Abstract
Daqu is not only a crucial starter in the production of baijiu, but it is also an important source of flavoring substances, so maintaining a stable quality is an important part of improving the quality of baijiu. Nonetheless, since the production of daqu is still a natural fermentation process, which is influenced by seasonal factors, the rapid testing of daqu quality is a problem that must be solved. In this study, headspace solid-phase microextraction technology (HS-SPME) was used to explore the volatile components in daqu, and a total of 115 volatile components were extracted. By constructing an untargeted statistical model, the variation in volatile compounds in dissimilar production processes of daqu was studied, and the differences between different maturation stages and the correlations between volatile compounds were analyzed. Subsequently, six compounds, including ethyl acetate, ethanol, phenylethanol, (R,R)-2,3-butanediol, ethyl caproate, and 2,3-butanediol, were further screened out by partial least squares discrimination analysis (PLS-DA), and the symbolic combination of daqu’s maturity was speedily judged in accordance with the changes in marker compound concentrations to lay the foundation for the mechanization of baijiu production.
1. Introduction
As a unique beverage in China with a long history of more than 2000 years, baijiu has always been deeply loved by people []. Baijiu can be divided into 12 flavor types in line with dissimilar production processes []. Traditionally, baijiu is made from grains as raw materials. After a series of complicated processes, such as cooking, adding qu, saccharification, fermentation, and distillation, a traditional Chinese brewed beverage with a complex aroma and rich flavor are ultimately obtained [,]. Baijiu uses a stater called “jiuqu” for saccharification and fermentation in the brewing process. In general, jiuqu can be categorized as daqu, xiaoqu, and fuqu []. One of the most commonly employed is daqu. In accordance with the culture temperature, daqu can be divided into three categories: low-temperature type, medium-temperature type, and high-temperature type [], which are adopted to produce diverse flavor types of baijiu. Light-flavor baijiu uses low-temperature daqu with the highest product temperature of 40–50 °C. Strong-flavor baijiu generally uses medium-temperature daqu with the highest product temperature of 50–60 °C, and sauce-flavor baijiu generally uses high-temperature daqu with the highest product temperature greater than 60 °C. Daqu is made of grain and is shaped like rectangular bricks. After artificial caiqu, these bricks are placed in a daqu house. At this time, bacteria, mold, yeast, and other microorganisms began to work. []. Within one month, the anqu duration will normally vary in line with the climate and temperature. When daqu is in the anqu process, rich flavors can be obtained through the fermentation and culture of microorganisms, making the flavor of baijiu diversified.
Apart from being employed as a fermentation starter during the production processes of baijiu, daqu can also be employed as a “flavoring agent” to directly or indirectly provide baijiu with various flavors []. Dissimilar fermentation environments will give rise to the diversification of microorganisms when producing daqu, which will affect the flavor and fermentation metabolism of daqu []. Strong-flavor baijiu is one of the important styles of baijiu, and some of its flavor components are provided by daqu. Discussing the changes in volatile components in the production process of daqu can provide ideas and references for the study of flavor substances in strong-flavor baijiu. Notwithstanding the fundamental fact that scholars have conducted a myriad of studies on the microbial community in daqu, primarily exploring the correlation between microbes and flavor components [,], but the variation in volatile compounds has been rarely discussed. In this study, the aim is to analyze the volatile compounds in the production process of daqu.
There are many methods currently applied to the study of volatile compounds in the field of baijiu. The direct injection method (DI) involves the direct injection of the sample into a gas chromatograph (GC) for separation and detection without or with simple treatment. Liquid–liquid extraction (LLE) is the extraction and concentration of trace components in baijiu using organic solvents to enrich volatile compounds. HS-SPME is a solvent-free extraction method for the extraction of volatile compounds from baijiu by coating adsorption. Solvent-assisted flavor evaporation (SAFE) is a combination of a distillation unit and a high vacuum pump to achieve the separation and analysis of flavor substances. In addition to the above methods, other methods for the extraction of volatile compounds include liquid–liquid microextraction (LLME), dynamic headspace adsorption (DHS), etc. Based on the characteristics of the various methods and the aim of this study, which is to achieve rapid detection of flavor and rapid determination of maturity level in the production process of daqu, HS-SPME, which is simple to operate and less expensive, was finally selected for this study.
