Cloning, Expression and Characterization of an Alginate Lyase in Bacillus subtilis WB600
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmid and Mediums
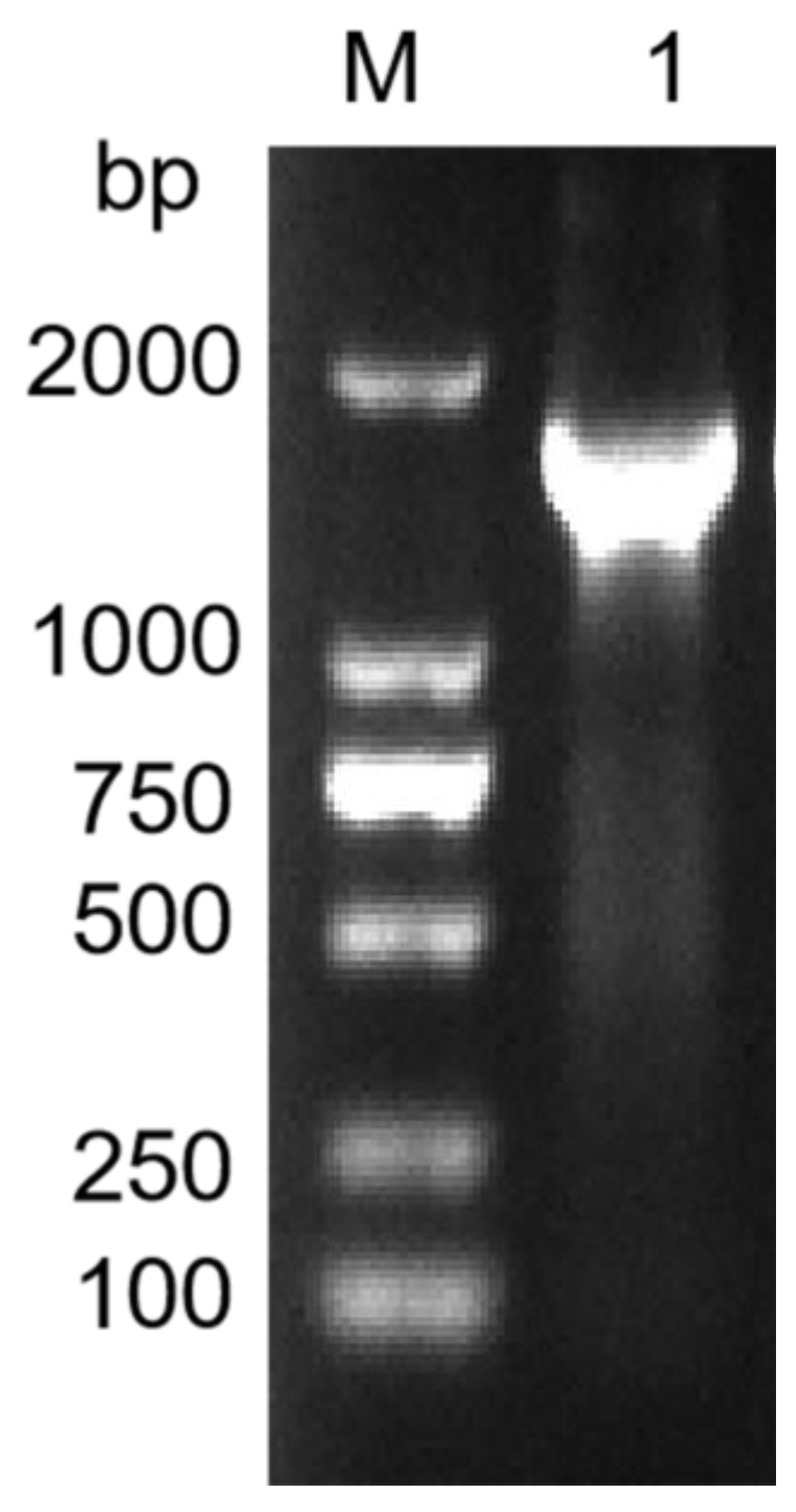
2.2. Cloning and Sequence Analysis of Alginate Lyase Alg62
2.3. Expression and Purification of Alg62
2.4. Enzymatic Activity Assay
2.5. Effect of Temperature on Enzyme Activity
2.6. Effect of pH on Enzyme Activity
2.7. Effects of Metal Ions on Enzyme Activity
2.8. Substrate Specificity
2.9. Optimization of the Fermentation Conditions for the Recombinant Expression of Alg62
3. Results
3.1. Cloning and Sequence Analysis of Alginate Lyase Alg62
3.2. Expression and Purification of Alg62
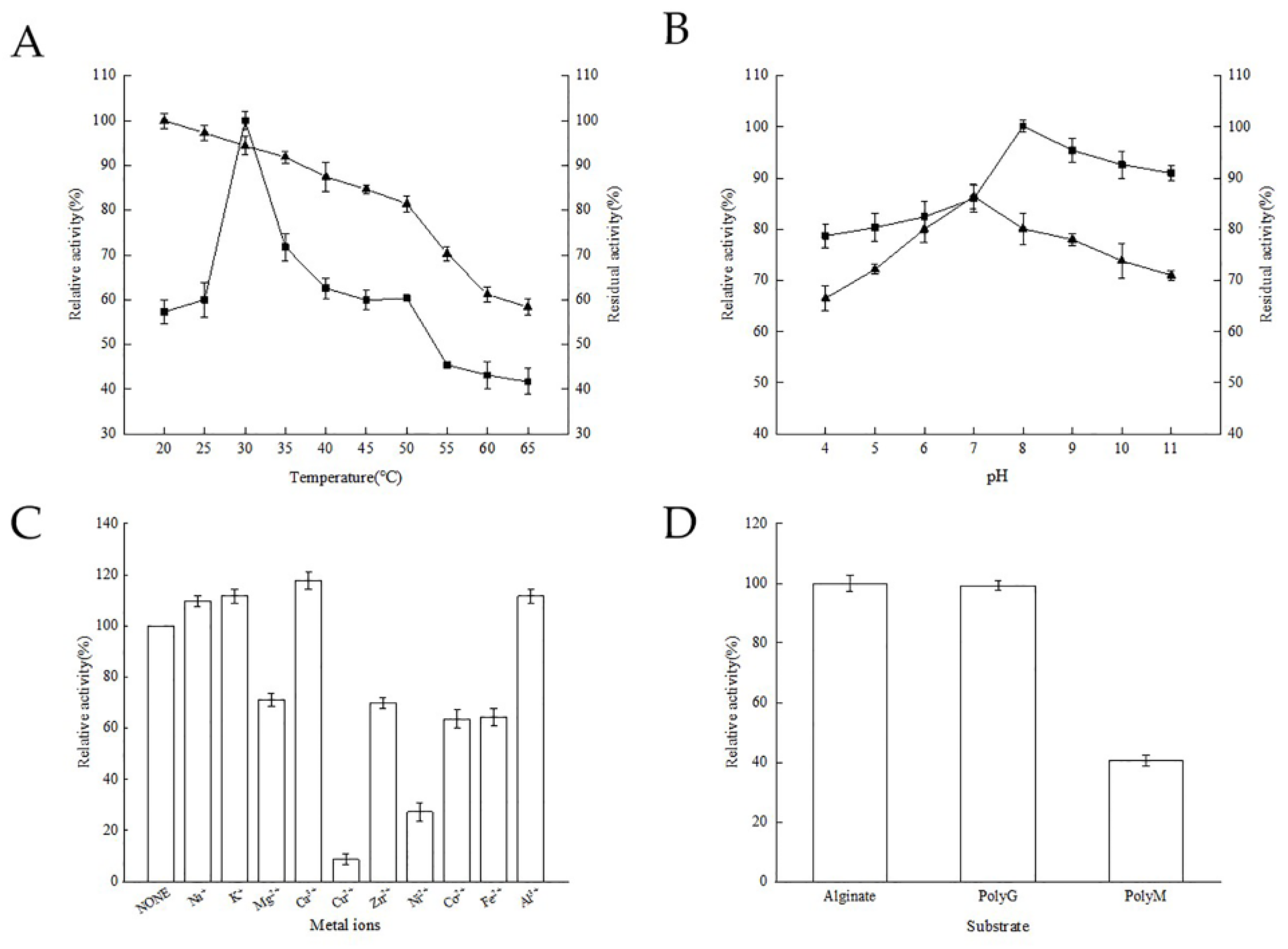
3.3. Effects of Temperature on the Recombinant Alginate Lyase
3.4. Effects of pH on Recombinant Alginate Lyase
3.5. Effects of Metal Ions on the Recombinant Alginate Lyase
3.6. Substrate Specificity
3.7. Optimization of Medium Components for Recombinant Alg62 Expression in B. subtilis WB600
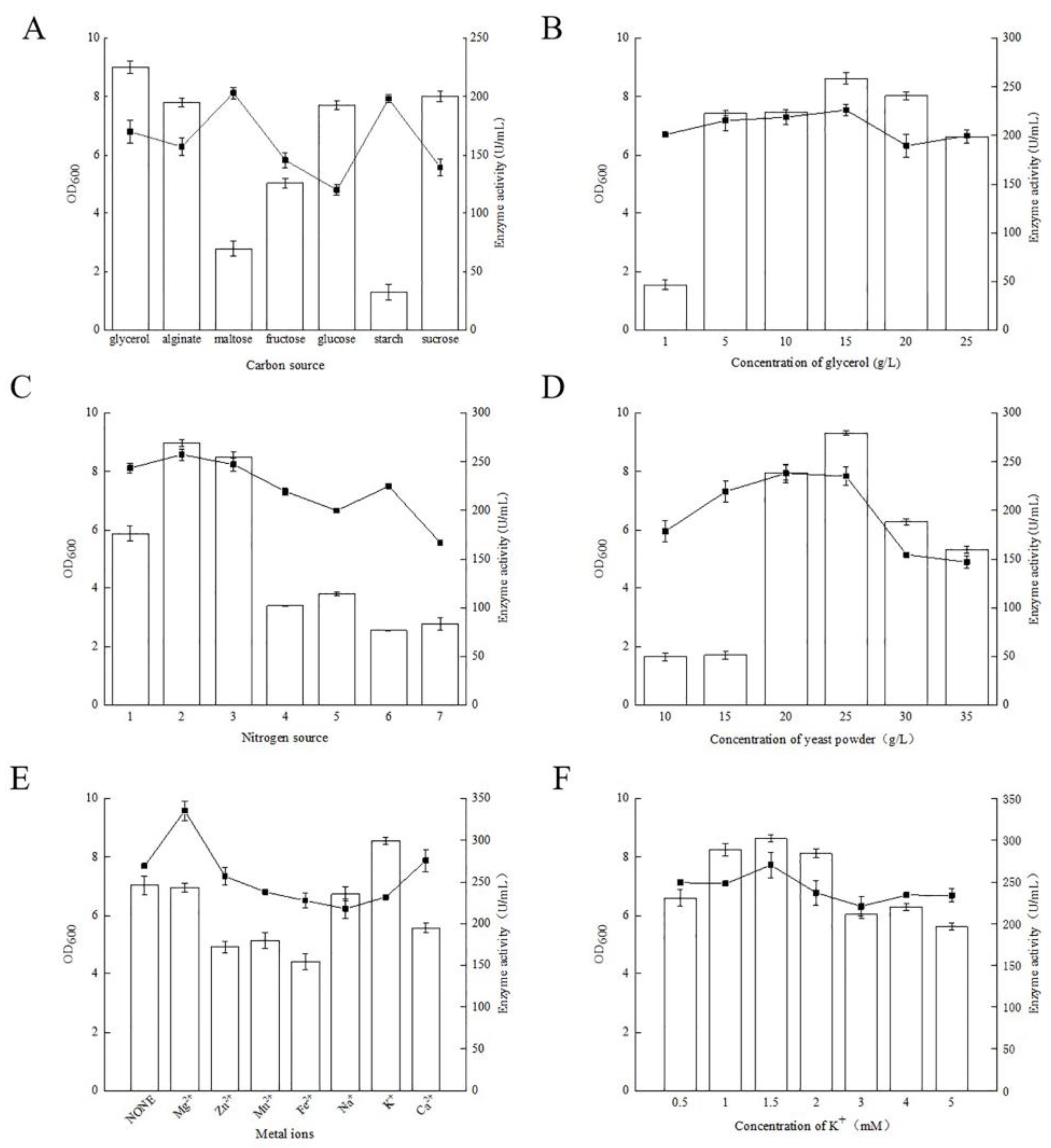
3.7.1. Effects of Carbon Sources
3.7.2. Effect of Nitrogen Sources
3.7.3. Effect of Adding Metal Ions
3.8. Optimization of Fermentation Conditions for Recombinant Alg62 Expression in B. subtilis WB600
3.8.1. Effect of Fermentation Temperature
3.8.2. Effect of Initial pH
3.8.3. Effect of the Inoculum Size
3.8.4. The Effect of Filling Volume
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Serge, M.; Bernard, K. Isolation and Analysis of the Cell Walls of Brown Algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.Q.; Li, X.T.; Yan, Q.J.; Reaney, M.J.T.; Jiang, Z.Q. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bi, D.C.; Li, C.; Fang, W.S.; Zhou, R.; Li, S.M.; Chi, L.L.; Wan, M.; Shen, L.M. Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Mar. Drugs 2015, 13, 1798–1818. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef]
- Chi, F.; Kulkarni, S.; Zulueta, M.; Hung, S. Synthesis of alginate oligosaccharides containing L-guluronic acids. Chem. Asian J. 2010, 4, 386–390. [Google Scholar] [CrossRef]
