Abstract
This study evaluated the effects of selenium yeast (SY) on rumen fermentation parameters, rumen bacterial diversity, and expression pathways in goats. A total of 18 Qianbei-pockmarked weather goats from Guizhou (body weight, 25.75 ± 1.75 kg; mean ± standard deviation) were assigned to three groups according to a completely randomized design. Control group (CON, n = 6) kids were fed a basal diet, while treatment 1 (LS, n = 6) and treatment 2 (HS, n = 6) kids were fed a basal diet with 2.4 and 4.8 mg/kg SY, respectively. The feeding trial lasted for 74 days. The results indicated that the ruminal fluid of LS goats had significantly higher levels of propionic, caproic, isobutyric, and isovaleric acids than that of the CON. The levels of butyric and valeric acids were higher in the HS group than in the CON. The acetate:propionate ratio was significantly higher in the CON than in the two treatments. In addition, the inclusion of 2.4 mg/kg SY can lead to a significant decrease in the relative abundances of Euryarchaeota, and Proteobacteria at the phylum level compared to the CON and the HS groups. At the genus level, the LS group had a significant decrease in the relative abundance of Methanobrevibacter and Sarcina, whereas it could lead to a significant increase in the relative abundance of Clostridium in the ruminal fluid relative of the other two groups. At the species level, the LS group had a significant decrease in the relative abundance of bacterium_P3, bacterium_P201, and Sarcina_sp._DSM_11001 compared to the other groups. Moreover, the CON group had a significant decrease in the relative abundance of bacterium_P201 compared to the other two treatments. Compared to the CON, the addition of 2.4 mg/kg SY significantly enriched carbohydrate metabolism pathways in the ruminal fluid for gene encoding. Additionally, goats receiving SY showed a significant upregulation of glycosyl transferase and carbohydrate binding module pathways. These results suggest that dietary supplementation with SY modulates fermentation parameters, and it affects microbial diversity and microbial metagenome in the rumen of Qianbei-pockmarked goats.
1. Introduction
The rumen is a complex microecosystem where bacteria, protozoa, and anaerobic fungi are the three major microorganism groups. There is a dynamic balance between rumen microorganisms and hosts [1], and rumen microorganisms play essential roles in feed fermentation and methane production, ultimately influencing the production, health, and welfare of ruminants [2]. However, many factors influence the rumen microbial community structure, including breed, lactation, and diet [3]. Thus, it is extremely important to identify a suitable method to improve rumen bacterial digestion and metabolism in ruminants.
Oxidative stress is the most important reason for cell damage, leading to the occurrence and the development of many diseases, reducing the production performance of ruminants [4]. Antioxidants play important roles in immunity function and growth, among many other processes [5,6], and their addition to feed can improve the antioxidant performance of ruminants [7]. Selenium (Se) is an antioxidant element that can enhance the immune system of ruminants. Se deficiency can result in oxidative stress status, downregulation of selenoproteins, and a cell stress response in ruminants [8]. Specifically, it is a component of selenoenzyme glutathione peroxidase (GSH-Px), which metabolizes hydrogen peroxide and lipid peroxide, thus neutralizing free radicals and promoting immunity [9]. Hence, dietary supplementation with Se could affect ruminal fluid microbial function through its antioxidative effects and the modulation of the immune system.
The diet of ruminants is usually of plant origin, and the Se concentration in plants can be extremely variable. Therefore, adding Se to the ruminant diet may be required [10]. There are two forms of Se supplements: inorganic mineral salts, such as sodium selenite or selenate, and organic forms such as selenium yeast (SY) [11]. Inorganic Se has a lower ruminal microbial uptake than organic Se sources because rumen microorganisms can reduce most dietary inorganic Se to unabsorbable elements or inorganic selenides [12,13]. Notably, SY is a source of organic Se, which has many advantages, as it can be absorbed and retained more readily than inorganic Se [14]. Dietary supplementation of 0.4 mg/kg SY improves antioxidant activity in ruminal fluid, which can promote ciliate development in the rumen of young ruminants [15]. Pino and Heinrichs [16] showed that SY affects bacteria in the ruminal fluid of dairy heifers. Cui et al. [17] reported the same in Tibetan sheep; in addition, SY supplementation ultimately affected microbial fermentation and led to the overexpression of genes and metabolic pathways associated with carbohydrates and amino acids in the rumen microbiota.
Generally, ruminants have a strong tolerance of Se, and signs of Se toxicity usually start to appear at levels of 5 to 8 mg/kg dry matter (DM) [18]. In preliminary studies, we found that the feeding of antioxidants enhanced antioxidant potential in Qianbei-pockmarked goats [19], and that SY improved meat quality, muscle antioxidant activity, and fatty acid and amino acid profiles [20]. However, there is little information on the effects of Se on rumen bacterial diversity in goats. In this study, we hypothesized that dietary supplementation with SY would influence the structure and the function of ruminal bacterial communities. We evaluated the effects of SY on rumen fermentation, rumen bacterial diversity, and expression pathways in Qianbei-pockmarked goats using the Kyoto Encyclopedia of Genes and Genomes [KEGG] and Carbohydrate-Active Enzymes [CAZyme] databases.
