreased biomass, and decreased chlorophyll-a, carotenoids, and phycocyanin contents [38].
Our results demonstrated that at a relatively high concentration of 200 mg L−1, ZnO NPs inhibited the growth of Chlamydomonas. Nevertheless, there were no observable effects of ZnO NPs on photosynthesis. There was a small decrease in photosynthetic pigment content. In CC-4414, chl b was suppressed to a considerably lesser extent than chl a, indicating that photosynthesis was still fully functioning and there was no need for cells to reduce the LHCII levels. Therefore, ROS production was not the issue in this case. This observation suggests that the presence of ZnO NPs did not have a direct influence on photosynthesis in this alga. The constant Fv/Fm ratio throughout the experiment also confirmed that PSII was unaffected. Our results are in accordance with a prior finding that ZnO NPs do not increase ROS production in Chlamydomonas even at a 100 mg mL−1 concentration [15]. Nevertheless, responses in terms of ROS generation could be different in different species, because a study reported that ZnO NPs did cause oxidative stress in Tetraselmis sp. but not in Desmodesus subspicatus [39]. Therefore, lower cell density and pigment content in our study were not due to a reduction in photosynthesis. Rather, the decline in growth was caused by the mechanical disturbance of the cell surface, which led to cell death, as it has been demonstrated that the toxicity of ZnO NPs causes cell wall rupture in Haematococcus pluvialis and the destabilization of the cell membrane in Pseudokirchneriella subcapitata [40,41]. CC-4414 is a fast-growing strain. It entered the stationary phase on Day 2 with a significantly higher cell density than CC-2344 (Figure 2a,b). The response between the two strains was already distinct from the beginning. CC-4414 was found to aggregate in the presence of ZnO NPs (Figure 5j). Cell aggregation has been suggested as a protection mechanism to minimize the surface area that comes into contact with ZnO NPs and to reduce the zinc ions released from the NPs [42]. CC-4414 could also potentially be more resistant to cell disruption due to variations in the cell wall and membranes of these two strains.
5. Conclusions
Examining two Chlamydomonas strains, we discovered that their reactions to norflurazon and ZnO NPs are distinct. CC-4414 initially demonstrated a high sensitivity to norflurazon, but it was able to adapt and thrive in its new habitat through a remarkable recovery. Very high concentrations of ZnO NPs reduced cell growth and pigment synthesis in both strains. CC-4414 demonstrated cell aggregation and greater resistance to ZnO NPs, leading to better cell survival. Our findings support the notion that various strains of the same species exhibit distinct behavioral responses. Selecting a strain that is compatible with particular components of the wastewater will help to maximize product yield.
Author Contributions
Conceptualization, T.I. and A.S.; methodology, T.I.; formal analysis, T.I. and A.S.; investigation, T.I. and A.S.; writing—original draft preparation, T.I. and A.S.; writing—review and editing, A.S.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by National Research Council of Thailand (NRCT) and Kasetsart University: N42A650287. The research and innovation activity were funded by the National Research Council of Thailand (NRCT). This research is supported in part by the Faculty of Science, Kasetsart University, International SciKU Branding (ISB) fund, Faculty of Science Kasetsart University, and Kasetsart University Research and Development Institute (KURDI).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, R. Diversity of eukaryotic algae. Biodivers. Conserv. 1992, 1, 267–292. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Rai, A.K. Biodiversity and biogeography of microalgae: Progress and pitfalls. Environ. Rev. 2011, 19, 1–15. [Google Scholar] [CrossRef]
- Jin, E.S.; Melis, A. Microalgal biotechnology: Carotenoid production by the green algaeDunaliella salina. Biotechnol. Bioprocess Eng. 2003, 8, 331–337. [Google Scholar] [CrossRef]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two phase microalgae growth in the open system for enhanced lipid productivity. Renew. Energy 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Barsanti, L.; Coltelli, P.; Evangelista, V.; Frassanito, A.M.; Passarelli, V.; Vesentini, N.; Gualtieri, P. Oddities and curiosities in the algal world. In Algal Toxins: Nature, Occurrence, Effect and Detection; Springer: Berlin/Heidelberg, Germany, 2008; pp. 353–391. [Google Scholar]
- Corcoran, E. Sick Water?: The Central Role of Wastewater Management in Sustainable Development: A Rapid Response Assessment; UNEP/Earthprint: Nairobi, Kenya, 2010. [Google Scholar]
- Boger, P. Carotenoid biosynthesis inhibitor herbicides-mode of action and resistance mechanism. Pestic. Outlook 1998, 9, 29–35. [Google Scholar]
- Park, J.-H.; Tran, L.H.; Jung, S. Perturbations in the photosynthetic pigment status result in photooxidation-induced crosstalk between carotenoid and porphyrin biosynthetic pathways. Front. Plant Sci. 2017, 8, 1992. [Google Scholar] [CrossRef]
