Evaluating Bio-Hydrogen Production Potential and Energy Conversion Efficiency from Glucose and Xylose under Diverse Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Medium
2.2. Bio-Hydrogen Potential Tests
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
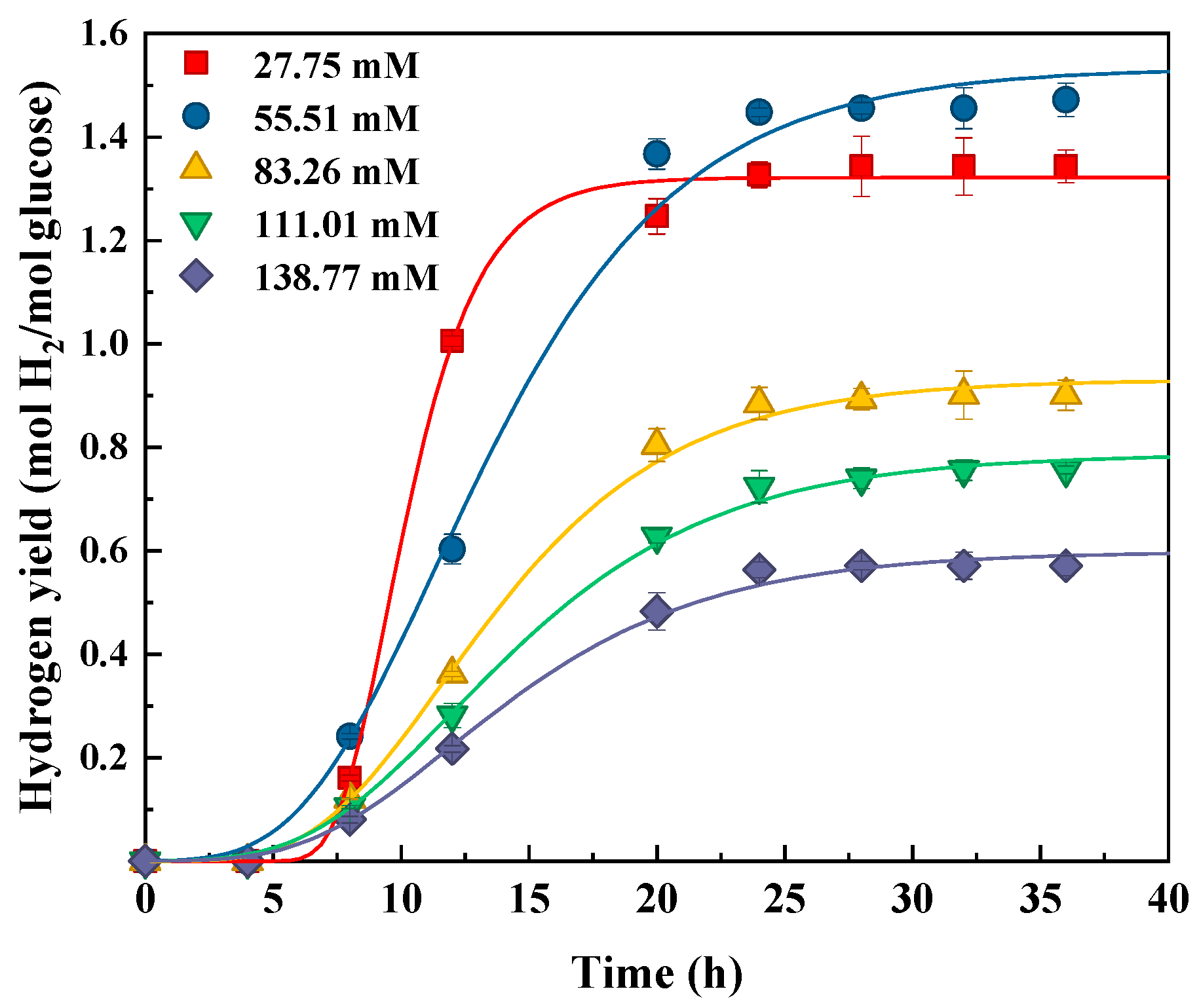
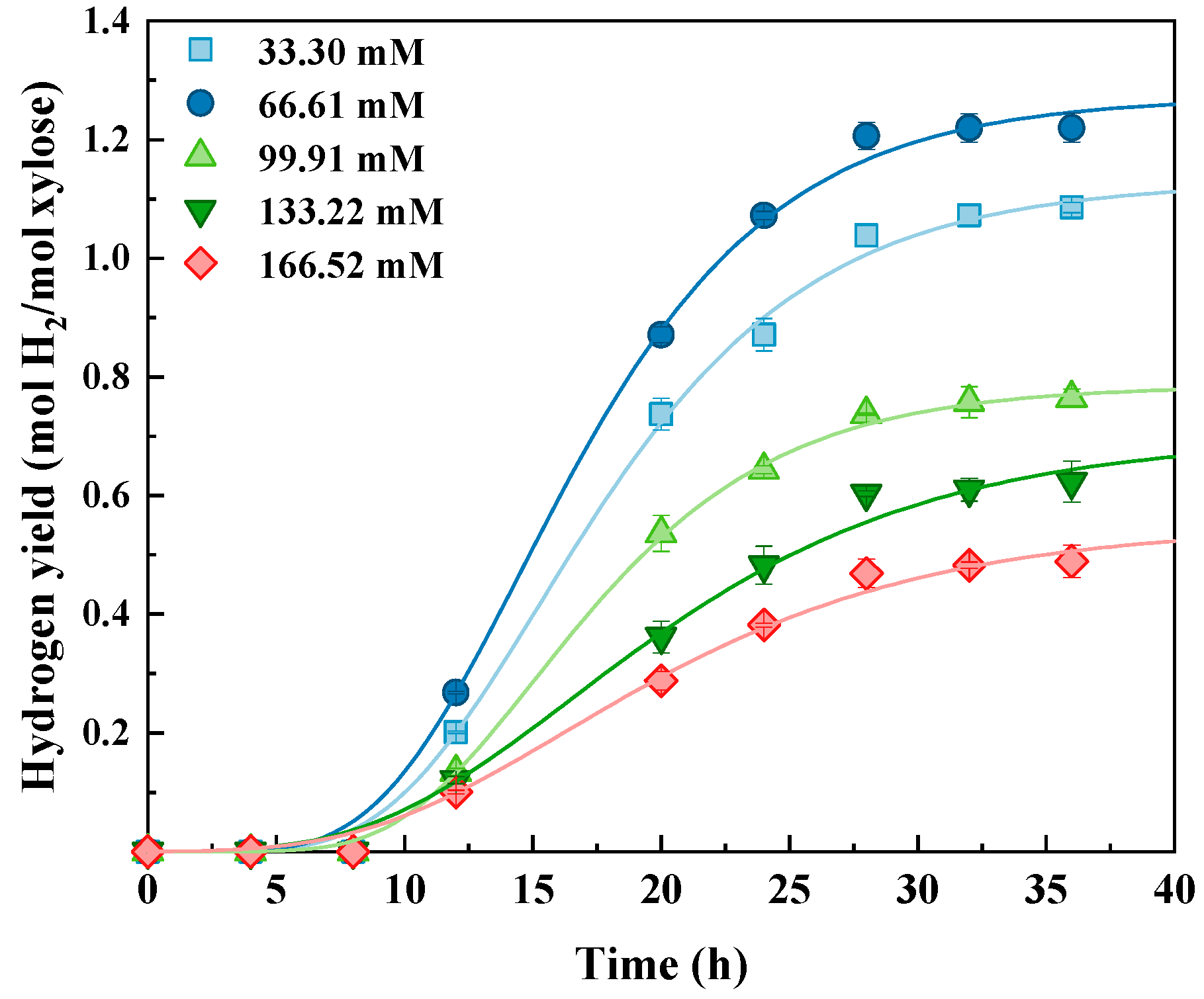
3.1. Bio-Hydrogen Production from Glucose, Xylose, and Glucose–Xylose
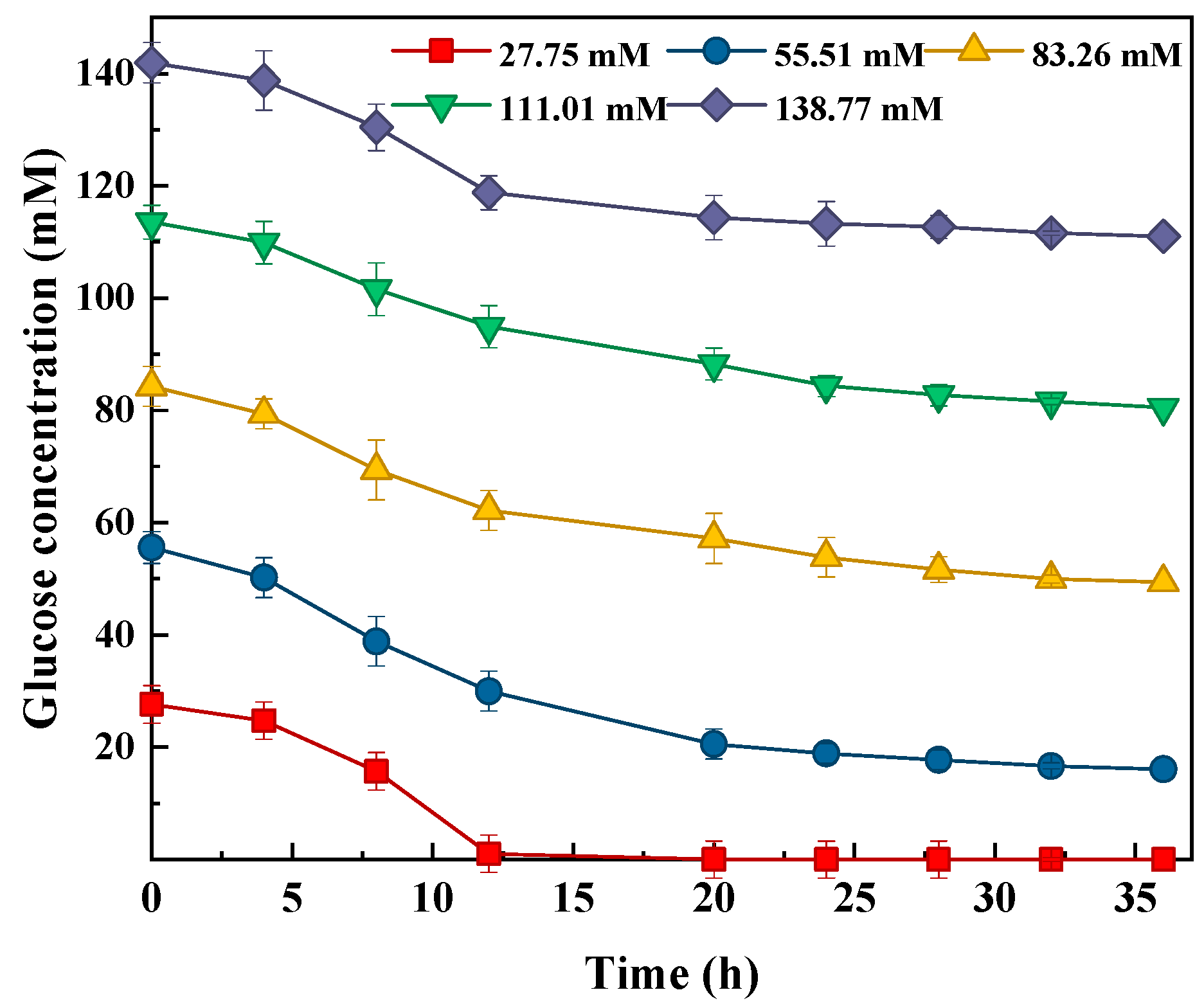
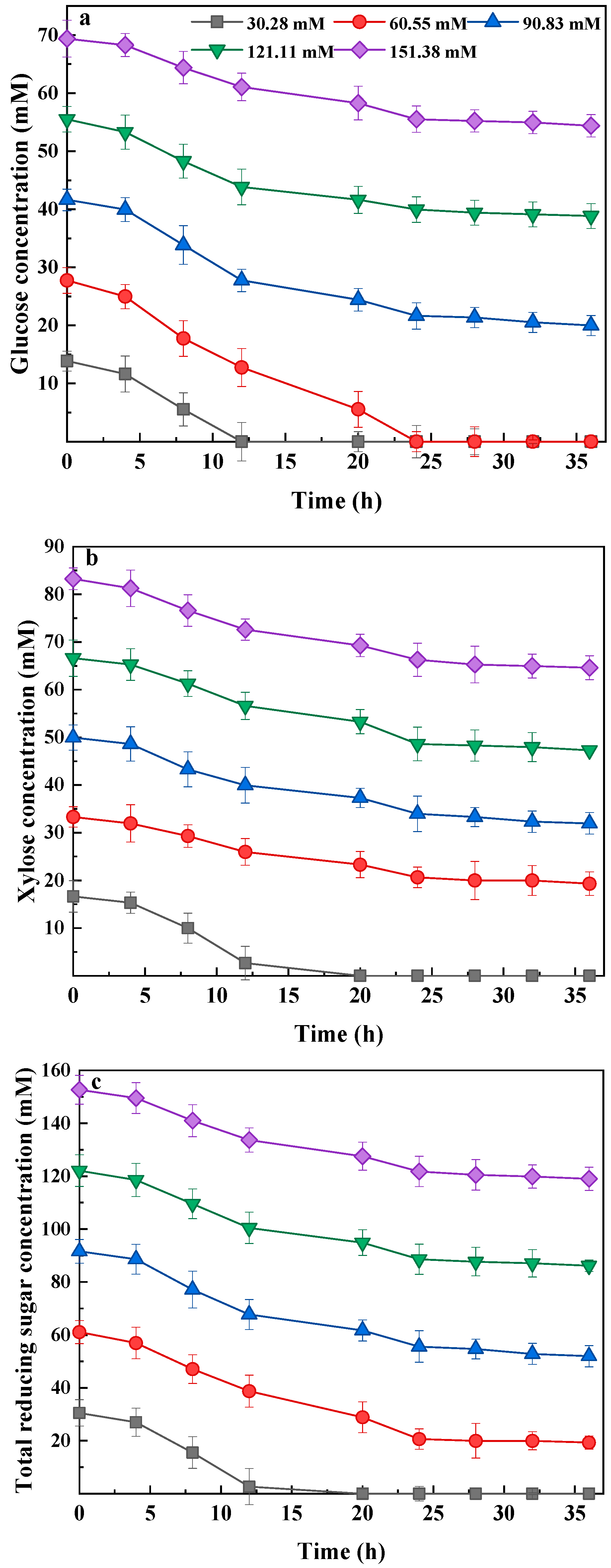
3.2. Glucose and Xylose Consumption during Bio-Hydrogen Production
3.3. Energy Conversion Efficiency of Bio-Hydrogen Production from Glucose, Xylose, and Glucose–Xylose
3.4. Implications of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel production from plant and algal biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Coupling dark fermentation with biochemical or bioelectrochemical systems for enhanced bio-energy production: A review. Int. J. Hydrogen Energy 2017, 42, 26667–26686. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrogen Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Lukajtis, R.; Holowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kaminski, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Marone, A.; Ayala-Campos, O.R.; Trably, E.; Carmona-Martinez, A.A.; Moscoviz, R.; Latrille, E.; Steyer, J.P.; Alcaraz-Gonzalez, V.; Bernet, N. Coupling dark fermentation and microbial electrolysis to enhance bio-hydrogen production from agro-industrial wastewaters and by-products in a bio-refinery framework. Int. J. Hydrogen Energy 2017, 42, 1609–1621. [Google Scholar] [CrossRef]