- Pritchard, M.F.; Powell, L.C.; Jack, A.A.; Powell, K.; Beck, K.; Florance, H.; Forton, J.; Rye, P.D.; Dessen, A.; Hill, K.E.; et al. A Low-Molecular-Weight Alginate Oligosaccharide Disrupts Pseudomonal Microcolony Formation and Enhances Antibiotic Effectiveness. Antimicrob. Agents Chemother. 2017, 61, 14. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yin, H.; Chen, D.W.; Yu, B.; He, J. Ameliorative effects of alginate oligosaccharide on tumour necrosis factor-alpha-induced intestinal epithelial cell injury. Int. Immunopharmacol. 2020, 89, 9. [Google Scholar] [CrossRef]
- Zhang, C.G.; Wang, W.X.; Zhao, X.M.; Wang, H.Y.; Yin, H. Preparation of alginate oligosaccharides and their biological activities in plants: A review. Carbohydr. Res. 2020, 494, 108056. [Google Scholar] [CrossRef]
- Feng, L.P.; Cao, Y.P.; Xu, D.X.; Wang, S.J.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mohdy, H.L. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2017, 10, S431–S438. [Google Scholar] [CrossRef]
- Dou, W.F.; Wei, D.; Li, H.; Li, H.; Rahman, M.M.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Purification and characterisation of a bifunctional alginate lyase from novel Isoptericola halotolerans CGMCC 5336. Carbohydr. Polym. 2013, 98, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, F.; Wang, M.Y.; Zhu, B.W.; Ni, F.; Yao, Z. Elucidation of degradation pattern and immobilization of a novel alginate lyase for preparation of alginate oligosaccharides. Int. J. Biol. Macromol. 2020, 146, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.Y.; Jiang, C.C.; Xu, J.C.; Liu, Z.; Mao, X.Z. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.R.; Srinivasan, R.; Sarath, R.; Ramya, M. Recent progress on engineering microbial alginate lyases towards their versatile role in biotechnological applications. Folia Microbiol. 2020, 65, 937–954. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef]