- Guardini, Z.; Dall’Osto, L.; Barera, S.; Jaberi, M.; Cazzaniga, S.; Vitulo, N.; Bassi, R. High carotenoid mutants of Chlorella vulgaris show enhanced biomass yield under high irradiance. Plants 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gerken, H.; Huang, J.; Chen, F. Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Process Biochem. 2013, 48, 788–795. [Google Scholar] [CrossRef]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random mutagenesis as a promising tool for microalgal strain improvement towards industrial production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Sirimanoonphan, A.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Submicron and nano formulations of titanium dioxide and zinc oxide stimulate unique cellular toxicological responses in the green microalga Chlamydomonas reinhardtii. J. Hazard. Mater. 2013, 260, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Prach, M.; Stone, V.; Proudfoot, L. Zinc oxide nanoparticles and monocytes: Impact of size, charge and solubility on activation status. Toxicol. Appl. Pharmacol. 2013, 266, 19–26. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Kim, H.; Hong, Y.; Ahn, J.H. A study on the management of micropollutants in water system considering climate change and other potential effects. Korean Chem. Eng. Res. 2013, 51, 645–654. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonas as a model organism. Annu. Rev. Plant Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef] [PubMed]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife 2018, 7, e39233. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Gallaher, S.D.; Fitz-Gibbon, S.T.; Glaesener, A.G.; Pellegrini, M.; Merchant, S.S. Chlamydomonas genome resource for laboratory strains reveals a mosaic of sequence variation, identifies true strain histories, and enables strain-specific studies. Plant Cell 2015, 27, 2335–2352. [Google Scholar] [CrossRef]
- Flowers, J.M.; Hazzouri, K.M.; Pham, G.M.; Rosas, U.; Bahmani, T.; Khraiwesh, B.; Nelson, D.R.; Jijakli, K.; Abdrabu, R.; Harris, E.H. Whole-genome resequencing reveals extensive natural variation in the model green alga Chlamydomonas reinhardtii. Plant Cell 2015, 27, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H. Chlamydomonas Sourcebook; Academic Press: San Diego, CA, USA, 1989; Volume 2. [Google Scholar]
- Pérez-Martín, M.; Pérez-Pérez, M.E.; Lemaire, S.D.; Crespo, J.L. Oxidative stress contributes to autophagy induction in response to endoplasmic reticulum stress in Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Zeier, J.; Marocco, A.; Lamb, C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA 2001, 98, 13454–13459. [Google Scholar] [CrossRef]
- Sirisha, V.; Sinha, M.; D’Souza, J.S. Menadione-induced caspase-dependent programmed cell death in the green chlorophyte C hlamydomonas reinhardtii. J. Phycol. 2014, 50, 587–601. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- MELIS, A.; Spangfort, M.; Andersson, B. Light-absorption and electron-transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochem. Photobiol. 1987, 45, 129–136. [Google Scholar] [CrossRef]
- Yogesh Taparia, A.Z.; Leu, S.; Zarivach, R.; Boussiba, S.; Khozin-Goldberg, I. A novel endogenous selection marker for the diatom Phaeodactylum tricornutumbased on a unique mutation in phytoene desaturase. Sci. Rep. 2019, 9, 8271. [Google Scholar]
- Zhekisheva, M.; Zarka, A.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga haematococcus pluvialis (Chlorophyceae) 1. J. Phycol. 2005, 41, 819–826. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar]
- Genevière, A.-M.; Derelle, E.; Escande, M.-L.; Grimsley, N.; Klopp, C.; Ménager, C.; Michel, A.; Moreau, H. Responses to iron oxide and zinc oxide nanoparticles in echinoderm embryos and microalgae: Uptake, growth, morphology, and transcriptomic analysis. Nanotoxicology 2020, 14, 1342–1361. [Google Scholar] [CrossRef]
- Suman, T.; Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Iswarya, V.; Archanaa, S.; Madhu, G.; Kumar, G.S.; Nagarajan, R.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat. Toxicol. 2015, 162, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cytotoxic effects of zinc oxide nanoparticles on cyanobacterium Spirulina (Arthrospira) platensis. PeerJ 2018, 6, e4682. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.C.O.; dos Santos, L.F.; Vicentini, D.S.; Matias, W.G.; Melegari, S.P. Evaluation of toxicity of zinc oxide nanorods on green microalgae of freshwater and marine ecosystems. Environ. Chem. Ecotoxicol. 2021, 3, 85–90. [Google Scholar] [CrossRef]
- Lee, W.-M.; An, Y.-J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cellular accumulation and cytotoxic effects of zinc oxide nanoparticles in microalga Haematococcus pluvialis. PeerJ 2019, 7, e7582. [Google Scholar] [CrossRef]
- Chen, P.; Powell, B.A.; Mortimer, M.; Ke, P.C. Adaptive interactions between zinc oxide nanoparticles and Chlorella sp. Environ. Sci. Technol. 2012, 46, 12178–12185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).