- Yadav, M.; Paritosh, K.; Vivekanand, V. Lignocellulose to bio-hydrogen: An overview on recent developments. Int. J. Hydrogen Energy 2020, 45, 18195–18210. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Mukasekuru, M.R.; Hu, J.G.; Zhao, X.Q.; Sun, F.F.; Pascal, K.; Ren, H.Y.; Zhang, J.H. Enhanced High-Solids Fed-Batch Enzymatic Hydrolysis of Sugar Cane Bagasse with Accessory Enzymes and Additives at Low Cellulase Loading. ACS Sustain. Chem. Eng. 2018, 6, 12787–12796. [Google Scholar] [CrossRef]
- Silva, J.S.; Mendes, J.S.; Correia, J.A.C.; Rocha, M.V.P.; Micoli, L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018, 286, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Hu, X.; Zhao, X.; Li, L.; Yang, J.; Zhao, Y.; Li, X. Fermentative hydrogen production with peanut shell as supplementary substrate: Effects of initial substrate, pH and inoculation proportion. Renew. Energy 2018, 127, 559–564. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Sánchez, A.; De León-Rodríguez, A. Simultaneous production of bioethanol and biohydrogen by Escherichia coli WDHL using wheat straw hydrolysate as substrate. Fuel 2017, 188, 19–27. [Google Scholar] [CrossRef]
- Hu, C.C.; Giannis, A.; Chen, C.L.; Qi, W.; Wang, J.Y. Comparative study of biohydrogen production by four dark fermentative bacteria. Int. J. Hydrogen Energy 2013, 38, 15686–15692. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lu, W.C.; Chen, C.Y.; Chang, J.S. Dark fermentative hydrogen production from enzymatic hydrolysate of xylan and pretreated rice straw by Clostridium butyricum CGS5. Bioresour. Technol. 2010, 101, 5885–5891. [Google Scholar] [CrossRef]