As a consequence, this study is intended to analyze the types and contents of volatile components in daqu samples to reveal the change law of volatile compounds during the production process of strong-flavor daqu and to screen out the index compounds by various statistical methods so as to achieve a rapid determination of the maturity level of daqu, which in turn will lead to the improvement of the quality and stability of daqu and baijiu, and then to lay the foundation for the realization of the mechanized production of baijiu.
2. Materials and Methods
2.1. Sample Collection
Daqu samples were taken from a distillery fermentation workshop. Representative time points in the production process were selected, which were, respectively, 0, 4, 8, 12, 13, and 24 days, corresponding to six stages in the production process, which indicated that daqu was gradually matured. In addition, the samples at each stage were fermented independently in a qufang, and the temperature and humidity of the qufang were not fixed with the degree of fermentation but did not exceed 60 °C. In this study, several medium-temperature strong-flavor daqu samples were analyzed: A (production for 0 days), B (production for 4 days), C (production for 8 days), D (production for 12 days), E (production for 13 days), and F (production for 24 days, finished qu). Daqu samples were ground into powder and screened with a 40-mesh sieve, then were stored at −20 °C before the experiment. The RI were calculated on the basis of the modified Kovats method [].
2.2. Chemicals
The following commercial standards (analytical reagent grade, ≥97% purity) were used: 4-octanol from Sigma-Aldrich (Shanghai, China) was employed as an internal standard. The ultra-pure water employed herein was obtained from a Milli-Q system. A C8−C40 n-alkane mixture (Sigma-Aldrich, Shanghai, China) was employed for determining linear retention indices (RI). Absolute ethanol was from China National Pharmaceutical Group Corp (Shanghai, China).
2.3. Isolation of the Volatiles
A total of 1 g of ground daqu sample was taken; then, 5 μL of a 4-octanol solution was added in a 20 mL headspace bottle. It was covered, sealed, and equilibrated for 20 min at 40 °C. The fiber was 50/30 μm, DVB/CAR/PDMS. The fiber tip was placed above the sample and extracted for 40 min while stirring below 40 °C, and was then immediately analyzed for 5 min with a GC injector at 250 °C.
2.4. Gas Chromatography–Mass Spectrometry (GC-MS)
The volatile compounds were further evaluated by adopting GC-MS (7890B GC System, 5977A MSD). Each sample (1 μL each) was employed for analysis on a DB-FFAP capillary column (60 m × 250 μm × 0.25 μm, Agilent Technologies, Santa Clara, CA, USA). Helium (99.999%) was used as a carrier gas at a constant flow rate of 2.0 mL/min, and the inlet temperature was 250 °C. The oven temperature was maintained at 40 °C initially and was held for 3 min, ramped to 150 °C at a rate of 2 °C/min and was held for 2 min, and then finally was raised to 230 °C at a rate of 7 °C/min and held was for 8 min. The temperature of the transfer line was 250 °C, and that of the MS ion source was set at 230 °C. The ionization energy of the electron impact mass spectra was 70 eV, and the acquisitions were over an m/z scan range of 35–450 amu. The injection was performed in splitless mode.
Three parallel experiments were conducted for each sample, and the volatile compounds were identified by comparing their mass spectra in the NIST (2020) baijiu flavor compounds database developed by our group and their RI from the DB-FFAP column with those of the authentic standards. The retention indices of the compounds were calculated using alkanes (C8–C40). The n-alkanes of C8–C40 were configured in the n-hexane solution, which was selected to take into account the protection of the column. The solution concentration was 20 µg/L. The same heating procedure as the sample was selected. The injection mode was splitless, and the injection volume was 1 µL. After the RI were calculated according to the formula, they were compared with the values in the literature so as to realize the further characterization of the compounds.
2.5. Statistical Analysis
Statistical analysis and hierarchical cluster analysis (HCA) of volatile compounds in strong-flavor daqu were conducted by Origin version 9.0 software (Origin Lab Co., Northampton, MA, USA). SIMCA version 14.0 (Umetrics, Umea, Sweden) performed the principal components analysis (PCA) and PLS-DA.
3. Results and Discussion
3.1. Qualitative and Quantitative Analysis of Volatile Compounds
A total of 115 volatile compounds were detected in the strong-flavor daqu, including 31 esters, 13 alcohols, 7 acids, 13 aldehydes, 13 nitrogenous compounds, and so on. Ester compounds are mainly formed by microorganisms in daqu using alcohols and carboxylic acids. Almost all of the 31 ester compounds detected in this study were found in the flavor analysis of strong-flavor baijiu [,,].