2. Materials and Methods
2.1. Selenium Yeast
SY was purchased from a commercial company (Jiangsu Qianbo Bioengineering Co., Ltd., Jiangsu, China). Its composition was as follows: Se concentration 2000 mg/kg, heavy metal content ≤ 3 ppm, moisture ≤ 6.0%, crude protein ≥ 40.0%, Pb ≤ 2.0 mg/kg, As ≤ 1.0 mg/kg, total bacterial amount ≤30,000 cfu/g, coliform group ≤ 90 MPN/100 g, and mold ≤ 25 cfu/g. The food additive production license number was SC20133062401397; it was a yellow powder with uniform fineness.
2.2. Animals, Diets, and Experimental Design
Animal feeding and management is described in Tian et al. [20]. Briefly, a feeding trial was performed at a stud farm in Guizhou Province (Xishui, China). A total of 18 Qianbei-pockmarked weather goats (body weight, 25.75 ± 1.75 kg; mean ± standard deviation) were assigned to three groups according to a completely randomized design. The control group (CON, n = 6) kids were fed a basal diet, while the treatment 1 (LS, n = 6) and treatment 2 group (HS, n = 6) kids were fed a basal diet with 2.4 mg/kg SY (account for 0.12% of basal diet) and 4.8 mg/kg SY (account for 0.24% of basal diet), respectively. The content of Se of the basal diet was 0.0062% DM. The feeding trial lasted for 74 days, consisting of a 14-day preparation period and a 60-day formal experimental period. The basal diet was a total mixed ration (TMR); SY was mixed in the concentrate and then mixed with the roughage evenly to prepare TMR. The nutrient requirements of the kids were determined according to the National Research Council (NRC; Table 1) [21]. Kids were fed twice a day (08:30 and 16:30) with free access to water. Power calculation had identified a required sample size of six goats per group, and it resulted in a power greater than 0.80 and a significance level of 0.05. Each animal was an experimental unit.

Table 1.
Ingredients and nutrient composition of basal diets (DM 1 basis).
2.3. Chemical Composition
An approximately 100 g base diet was collected every week and kept at −20 °C, and all samples were pooled together at the end of the feeding trial. Next, 500 g samples of mixture were collected and dried at 65 °C in a vacuum oven for 72 h prior to grinding. The dried samples were ground, run through a 1 mm sieve, and stored at 4 °C until the assay was performed. DM, crude protein (CP), ether extract (EE), and ash were measured according to the Association of Official Analytical Chemists [22]. The metabolic energy (ME) value was calculated based on the database of the NRC [23], and neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined as per the method of Van Soest et al. [24].
2.4. Rumen Fermentation Parameters
This research was part of a larger project, and the objective of this study was to observe the effects of selenium on rumen fermentation parameters and microbial metagenome in goats. At the end of the feeding trial, all kids were slaughtered and ruminal fluids were collected immediately, and divided into two portions. One aliquot (about 10 mL) of homogenized ruminal fluid was collected in triplicate and kept in dry ice and transferred to a –80 °C refrigerator until analysis of the microbial DNA. The other aliquot was screened through a four-layer cheesecloth, and the pH value was determined immediately using a portable pH meter (pH 818, Guangdong, China). Then, a 20 mL sample and 4 mL 25% (w/v) of metaphosphoric acid were mixed, placed in a container, and stored at −80 °C until further analysis to determine the levels of ammonia nitrogen (NH3-N) and volatile fatty acids (VFAs).
The NH3-N was assayed using the steam distillation method according to Bremner and Keeney [25]. The concentrations of individual VFAs were detected on a Thermo TRACE 1310-ISQ gas chromatography-mass spectrometer (GC-MS; Thermo Fisher Scientific, Waltham, MA, USA) according to Tian et al. [26]. Briefly, the GC parameters were as follows: Agilent HP-INNOWAX capillary column, 30 m × 0.25 mm × 0.25 µm; 1 µL injection volume; 250 °C injection port temperature; 230 °C ionization temperature; 250 °C transmission line temperature; quadrupole temperature 150 °C; and carrier gas He. The MS parameters were as follows: 70 eV ionization and single-ion scanning mode. The total ion current chromatograms of individual VFAs of ruminal fluid are displayed in Supplementary Figure S1. The linear regression equation of standard is shown in Supplementary Table S1. Individual VFAs can be distinguished and the peak time of the internal standard (isocaproic acid) was obviously separated from other VFAs; moreover, all correlation coefficients of the linear regression equation were greater than 0.99. The experimental results indicate that the assay exhibited excellent accuracy and precision.
2.5. Shotgun Metagenome
Shotgun metagenome data were analyzed using the online platform Majorbio Cloud (www.majorbio.com, accessed on 9 June 2021).
Total genomic DNA was extracted from ruminal fluid samples using the FastDNA Spin Kit for Soil ((MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. The concentration and the purity of extracted DNA were determined on a TBS-380 and NanoDrop2000, respectively. The quality of DNA was checked on a 1% agarose gel. DNA extracts were fragmented to an average size of about 400 bp using the Covaris M220 (Gene Company Limited, China) device for paired-end library construction. The library was constructed by NEXTflexTM Rapid DNA-Seq (Bioo Scientific, Austin, TX, USA). Adapters containing the full complement of primer hybridization sites were ligated to the blunt-ends of fragments. Paired-end sequencing was performed on an Illumina Novaseq 6000 (Illumina Inc., San Diego, CA, USA) at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) using NovaSeq reagent kits according to the manufacturer’s instructions.