- Tian, Q.Q.; Liang, L.; Zhu, M.J. Enhanced biohydrogen production from sugarcane bagasse by Clostridium thermocellum supplemented with CaCO3. Bioresour. Technol. 2015, 197, 422–428. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.L.; Sheng, T.; Ren, H.Y.; Wang, A.J.; Zhang, J.; Zhong, Y.J.; Ren, N.Q. Bio-immobilization of dark fermentative bacteria for enhancing continuous hydrogen production from cornstalk hydrolysate. Bioresour. Technol. 2017, 243, 548–555. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Jing, Y.; Tahir, N.; Zhang, Y.; Zhang, Q. Comparative study on bio-hydrogen production from corn stover: Photo-fermentation, dark-fermentation and dark-photo co-fermentation. Int. J. Hydrogen Energy 2020, 45, 3807–3814. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Ren, H.Y.; Chen, C.; Nan, J.; Cao, G.L.; Yang, S.S.; Ren, N.Q. Residue cornstalk derived biochar promotes direct bio-hydrogen production from anaerobic fermentation of cornstalk. Bioresour. Technol. 2021, 320, 124338. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Alhazmi, A.; Mohammad, A.; Srivastava, N.; Haque, S.; Sharma, S.; Singh, R.; Yoon, T.; Gupta, V.K. Integrated biohydrogen production via lignocellulosic waste: Opportunity, challenges & future prospects. Bioresour. Technol. 2021, 338, 12. [Google Scholar]
- Saratale, G.D.; Kshirsagar, S.D.; Saratale, R.G.; Govindwar, S.P.; Oh, M.-K. Fermentative hydrogen production using sorghum husk as a biomass feedstock and process optimization. Biotechnol. Bioprocess Eng. 2015, 20, 733–743. [Google Scholar] [CrossRef]