- Zhu, B.W.; Huang, L.S.X.; Tan, H.D.; Qin, Y.Q.; Du, Y.G.; Yin, H. Characterization of a new endo-type polyM-specific alginate lyase from Pseudomonas sp. Biotechnol. Lett. 2015, 37, 409–415. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, E.W.; Yu, W.G.; Han, F. Purification and characterization of a new alginate lyase from a marine bacterium Vibrio sp. Biotechnol. Lett. 2013, 35, 703–708. [Google Scholar] [CrossRef]
- Li, H.F.; Wang, S.L.; Zhang, Y.Y.; Chen, L.H. High-Level Expression of a Thermally Stable Alginate Lyase Using Pichia pastoris, Characterization and Application in Producing Brown Alginate Oligosaccharide. Mar. Drugs 2018, 16, 158. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shao, Y.; Jiao, C.; Yang, Q.M.; Weng, H.F.; Xiao, A.F. Characterization and Application of an Alginate Lyase, Aly1281 from Marine Bacterium Pseudoalteromonas carrageenovora ASY5. Mar. Drugs 2020, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Li, K.K.; Wang, W.X.; Ning, L.M.; Tan, H.D.; Zhao, X.M.; Yin, H. Preparation of trisaccharides from alginate by a novel alginate lyase Alg7A from marine bacterium Vibrio sp. W13. Int. J. Biol. Macromol. 2019, 139, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; Lluch-Senar, M. Characterization of different alginate lyases for dissolving Pseudomonas aeruginosa biofilms. Sci. Rep. 2020, 10, 9390. [Google Scholar] [CrossRef]
- Atalah, J.; Caceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef]
- Kawai, S.; Ohashi, K.; Yoshida, S.; Fujii, M.; Mikami, S.; Sato, N.; Murata, K. Bacterial pyruvate production from alginate, a promising carbon source from marine brown macroalgae. J. Biosci. Bioeng. 2014, 117, 269–274. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Z.L.; Li, S.Y.; Su, H.; Tang, L.Y.; Tan, Y.L.; Yu, W.G.; Han, F. Characterization of a new endo-type polysaccharide lyase (PL) family 6 alginate lyase with cold-adapted and metal ions-resisted property. Int. J. Biol. Macromol. 2018, 120, 729–735. [Google Scholar] [CrossRef]