Raw reads were used to generate clean reads by removing adaptor sequences and low-quality reads (those with N bases, a minimum length threshold of 50 bp, and a minimum quality threshold of 20 using fastp) and trimming on the free online platform of Majorbio Cloud (cloud.majorbio.com, accessed on 9 June 2021). The clean reads were mapped to the human ARS1 reference genome using BWA to identify and the remove reads originating from the goat host. The remaining high-quality reads were assembled to contigs using MEGAHIT (parameters: kmer_min = 47, kmer_max = 97, step = 10), which makes use of succinct de Bruijn graphs. Contigs with lengths ≥ 300 bp were selected as final assembling results.
Open reading frames (ORFs) in contigs were identified using MetaGene. Predicted ORFs ≥ 100 bp were retrieved and translated into amino acid sequences using the National Center for Biotechnology Information (NCBI) translation table. A nonredundant gene catalog was constructed using CD-HIT with a 90% sequence identity and 90% coverage. Reads after quality control were mapped to the nonredundant gene catalog with 95% identity using SOAPaligner, and the gene abundance in each sample was evaluated. Representative sequences from the nonredundant gene catalog were annotated based on the NCBI NR database using blastp as implemented in DIAMOND v0.9.19 with an e-value cutoff of 1 × 10−5 using Diamond for taxonomic annotations. KEGG annotation was conducted using Diamond against the KEGG database with an e-value cutoff of 1 × 10−5. CAZyme annotation was conducted using hmmscan against the CAZy database with an e-value cutoff of 1 × 10−5.
2.6. Statistical Analysis
The effects of SY on rumen fermentation parameters were analyzed using SAS 9.1.3 (SAS Institute, Cary, NC, USA), using the one-way analysis of variance method. The least squares means were reported by a Tukey’s test. The ruminal fluid microbiota were analyzed using the Wilcoxon rank-sum test. The ruminal fluid of the abundances of microbial metabolic pathways, modules, KEGG enzymes, and CAZyme were compared among the three groups by a multilevel discriminant analysis (LEfSe), with significant differences considered by a linear discriminant analysis (LDA) score > 2 and p-value < 0.05. All p-values for significance (non-significance) are p < 0.05 (p > 0.05) unless otherwise noted.
3. Results
3.1. Rumen Fermentation Parameters
There were no significant (p > 0.05) differences in the ruminal fluid pH, NH3-N, total VFA, or acetic acid among the three groups (Table 2). However, the levels of propionic, isobutyric, and isovaleric acids were higher (p < 0.05) in LS goats than in the control and the HS groups. Levels of butyric and valeric acids were higher (p < 0.05) in the HS group than in the control and the LS groups. In contrast, the acetate:propionate ratio was significantly higher (p < 0.05) in the control than in the two treatment groups.

Table 2.
Effect of selenium yeast on ruminal fluid fermentation parameters of goats 1.
3.2. Macrogenome Sequencing Results
Metagenomic data were collected from 18 DNA samples of ruminal digesta (six from each group) and 132 gigabyte (GB) raw base was obtained (Supplementary Table S2). Metagenome sequencing generated 938,937,292 raw reads, with an average of 52,163,183 ± 1,706,771 raw reads (mean ± standard error of the mean) per sample. In all, we obtained 245,971,362 sequences for the rumen metagenome, broken down as follows: 96.72% bacteria, 1.76% archaea, 1.33% eukaryotes, and 0.19% others (Supplementary Figure S2). At the domain level, the differences in the relative abundances of bacteria and archaea were statistically significant among the three groups (Figure 1). As a result, the effect of SY on the ruminal fluid microbial taxa mainly involved bacteria and archaea. In addition, bacteria were the main types of rumen microorganisms.
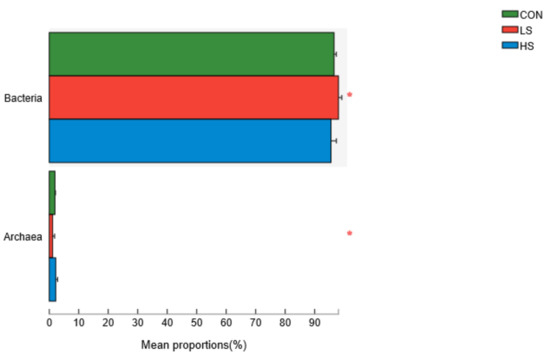
Figure 1.
Differently rumen microbial domains among the three groups. CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY. * p < 0.05.
3.3. Relative Abundances of Rumen Microorganisms
The dominant bacterial phyla included Bacteroidetes (45.06 ± 1.60%), Firmicutes (34.25 ± 2.07%), unclassified_d__Bacteria (10.05 ± 0.33%), and Fibrobacteres (2.14 ± 0.62%); and, other phyla included Euryarchaeota, Actinobacteria, Proteobacteria, Spirochaetes, Ciliophora, and Tenericutes (Supplementary Figure S3). The differences in the relative abundances of unclassified_d__Bacteria, Euryarchaeota, and Proteobacteria were statistically significant (Figure 2) and they decreased in the LS group relative to the other two groups at the phylum level.
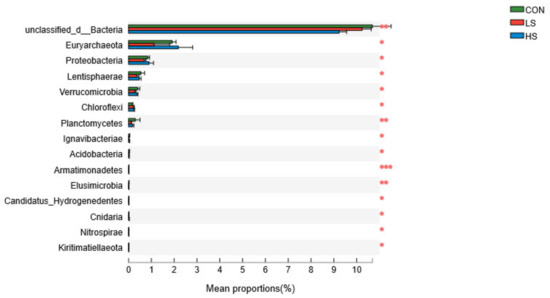
Figure 2.