- Ren, N.; Cao, G.; Wang, A.; Lee, D.; Guo, W.; Zhu, Y. Dark fermentation of xylose and glucose mix using isolated Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 2008, 33, 6124–6132. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.H.; Wu, J.T.; Ren, H.Y.; Yang, S.S.; Nan, J.; Cao, G.L.; Sheng, Y.C.; Wang, A.J.; Ren, N.Q. Co-fermentation of a mixture of glucose and xylose to hydrogen by Thermoanaerobacter thermosaccharolyticum W16: Characteristics and kinetics. Int. J. Hydrogen Energy 2019, 44, 9248–9255. [Google Scholar] [CrossRef]
- Li, W.M.; He, L.; Cheng, C.; Cao, G.L.; Ren, N.Q. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 8. [Google Scholar] [CrossRef]
- Lei, Z.; Guo, W.Q.; Guo, X.C.; Ren, H.Y.; Wu, J.T.; Cao, G.L.; Wang, A.J.; Ren, N.Q.J.R.A. Continuous hydrogen production from glucose/xylose by an anaerobic sequential batch reactor to maximize the energy recovery efficiency. Rsc. Adv. 2018, 8, 20712–20718. [Google Scholar]
- Hitit, Z.Y.; Lazaro, C.Z.; Hallenbeck, P.C.J. Single stage hydrogen production from cellulose through photo-fermentation by a co-culture of Cellulomonas fimi and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2016, 42, 6556–6566. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Duber, A.; Seifert, K. Hydrogen production during direct cellulose fermentation by mixed bacterial culture: The relationship between the key process parameters using response surface methodology. J. Clean. Prod. 2021, 314, 127971. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.L.; Wang, A.J.; Guo, W.Q.; Ren, H.Y.; Ren, N.Q. Simultaneous saccharification and fermentation of fungal pretreated cornstalk for hydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Bioresour. Technol. 