- Kumar, C.S.; Hazarika, N.M.J.; Kumar, S. Analysis of synonymous codon usage in the VP2 protein gene of infectious bursal disease virus. Arch. Virol. 2015, 160, 2359–2366. [Google Scholar] [CrossRef]
- Cui, W.J.; Han, L.C.; Suo, F.Y.; Liu, Z.M.; Zhou, L.; Zhou, Z.M. Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J. Microbiol. Biotechnol. 2018, 34, 19. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wang, Z.P.; Cao, M.; Li, B.; Ji, X.F.; Zhang, X.Y.; Zhang, Y.Q.; Wang, H.Y. Cloning, Secretory Expression and Characterization of a Unique pH-Stable and Cold-Adapted Alginate Lyase. Mar. Drugs 2020, 18, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.C.; Fu, Z.; Tang, L.Y.; Zhang, Z.L.; Han, F.; Yu, W.G. Biochemical Characterization of a Novel Exo-Type PL7 Alginate Lyase VsAly7D from Marine Vibrio sp. QY108. Int. J. Mol. Sci. 2021, 22, 8402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, F.; Ning, L.; Sun, Y.; Yao, Z. Cloning and characterization of a new pH-stable alginate lyase with high salt tolerance from marine Vibrio sp. NJ-04. Int. J. Biol. Macromol. 2018, 115, 1063–1070. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, K.; Liu, X.; Liu, W.; Lyu, Q.; Ji, A. Characterization of a Novel PolyM-Preferred Alginate Lyase from Marine Vibrio splendidus OU02. Mar. Drugs 2018, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cui, D.D.; Ma, S.; Chen, W.K.; Chen, D.W.; Shen, H. Characterization of a novel PL 17 family alginate lyase with exolytic and endolytic cleavage activity frommarine bacterium Microbulbifer sp. SH-1. Int. J. Biol. Macromol. 2021, 169, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Huang, M.; Huang, X.; Huang, Y.; Shao, E.; Guan, X.; Huang, Z. Cloning and Characterization of a Novel Endo-Type Metal-Independent Alginate Lyase from the Marine Bacteria Vibrio sp. Ni1. Mar. Drugs 2022, 20, 479. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Wu, L.Y.; Chen, Y.H.; Ni, H.; Xiao, A.F.; Cai, H.N. Characterization of an extracellular biofunctional alginate lyase from marine Microbulbifer sp ALW1 and antioxidant activity of enzymatic hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef]