Effect of selenium yeast on rumen dominant bacteria at phylum level (>1%; as a percentage of the total sequence). CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY. * p < 0.05, ** p < 0.01, *** p < 0.001.
The dominant bacterial genus was Prevotella (18.31 ± 1.41%; Supplementary Figure S4), followed by unclassified_o__Clostridiales (10.14 ± 0.97%), unclassified_d__Bacteria (10.05 ± 0.33%), unclassified_o__Bacteroidales (8.44 ± 0.57%), Bacteroides (4.21 ± 0.20%), unclassified_f__Rikenellaceae (3.59 ± 0.27%), and unclassified_f__Lachnospiraceae (3.52 ± 0.23%). Supplementation with 2.4 mg/kg SY had a significant (Figure 3) decrease in the relative abundances of Methanobrevibacter and Sarcina, whereas it could lead to a significant increase in the relative abundance of Clostridium in the ruminal fluid relative to the other two groups at the genus level.
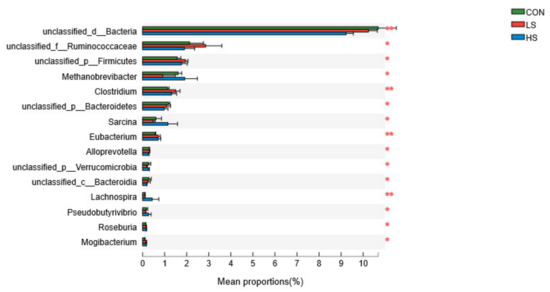
Figure 3.
Effect of selenium yeast on rumen dominant bacteria at genus level (>1%; as a percentage of the total sequence). CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY. * p < 0.05, ** p < 0.01.
The dominant bacterial species was Clostridiales_bacterium (9.36 ± 0.93%; Supplementary Figure S5), Bacteroidales_bacterium (4.56 ± 0.25%), Rikenellaceae_bacterium (3.59 ± 0.27%), bacterium_F082 (3.43 ± 0.21%), and bacterium_P3 (2.02 ± 0.31%). At the species level, goats receiving 2.4 mg/kg SY showed significantly (p < 0.05; Figure 4) higher relative abundances of bacterium_P3, bacterium_P201, and Sarcina_sp._DSM_11001 compared to the other groups. Differently, the inclusion of SY had a significant (p < 0.05) increase on the relative abundance of bacterium_P201 compared to the CON group.
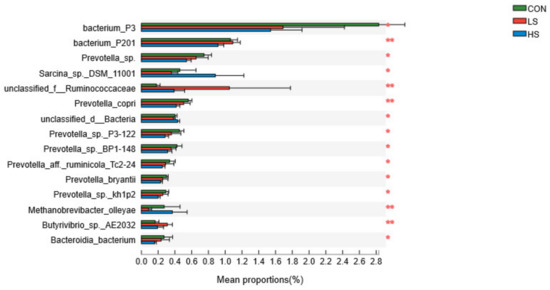
Figure 4.
Effect of selenium yeast on rumen dominant bacteria at species level (>1%; as a percentage of the total sequence). CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY. * p < 0.05, ** p < 0.01.
3.4. Relative Abundances of KEGG Pathways
Regarding KEGG profiles, 428 endogenous third-level pathways were considered rumen microbial metabolic pathways (Supplementary Table S3). These pathways belonged to six first-level categories, including metabolism (75.22%), genetic information processing (8.51%), environment information processing (4.74%), cellular processes (4.16%), human diseases (3.92%), and organismal systems (3.46%). At the second level, 46 categories were observed, with global and overview maps (37.98%), carbohydrate metabolism (10.88%), amino acid metabolism (6.60%), replication and repair (4.25%), energy metabolism (3.68%), and metabolism of cofactors and vitamins (3.31%) being the most abundant.
When the identified KEGG pathways were compared, a total of seven third-level pathways, including other glycan degradation, sphingolipid metabolism, galactose metabolism, fatty acid biosynthesis, fatty acid metabolism, phenylpropanoid biosynthesis, and folate biosynthesis, were significantly enriched in the rumen microbiomes of control (Figure 5). Additionally, 18 pathways, including 12 metabolism pathways, 5 genetic information processing pathways, and 1 cellular processes pathway were significantly enriched in LS kids. Moreover, one human diseases pathway and one metabolism pathway were significantly enriched in the rumen of HS kids (LDA > 2).
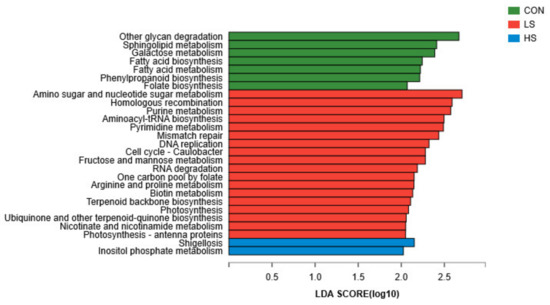
Figure 5.
Effect of selenium yeast on the differential kyoto encyclopedia of genes and genomes functions of significantly enriched metabolic pathways. CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY.