Figure 1 shows the variation in volatile compounds at dissimilar stages of the production process of strong-aroma daqu. As illustrated in Figure 1a, when elongating the production duration, the maximum change in volatile compound type was observed after 4 days of daqu production, which belongs to the material rapid growth stage. This is probably owing to the fact that multitudinous new substances are produced by microbial fermentation [,], such as through the Maillard reaction, and the reaction substrate is sufficient at this stage, thus achieving speedy growth. In the last four periods, the species of compounds changed were unremarkable, all within 10 species. At this time, the variety of components was comparatively stable, but the concentrations of the compounds still changed. As depicted in Figure 1b, the species of acid compounds reached a maximum value in the first four periods after constant growth and then exhibited a decreasing trend before reaching relative stability. A similar trend existed for aldehydes, esters, and alcohols, which presented a comparatively balanced and harmonious level in the final period, suggesting that the daqu gradually matured. It is noteworthy that nitrogenous and sulphur-containing compounds are two extremely important categories in the study of baijiu flavor, with nitrogenous compounds usually showing a roasted aroma. Fan et al. [] detected a variety of pyrazine compounds such as 2,6-dimethylpyrazine and 2-ethylpyrazine in strong-flavor baijiu such as Gujinggong and Jiannanchun. These pyrazines are often produced by the Maillard reaction. In addition to the above aroma, there also are certain health care effects []. Sulphur-containing compounds, on the other hand, have a considerably low olfactory threshold and usually have tremendously high odor activity values [], thus making a considerably high contribution to the overall flavor development of baijiu. In this study, the nitrogenous compounds only appeared at stage C, and 13 species in total were detected. The sulfur compounds were only produced in the form of methyl mercaptan in the final stage, which indicates that in the latter stages, although the total types and concentrations of esters and alcohols changed very little, the overall flavor still changed significantly.
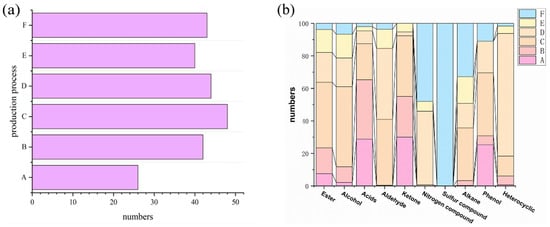
Figure 1.
Variation in volatile compounds in dissimilar production process stages of strong-flavor daqu. (a) Changes in the total amount of volatile compounds when producing strong-flavor daqu. (b) Changes in the types and quantities of volatile compounds in dissimilar production processes of strong-flavor daqu. A, B, C, D, E, and F represent samples from different periods, respectively.
The concentration changes in volatile compounds in the different production processes of strong-flavor daqu are exhibited in Table 1. We found that some compounds could be detected in all the daqu production processes, such as ethyl acetate, ethyl caproate, ethyl pentadecanoate, ethyl octadecenoate, etc. Ester compounds give baijiu an important fruit aroma but also have certain health effects, such as ethyl acetate, which has a protective effect on acute liver injury. Similar to dibutyl phthalate, nonanoic acid, octanoic acid, 4-hydroxy-2-butanone, and 2,5-di-tert-butylphenol could only be detected in A; 12 species, such as ethyl valerate, hexyl formate, and ethyl heptanoate, were only present in stage B; 10 compounds, including isopentyl formate, 2,8-dimethylundecanoic acid, and methyl ester, were only detected in phase C. The mere compounds in D were methallyl alcohol, crotonyl alcohol, 2-methylhexadecanoic acid, 11,14-eicosadienoic acid, methacrolein, 2-vinyl-2-butenal, (E,E)-2,4-hexadienal, 2-Ethyl-5-methyl-pyridine, ethyl 5-amino-1,2,3-thiadiazole-4-carboxylate, 3,5-dimethylpyrazole, methyl anthranilate, 3,8-dimethyl-undecane, and 2-ethyl-furan. The certain number of aldehydes in baijiu plays an indispensable role in regulating its aroma release [], but excessive amounts will lead to a rough and unpleasant taste. Acid compounds not only directly affect the overall flavor of baijiu, for example, the existence of excessive lactic acid will cause the body taste to be sour and astringent, but also, acid compounds are precursors to the formation of ester compounds []. In the maturing process of daqu, acid compounds react with alcohol compounds to form ester compounds and further regulate the overall smell and taste of the baijiu body. They play an important role in the formation of daqu and baijiu flavor. 