- Yang, J.; Cui, D.; Chen, D.; Chen, W.; Ma, S.; Shen, H. Purification and Characterization of a Novel Endolytic Alginate Lyase from Microbulbifer sp. SH-1 and Its Agricultural Application. Mar. Drugs 2020, 18, 184. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Q.; Lu, M.; Xu, C.; Li, F.; Zhang, R.; Liao, W.; Huang, S. AlgM4: A New Salt-Activated Alginate Lyase of the PL7 Family with Endolytic Activity. Mar. Drugs 2018, 16, 120. [Google Scholar] [CrossRef]
- Xu, F.; Dong, F.; Wang, P.; Cao, H.Y.; Li, C.Y.; Li, P.Y.; Pang, X.H.; Zhang, Y.Z.; Chen, X.L. Novel Molecular Insights into the Catalytic Mechanism of Marine Bacterial Alginate Lyase AlyGC from Polysaccharide Lyase Family 6. J. Biol. Chem. 2017, 292, 4457–4468. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Guo, Y.X.; Wang, X.X.; Li, H.B.; Ni, H.; Li, L.J.; Xiao, A.F.; Zhu, Y.B. Molecular cloning and characterization of AlgL17, a new exo-oligoalginate lyase from Microbulbifer sp. ALW1. Protein Expr. Purif. 2019, 161, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska-Powalowska, D.; Leja, K. An increasing of the efficiency of microbiological synthesis of 1,3-propanediol from crude glycerol by the concentration of biomass. Electron. J. Biotechnol. 2014, 17, 72–78. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Deit, H.L.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Bibi, R.; Ahmad, Z.; Imran, M.; Hussain, S.; Ditta, A.; Mahmood, S.; Khalid, A. Algal bioethanol production technology: A trend towards sustainable development. Renew. Sust. Energ. Rev. 2017, 71, 976–985. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, T.; Huang, X.; He, Y.L.; Zhou, Y.Z.; Wu, L.Y.; Wu, K.W.; Fan, M.; Zhu, L.L. Dissolved oxygen concentration in the medium during cell culture: Defects and improvements. Cell Biol. Int. 2016, 40, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Badur, A.H.; Jagtap, S.S.; Yalamanchili, G.; Lee, J.K.; Zhao, H.; Rao, C.V. Alginate Lyases from Alginate-Degrading Vibrio splendidus 12B01 Are Endolytic. Appl. Environ. Microbiol. 2015, 81, 1856–1864. [Google Scholar] [CrossRef]