3.5. Relative Abundance of CAZy Enzymes
Regarding CAZyme profiles, a total of 511 genes encoding CAZymes were identified (Supplementary Table S4), including 19 auxiliary activities (AAs), 63 carbohydrate-binding modules (CBMs), 16 carbohydrate esterases (CEs), 258 glycoside hydrolases (GHs), 81 glycosyltransferases (GTs), and 75 polysaccharide lyases (PLs). Among them, genes encoding GH2 (6.36%) were the most dominant, followed by those encoding GT2_Glycos_transf_2 (5.61%), CE1 (4.49%), CE10 (3.71%), GT4 (3.39%), and GT41 (3.05%). Furthermore, among the genes encoding CAZymes involved in deconstructing carbohydrates, 18 were significantly enriched (Figure 6) in the rumen of control (13 GH, 2 PL, and 3 GT), 11 were enriched in LS goats (5 GT, 4 GH, 1 CE, and 1 PL), and 13 were enriched in HS goats (2 GT, 6 GH, 1 CE, and 4 CBM).
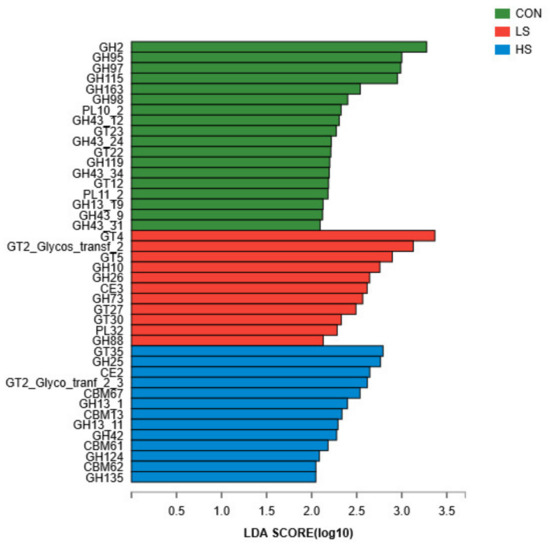
Figure 6.
Linear discriminant analysis value distribution of difference function for the carbohydrate-active enzymes among the three groups. CON = basal diet; LS = basal diet + 2.4 mg/kg SY; HS = basal diet + 4.8 mg/kg SY.
4. Discussion
VFA levels are one of the most important fermentation indexes in ruminants. Indeed, the absorption of carbohydrates by ruminants is mainly in the form of VFAs, which account for approximately 70% of the total energy absorbed by ruminants [27]. Thus, VFAs represent an energy source for different bacterial species in the rumen, and they regulate various signaling mechanisms that regulate immunity and growth. Xun et al. [28] reported that feeding 4 mg/kg SY to sheep increased total VFA levels in ruminal fluid. This was not the case in our study, possibly because the SY did not increase the relative abundances of the microorganisms that mainly degrade carbohydrates, which include Ruminococcus albus, Ruminococcus flflavefaciens, Fibrobacter succinogenes, and Butyrivibrio fifibrisolvens.
Endogenous glucose is the main source of glucose for goats, and propionic acid is the main precursor for endogenous gluconeogenesis in ruminants [29]. Poor digestibility and deficient fermentation lead to environmental pollution and less meat production [30]. In addition, more propionate is required to provide energy for growing ruminants. In this study, the feeding of SY increased the proportion of propionate and decreased the ratio of acetate to propionate of ruminal fluid, showing that SY supplementation improved propionate production. This is because SY increases the bacterial intake of Se, and bacterial digestion later in the digestive tract increases the ruminants’ supply of Se, which shows antioxidant and anti-inflammatory activities [31]. A high content of propionic acid in the rumen may lead to less methane production, indicating increased energy utilization. Adding Se to the ruminant diet may impact the molar proportion of propionate by decreasing the total methanogen population [31]. Pan et al. [32] reported that sheep given Se showed increased energy utilization because methane output as a proportion of gross energy, digestive energy, and ME intake decreased compared to control. These results suggest that the inclusion of SY in the goat diet may improve feed energy and inhibit methane in the form of VFAs during microbial fermentation. Our results are consistent with Wang et al. [33], who found that feeding SY increased total VFA production in ruminal fluid, while decreasing the ratio of acetate to propionate in lactating dairy cows.
Metagenomics is a suitable method for determining rumen microorganisms [34]. We found bacteria were the dominant kingdom in the ruminal fluid of our studied goats. This is in agreement with the results of earlier papers, which showed that feed additives can induce a shift in the structure and the relative abundance of ruminal fluid microbiota in Qianbei-pockmarked goats [26,35]. Various factors affect the composition of rumen microorganisms, including the available substrate (i.e., host diet), energy requirements, and resistance to certain metabolic end-products that may be toxic to some species [36]. Mihaliková et al. [37] found that SY supplementation improved the development of some rumen ciliate species by increasing populations of Ophryoscolex caudatus f. tricoronatus, Diploplastron, Dasytricha ruminantium, and Polyplastron multivesiculatum in crossbred Slovak Valashka lambs. Prevotella species are part of the normal microbial flora in animals, and they are associated with various oral diseases and infections in some other parts of the body. Se is one of the most important essential micronutrients in ruminants. Several ruminal fluid bacteria can incorporate Se into their structures, including Lactobacillus sp., Selenomonas ruminantium, Butyvibrio fibrisolvens, Streptococus sp., and Prevotella ruminicola [38]. Dietary supplementation with Se not only increases antioxidant activity but also inhibits bacterial growth in ruminal fluid to improve rumen dysfunction [31]. Hence, in our study, goats that received SY showed a significant decrease in the relative abundance of Prevotella. These results are consistent with Zhang et al. [39], who demonstrated that adding Se to the diet of Holstein dairy cows decreased Prevotella abundance.