2013, 145, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, G.L.; Wang, A.J.; Ren, H.Y.; Dong, D.; Liu, Z.N.; Guan, X.Y.; Xu, C.J.; Ren, N.Q. Fungal pretreatment of cornstalk with Phanerochaete chrysosporium for enhancing enzymatic saccharification and hydrogen production. Bioresour. Technol. 2012, 114, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, Y.Y.; Lu, C.Y.; Kongjan, P.; Wang, J.; Awasthi, M.K.; Tahir, N.; Zhang, Q.G. A syntrophic co-fermentation model for bio-hydrogen production. J. Clean. Prod. 2021, 317, 9. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.X.; Tokudome, H.; Zhong, M.; Wang, C.Z.; Pan, Z.H.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]



| Microorganism | Substrate Concentration (mM) | Hydrogen Yield (mol H2·mol−1 Substrate) | References | |
|---|---|---|---|---|
| Glucose | Xylose | |||
| Clostridium butyricum | 27.75–111.01 | 0 | 2.4–3.1 | [17] |
| Clostridium butyricum CGS5 | 0 | 87.26/61.28 | 0.70–0.76 | [18] |
| C. thermocellum | 69.38 | 25.31 | 0.85 | [19] |
| Clostridium sp. T2 | 84.37 | 31.97 | 1.70 | [20] |
| Enterobacter aerogenes | 11.66 | 6.66 | 0.27 | [21] |
| Clostridium beijerinckii | 15.26 | 7.99 | 1.051 | [24] |
| Clostridium roseum ATCC 17,797 | 145.32 | 125.89 | 0.014 | [14] |
| Escherichia coli WDHL | 2.78 | 86.59 | 0.95 | [16] |
| Substrate Types | Substrate Concentration (g·L−1) | Substrate Concentration (mM) | P (mol H2·mol−1 Substrate) | Rm (mol H2·mol−1 Substrate h−1) | λ (h) | R2 |
|---|---|---|---|---|---|---|