| Enzyme | Origin | Molecular Weight (kDa) | Optimum pH/Temperature (°C) | Substrate Specificity | Activating Cation | Reference |
|---|---|---|---|---|---|---|
| AlyA-OU02 | Vibrio sp. OU02 | 65 | 8.0/30 | polyM | Fe2+, Mg2+ | [34] |
| TsAly6A | Thalassomonas sp. LD5 | 84 | 8.0/35 | Alginate, polyG | Ca2+, Mg2+ | [27] |
| AlgB | Vibrio sp. Ni1 | 67 | 8.0/35 | polyM, Alginate | K+ | [36] |
| AlgNJ04 | Vibrio sp. NJ-04 | 50 | 7.0/40 | polyG | Ca2+, K+, Na+ | [33] |
| ALW1 | Microbulbifer sp. ALW1 | 26 | 7.0/45 | Alginate, polyG | Na+ | [37] |
| AlgSH7 | Microbulbifer sp. SH-1 | 66.4 | 9.0/40 | polyM | Na+, K+, Al3+, Fe3+ | [38] |
| AlgSH17 | Microbulbifer sp. SH-1 | 82.54 | 7.0/30 | polyM | Mg2+, Fe2+, Mn2+ | [35] |
| Alyw201 | Vibrio sp. W2 | 38 | 8.0/30 | / | Mn2+, Co2+ | [31] |
| AlgM4 | Vibrio sp. M0101 | 55 | 8.5/30 | Alginate, polyG, polyM | Mg2+, Na+ | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Zhu, Y.; An, Z.; Lin, J.; Shan, S.; Zhang, H. Cloning, Expression and Characterization of an Alginate Lyase in Bacillus subtilis WB600. Fermentation 2023, 9, 144. https://doi.org/10.3390/fermentation9020144
Zheng K, Zhu Y, An Z, Lin J, Shan S, Zhang H. Cloning, Expression and Characterization of an Alginate Lyase in Bacillus subtilis WB600. Fermentation. 2023; 9(2):144. https://doi.org/10.3390/fermentation9020144
Chicago/Turabian StyleZheng, Kaixuan, Yaqing Zhu, Zhiqiang An, Jian Lin, Shoushui Shan, and Hailing Zhang. 2023. "Cloning, Expression and Characterization of an Alginate Lyase in Bacillus subtilis WB600" Fermentation 9, no. 2: 144. https://doi.org/10.3390/fermentation9020144
APA StyleZheng, K., Zhu, Y., An, Z., Lin, J., Shan, S., & Zhang, H. (2023). Cloning, Expression and Characterization of an Alginate Lyase in Bacillus subtilis WB600. Fermentation, 9(2), 144. https://doi.org/10.3390/fermentation9020144