The KEGG database can be used to systematically analyze the metabolic pathways of gene products in cells and the functions of these gene products [40]. In the present study, the main KEGG terms annotated were metabolism, genetic information processing, environment information processing, cellular processes, human diseases, and organismal systems. Among these, genes with the highest abundance were associated with metabolism. These findings are consistent with Zhang et al. [41], who demonstrated that genes in the metabolism category had the highest relative abundance in Hu sheep. In the current study, KEGG functions related to carbohydrate metabolism pathways were enriched in the control kids, and they included other glycan degradation and galactose metabolism. These data suggest that goats who ate a control diet may have had a better ability to degrade carbohydrates, and they may have produced more hydrolysates and pyruvate [42,43]. This may have been due to the control diet increasing the relative abundance of Prevotella in the rumen relative to the two treatment groups.
Vitamin B and vitamin K are believed to be synthesized by bacteria in the rumen in adequate amounts to meet the requirements of the ruminant. However, the addition of biotin can decrease hoof lesions and lameness and in many instances increase milk yield in dairy cows [44]. Moreover, pyrimidine is involved in the synthesis of some vitamins, for example, vitamin B [45]. Jin [46] found that adequate vitamin intake improved growth and development in rats by increasing Se retention in tissues. In our study, the functions of the vitamin pathways, including pyrimidine metabolism, one carbon pool by folate, biotin metabolism, ubiquinone and other terpenoid-quinone biosynthesis, and nicotinate and nicotinamide metabolism were more abundant in the LS rumen microbiome, suggesting that goats receiving 2.4 mg/kg SY exhibited the expression of more genes involved in vitamin metabolic pathways, promoting the synthesis of vitamins in the rumen.
Dietary supplement of antioxidants could increase the ability of antioxidatives in small ruminants [47,48], indicating that antioxidants may regulate rumen microorganisms to participate in related immune metabolic pathways. Se has antioxidant properties, and it is involved in the immune system. The addition of Se can improve growth performance, oxidant status, and Se concentration in blood and tissues in growing male goats [49]. Thus, the feeding of SY may not only enhance antioxidant status but also improve immune status in goats [50]. In our study, functions of the immune function pathways were more abundant in the HS ruminal fluid microbiome. This was probably because the ruminal fluid microorganisms in HS goats affected the immune function [51].
The rumen of ruminants such as goats are one of the most efficient natural systems for cellulose degradation. Rumen microorganisms are a rich source of CAZymes, which can be used to transform cellulose, including into biofuels. They participate in many biological processes, such as carbohydrate metabolism, protein glycosylation, and plant biomass synthesis and degradation in different ecosystems [52]. GHs are a class of enzymes found in all living organisms that hydrolyze the glycosidic bonds of various sugar-containing compounds (including monosaccharides, oligosaccharides, polysaccharides, saponins, and glycoproteins) to produce monosaccharides, oligosaccharides, or sugar complexes [53]. Specifically, the higher ruminal fluid propionate proportion may increase α-amylase activity, as well as increase Butyrivibrio fifibrisolvens and Ruminobacter amylophilus populations, thereby increasing the degradation ability of non-structural carbohydrates in ruminants [54]. In our study, GH2, GH95, GH97, GH115, GH163, and GH98 were enriched in the control, suggesting that those goats were better at degrading complex substrates.
GTs catalyze the formation of glycosidic bonds using sugar donors containing a nucleoside phosphate or a lipid phosphate group, resulting in glucuronidation of many endogenous substrates such as bile acids, steroids, and so forth, participating in their metabolism and regulation [55]. Hendawy et al. [31] suggested that Se can change the microbial activity and composition of ruminal fluid, which has the ability to increase the energy supply for the ruminant. Rumen bacteria may utilize Se as an energy source by competing with methanogens for hydrogen, and thus affecting the production of SCFAs in favor of butyrate production [56]. Hence, in our study, the abundances of genes encoding CAZymes of GT4 and GT35 were enriched in the LS and the HS groups, respectively, in contrast to the control group. This suggests that the rumen microbiomes of the SY groups may be more able to effectively use hydrolysates to produce VFAs, providing more energy for the goats. This may be because Se inhibited the growth of methanogens, leading to a corresponding reduction in the amount of methane produced. In general, improvements in feed conversion efficiency can be used to produce less methane in ruminants [57]. Future studies are needed to assess the relationship between Se concentrations and methane emissions to verify our assumptions.
5. Conclusions
The current study suggested that the addition of 2.4 mg/kg SY could modulate rumen fermentation by increasing the ruminal fluid propionic acid concentration and decreasing the acetate:propionate level; in addition, it could increase the relative abundance of the genes involved in carbohydrate metabolism pathways, upregulating GT and CBM pathways in Qianbei-pockmarked weather goats. Taken together, the appropriate addition level of SY in a growing goat’s diet is 2.4 mg/kg under the experimental condition. However, the underlying mechanisms require further investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8050240/s1, Table S1: Linear regression equation of standard for volatile fatty acids; Table S2: Summary of sequence data generated from rumen samples; Table S3: 428 endogenous third-level pathways were considered rumen microbial metabolic pathways in this study; Table S4: 511 genes encoding CAZymes in this study; Figure S1: Total ion current chromatograms of volatile fatty acids of mixed standard in ruminal fluid of goats; Figure S2: Comparison of microbial domains among the three groups; Figure S3: The percent of community abundance on phylum level; Figure S4: The percent of community abundance on genus level; Figure S5: The percent of community abundance on species level.