| Glucose | 5 | 27.75 | 1.32 ± 0.11 | 0.25 ± 0.02 | 7.4 ± 0.4 | 0.997 |
| 10 | 55.51 | 1.53 ± 0.12 | 0.11 ± 0.00 | 6.0 ± 0.3 | 0.997 | |
| 15 | 83.26 | 0.93 ± 0.07 | 0.07 ± 0.00 | 6.6 ± 0.4 | 0.999 | |
| 20 | 111.01 | 0.79 ± 0.06 | 0.05 ± 0.00 | 6.3 ± 0.3 | 0.991 | |
| 25 | 138.77 | 0.60 ± 0.05 | 0.04 ± 0.00 | 6.3 ± 0.5 | 0.992 | |
| Xylose | 5 | 33.30 | 1.13 ± 0.09 | 0.07 ± 0.01 | 9.3 ± 0.8 | 0.995 |
| 10 | 66.61 | 1.27 ± 0.10 | 0.08 ± 0.01 | 8.9 ± 0.7 | 0.988 | |
| 15 | 99.91 | 0.79 ± 0.05 | 0.05 ± 0.00 | 9.7 ± 0.8 | 0.996 | |
| 20 | 133.22 | 0.70 ± 0.05 | 0.03 ± 0.00 | 8.6 ± 0.7 | 0.998 | |
| 25 | 166.52 | 0.55 ± 0.03 | 0.03 ± 0.00 | 8.3 ± 0.6 | 0.994 | |
| Glucose–xylose | 5 | 30.28 | 1.19 ± 0.09 | 0.24 ± 0.02 | 7.5 ± 0.5 | 0.994 |
| 10 | 60.55 | 1.39 ± 0.11 | 0.09 ± 0.01 | 5.7 ± 0.4 | 0.993 | |
| 15 | 90.83 | 0.86 ± 0.07 | 0.06 ± 0.00 | 6.2 ± 0.5 | 0.998 | |
| 20 | 121.11 | 0.72 ± 0.05 | 0.04 ± 0.00 | 5.9 ± 0.3 | 0.998 | |
| 25 | 151.38 | 0.56 ± 0.04 | 0.03 ± 0.00 | 5.5 ± 0.3 | 0.999 |
| Substrate Types | Substrate Concentration (g·L−1) | Substrate Concentration (mM) | Acetate (mM) | Butyrate (mM) | Ethanol (mM) | Butanol (mM) |
|---|---|---|---|---|---|---|
| Glucose | 5 | 27.75 | 13.4 ± 1.0 | 40.4 ± 3.3 | n.d. | n.d. |
| 10 | 55.51 | 19.3 ± 1.4 | 39.5 ± 3.1 | n.d. | n.d. | |
| 15 | 83.26 | 20.3 ± 1.5 | 35.1 ± 2.8 | n.d. | n.d. | |
| 20 | 111.01 | 20.1 ± 1.4 | 37.3 ± 3.1 | n.d. | n.d. | |
| 25 | 138.77 | 28.3 ± 2.2 | 45.8 ± 3.7 | n.d. | n.d. | |
| Xylose | 5 | 33.30 | 10.6 ± 0.8 | 38.1 ± 3.1 | n.d. | n.d. |
| 10 | 66.61 | 15.9 ± 1.2 | 36.1 ± 2.9 | n.d. | n.d. | |
| 15 | 99.91 | 16.9 ± 1.3 | 32.6 ± 2.6 | n.d. | n.d. | |