Author Contributions
Data curation, writing—original draft preparation, methodology, project administration, X.T.; investigation, data curation, X.W., J.L. and Q.L. (Qingyuan Luo); supervision, C.B.; methodology, writing—reviewing, and editing, project administration, Q.L. (Qi Lu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Guizhou Province (Qiankehe foundation-ZK [2021] General 164), the Youth Science and Technology Talent Development Project of Guizhou Province (Qianjiaohe KY [2022]150), the Cultivating Project of Guizhou University (2019-33), the Start-up Fund of Guizhou University (2016-76; 2019-26), and the State Key Laboratory of Animal Nutrition (2004DA125184F1914).
Institutional Review Board Statement
Animals were cared for and handled in accordance with the Experimental Animal Ethics Committee of Guizhou University (EAE-GZU-2020-7009), and the Guizhou University Animal Care Committee (Guiyang, China) reviewed and approved all experiments and procedures carried out in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence files associated with each sample have been submitted to the NCBI under study accession number PRJNA828448.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, H.M.; Yang, C.; Hassan, F.U. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L. Role of endothelial cells in bovine mammary gland health and disease. Anim. Health Res. Rev. 2015, 16, 135–149. [Google Scholar] [CrossRef]
- Tian, X.Z.; Lu, Q.; Paengkoum, P.; Paengkoum, S. Short communication: Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J. Dairy Sci. 2020, 103, 7808–7812. [Google Scholar] [CrossRef]
- Tian, X.Z.; Wang, X.; Ban, C.; Luo, Q.Y.; Li, J.X.; Lu, Q. Effect of purple corn anthocyanin on antioxidant activity, volatile compound and sensory property in milk during storage and light prevention. Front. Nutr. 2022, 9, 862689. [Google Scholar] [CrossRef]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Thongpea, S.; Ban, C. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J. Integr. Agr. 2018, 17, 2082–2095. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.S.; Celi, P.; Ponnampalam, E.N.; Leury, B.J.; Liu, F.; Dunshea, F.R. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: Role of vitamin E and selenium. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Silveira, R.M.F.; Silva, B.E.B.E.; Vasconcelos, A.M.D.; Façanha, D.A.E.; Martins, T.P.; Rogério, M.C.P.M.; Ferreira, J. Does organic selenium supplement affect the thermoregulatory responses of dairy goats? Biol. Rhythm. Res. 2021, 52, 1–13. [Google Scholar] [CrossRef]
- Ceballos, A.; Sanchez, J.; Stryhn, H.; Montgomery, J.B.; Barkema, H.W.; Wichtel, J.J. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009, 92, 324–342. [Google Scholar] [CrossRef] [Green Version]
- Juniper, D.T.; Phipps, R.H.; Givens, D.I.; Jones, A.K.; Green, C.; Bertin, G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J. Anim. Sci. 2008, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mainville, A.M.; Odongo, N.E.; Bettger, W.J.; Mcbride, B.W.; Osborne, V.R. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 2009, 89, 105–110. [Google Scholar] [CrossRef]
- Milewski, S.; Sobiech, P.; Baejak-Grabowska, J.; Wójcik, R.; Zbek, K. The efficacy of a long-acting injectable selenium preparation administered to pregnant ewes and lambs. Animals 2021, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.M.; Rode, L.M.; Cohen, R.; Buckley, W.T. Effects of diet and chemical form of selenium on selenium metabolism in sheep. J. Anim. Sci. 1997, 75, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Kišidayová, S.; Mihaliková, K.; Siroka, P.; Čobanová, K.; Váradyová, Z. Effects of inorganic and organic selenium on the fatty acid composition of rumen contents of sheep and the rumen bacteria and ciliated protozoa. Anim. Feed Sci. Technol. 2014, 193, 51–57. [Google Scholar] [CrossRef]
- Pino, F.; Heinrichs, A.J. Effect of trace minerals and starch on digestibility and rumen fermentation in diets for dairy heifers. J. Dairy Sci. 2016, 99, 2797–2810. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, Z.; Tan, Y.; Chang, S.; Zheng, H.; Wang, H.; Yan, T.; Guru, T.; Hou, F. Selenium yeast dietary supplement affects rumen bacterial population dynamics and fermentation parameters of Tibetan sheep (Ovis aries) in alpine meadow. Front. Microbiol. 2021, 12, 663945. [Google Scholar] [CrossRef]
- Arshad, M.A.; Ebeid, H.M.; Hassan, F. Revisiting the effects of different dietary sources of selenium on the health and performance of dairy animals: A review. Biol. Trace Elem. Res. 2021, 199, 3319–3337. [Google Scholar] [CrossRef]
- Tian, X.Z.; Lu, Q.; Zhao, S.G.; Li, J.X.; Luo, Q.Y.; Wang, X.; Zhang, Y.; Zheng, N. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of longissimus thoracis et lumborum muscle in goats. Animals 2021, 11, 2407. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Wang, X.; Xiao, M.M.; Zhou, D.; Lu, Q.; Chen, Q. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Vet. Sci. 2022, 8, 813672. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Goats: Angora, Dairy, and Meat Goats in Temperate and Tropical Countries; The National Academies Press: Washington, DC, USA, 1981. [Google Scholar]
- Association of Official Analytical Chemists. Association of Official Analytical Chemists Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids, 1st ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bremner, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Zhou, D.; Long, Q.M.; Wang, X.; Lu, Q.; Wen, G.L. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and rumen microbiota in goats. Front. Vet. Sci. 2021, 8, 715710. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Xin, H.L.; Paengkoum, P.; Paengkoum, S.; Ban, C.; Sorasak, T. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J. Anim. Sci. 2019, 97, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Yue, W.; Zhang, C.; Ren, Y.; Qiang, L. Effect of high-dose nano-selenium and selenium-yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Elem. Res. 2012, 150, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lemosquet, S.; Delamaire, E.; Lapierre, H.; Blum, J.W.; Peyraud, J.L. Effects of glucose, propionic acid, and nonessential amino acids on glucose metabolism and milk yield in Holstein dairy cows. J. Dairy Sci. 2009, 92, 3244–3257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriyapha, C.; Cherdthong, A.; Suntara, C.; Polyorach, S. Utilization of yeast waste fermented citric waste as a protein source to replace soybean meal and various roughage to concentrate ratios on in vitro rumen fermentation, gas kinetic, and feed digestion. Fermentation 2021, 7, 120. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Sugimura, S.; Sato, K.; Mansour, M.M.; Abd El-Aziz, A.H.; Samir, H.; Islam, M.A.; Bostami, A.B.M.R.; Mandour, A.S.; Elfadadny, A.; et al. Effects of selenium supplementation on rumen microbiota, rumen fermentation, and apparent nutrient digestibility of ruminant animals: A review. Fermentation 2022, 8, 4. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Lou, S.; Wanapat, M.; Wang, Z.; Zhu, W.; Hou, F. Selenium supplementation improves nutrient intake and digestibility, and mitigates CH4 emissions from sheep grazed on the mixed pasture of alfalfa and tall fescue. J. Anim. Physiol. Anim. Nutr. 2021, 105, 611–620. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest. Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Walker, A.W.; Watson, M. Compendium of 4941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019, 37, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Tian, X.; Ma, Z.; Wu, W. Feeding a negative dietary cation–anion difference to female goats is feasible, as indicated by the non-deleterious effect on rumen fermentation and rumen microbial population and increased plasma calcium level. Animals 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Chloe, M.; Fiona, C.; Eva, L.; Michael, R.; O’Toole, W.P.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2018, 10, 115–132. [Google Scholar]
- Mihaliková, K.; Grešáková, L.; Boldižárová, K.; Faix, Š.; Leng, L.; Kišidayová, S. The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol. 2005, 50, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lenártová, V.; Holovská, K.; Javorský, P. The influence on the antioxidant enzyme activity of rumen bacteria Streptococcus bovis and Selenomonas ruminantium. FEMS Microbiol. Ecol. 1998, 27, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.D.; Wang, C.; Du, H.S.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Anim. Int. J. Anim. Biosci. 2020, 14, 2091–2099. [Google Scholar] [CrossRef]
- Minoru, K.V.; Miho, F.; Mao, T.; Yoko, S.; Kanae, M. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animals 2021, 15, 100161. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Singh, K.M.; Reddy, B.; Patel, D.; Patel, A.K.; Joshi, C.G. High potential source for biomass degradation enzyme discovery and environmental aspects revealed through metagenomics of Indian buffalo rumen. Biomed. Res. Int. 2014, 5938, 267189. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Invited review: Mineral and vitamin nutrition in ruminants. Prof. Anim. Sci. 2014, 30, 180–191. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Yan, C.; Baylon, J.L.; Yan, N. Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res. 2017, 27, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.S.; Yin, S.A. Effect of vitamin B6 status on selenium retention in the tissues in rats fed selenium from sodium selenate. J. Hyg. Res. 2005, 34, 422–424. [Google Scholar]
- Tian, X.Z.; Paengkoum, P.; Paengkoum, S.; Chumpawadee, S.; Ban, C.; Thongpea, S. Short communication: Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J. Dairy Sci. 2019, 102, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Li, J.; Luo, Q.; Wang, X.; Wang, T.; Zhou, D.; Xie, L.; Ban, C.; Lu, Q. Effects of purple corn anthocyanin on growth performance, meat quality, muscle antioxidant status, and fatty acid profiles in goats. Foods 2022, 11, 1255. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Lei, S.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, se-yeast and nano-elemental selenium on growth performance, se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Shi, L.; Ren, Y.; Zhang, C.; Yue, W.; Lei, F. Effects of organic selenium (Se-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang black goats. Anim. Feed. Sci. Tech. 2018, 238, 57–63. [Google Scholar] [CrossRef]
- Badgar, K.; Prokisch, J. The effects of selenium nanoparticles (SeNPs) on ruminant. Proc. Mong. Acad. Sci. 2020, 60, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.H.; Chen, Y.X.; Cheng, Z.H.; Dong, H.M. Age-related response of rumen microbiota to mineral salt and effects of their interactions on enteric methane emissions in cattle. Microb. Ecol. 2016, 73, 1–12. [Google Scholar] [CrossRef]
- Geng, A.; Jin, M.; Li, N.; Zhu, D.; Xie, R.; Wang, Q.; Liu, H.; Sun, J. New insights into the co-occurrences of glycoside hydrolase genes among prokaryotic genomes through network analysis. Microorganisms 2021, 9, 427. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. Rumen microbes, enzymes and feed digestion—A review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Soest, P.; Combs, G.F. Studies on the effects of selenium on rumen microbial fermentation in vitro. Biol. Trace Elem. Res. 1997, 56, 203–213. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Hegarty, R.S. Lowering ruminant methane emissions through improved feed conversion efficiency. Anim. Feed Sci. Technol. 2011, 166, 291–301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).