| 20 | 133.22 | 17.8 ± 1.3 | 35.0 ± 2.8 | n.d. | n.d. | |
| 25 | 166.52 | 24.9 ± 1.9 | 41.2 ± 3.3 | n.d. | n.d. | |
| Glucose and xylose | 5 | 30.28 | 4.1 ± 0.2 | 12.3 ± 0.8 | 34.0 ± 2.2 | 22.5 ± 1.4 |
| 10 | 60.55 | 6.0 ± 0.4 | 19.4 ± 1.1 | 25.9 ± 1.9 | 21.6 ± 1.4 | |
| 15 | 90.83 | 10.8 ± 0.3 | 26.9 ± 1.6 | 20.7 ± 1.2 | 20.2 ± 2.3 | |
| 20 | 121.11 | 15.4 ± 0.4 | 29.8 ± 2.4 | 20.4 ± 0.9 | 20.0 ± 1.6 | |
| 25 | 151.38 | 25.8 ± 0.8 | 31.8 ± 2.1 | 17.5 ± 1.1 | 16.4 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-H.; Tan, J.-Y.; Zhang, Y.-T.; Ren, N.-Q.; Zhao, L. Evaluating Bio-Hydrogen Production Potential and Energy Conversion Efficiency from Glucose and Xylose under Diverse Concentrations. Fermentation 2022, 8, 739. https://doi.org/10.3390/fermentation8120739
Wang Z-H, Tan J-Y, Zhang Y-T, Ren N-Q, Zhao L. Evaluating Bio-Hydrogen Production Potential and Energy Conversion Efficiency from Glucose and Xylose under Diverse Concentrations. Fermentation. 2022; 8(12):739. https://doi.org/10.3390/fermentation8120739
Chicago/Turabian StyleWang, Zi-Han, Jing-Yan Tan, Yu-Tong Zhang, Nan-Qi Ren, and Lei Zhao. 2022. "Evaluating Bio-Hydrogen Production Potential and Energy Conversion Efficiency from Glucose and Xylose under Diverse Concentrations" Fermentation 8, no. 12: 739. https://doi.org/10.3390/fermentation8120739
APA StyleWang, Z.-H., Tan, J.-Y., Zhang, Y.-T., Ren, N.-Q., & Zhao, L. (2022). Evaluating Bio-Hydrogen Production Potential and Energy Conversion Efficiency from Glucose and Xylose under Diverse Concentrations. Fermentation, 8(12), 739. https://doi.org/10.3390/fermentation8120739