2-Butyloctanol, methyl 2-(propanoylamino)benzoate, 7,9-dimethyl-hexadecane, 2,6,10-trimethyl-dodecane, 1-methyl-2-(3-methylpentyl)-cyclopropane, 1,3,5,7-cyclooctatetraene, decene, and dihydroaplotaxene were merely found in E, while ethyl isobutyrate, ethyl isovalerate, diethyl phthalate, isobutyric acid, 2-pyrrolecarbaldehyde, 2-methyl-pyrazine, 4,6-dimethyl-pyrimidine, 2,3-dimethyl-pyrazine, trimethyl-pyrazine, 1,2-dimethyl-hydrazine, methanethiol, 2,2,4,6,6-pentamethyl-heptane, 2,2,11,11-tetramethyl-dodecane, pentadecane, 4,6-dimethyldodecane, 4-methyl-nonane, 2,3,3-trimethyl-pentane, 2,3,4-trimethyl-hexane, and (E)-2,3-epoxydecane are volatile compounds that could merely be detected in finished products. Through these compounds, we can also find that the volatile compounds in strong-flavor daqu have undergone tremendous changes with the prolonged production duration. The concentration of different volatile compounds also varied considerably at different stages of maturation. Ethyl acetate, ethyl butyrate, ethyl caproate, ethyl caproate, and 22 other compounds showed an increasing and then decreasing pattern of change in their concentration during the maturation of the daqu. Ethyl valerate isopentyl formate, hexyl formate, ethyl heptanoate, ethyl nonanoate, diethyl succinate, and 46 other compounds were only present in the middle stages, while ethyl isobutyrate, ethyl isovalerate, and methanethiol were only produced in the final stage. Based on the complex changes in the above compounds and their crucial flavor contributions to the existence of daqu and baijiu, it is necessary to deeply analyze the law between the changes in substances and the maturity level of daqu.

Table 1.
Volatile compounds identified by HS-SPME-GC-MS in daqu.
3.2. The Variation Rule of Volatile Compounds in Daqu Production
3.2.1. PCA Analysis
In order to identify the distribution correlation of the sample, principal component analysis was conducted in accordance with the data of volatile compounds in daqu detected by HS-SPME-GC-MS. The principal component analysis score and load diagram are depicted in Figure 2. The score graph of the data obtained in the positive–negative mode indicates the trend of separation between groups. PC1 and PC2 are in Figure 2a. PC1 accounted for 32.7% of the total variance, while PC2 accounted for 21.9% of the total variance. The cumulative contribution rate of the first two PCs accounted for 54.6%, which demonstrates that they are both sufficient to explain the total variance in the dataset. PC1 and PC2 are positively correlated with the C and D daqu samples and are negatively correlated with the A, B, E, and F daqu samples, as illustrated in Figure 2a. As demonstrated by the experimental results, with the prolongation of the production duration, the variability between samples heightens. In the middle stage of production (8–12 days), the distance between C and the other data points in the PAC diagram is the greatest, not only indicating that the content of volatile components in the strong-aroma daqu changed most noticeably, but also indicating that the substance concentration changed considerably during the maturation process, which is extremely important for the maturation of daqu. Combined with Figure 2b, it was further resolved that the special state of the C-stage daqu was correlated with dozens of volatile compounds, such as acetic acid, ethyl acetate, 2,3-butanediol, isoamyl acetate, ethyl palmitate, and isobutyl alcohol. In the early stages of maturation, i.e., the A and B periods, which are in the same quadrant of the PCA diagram, there is an overall consistency, but there are still some gaps between them because of the large variation in compound species at this time. In contrast, D and E are both on the axes, again with some similarity, while F is more independently positioned, indicating that matured macrographs are easily distinguished from those in process, which is related to substances such as acetaldehyde, crotonaldehyde, 2-acetyl pyrrole, and 2,5-dimethyl-pyrazine. PCA analysis further proved the evidence of compound types and content changes in daqu samples at each maturity stage and associated them with specific compounds.
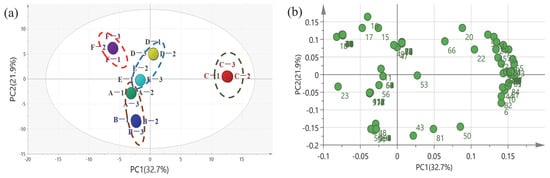
Figure 2.
PCA analysis based on volatile compounds. (a) Scores and (b) loading plot of PCA for classification of samples of strong-flavor daqu with different production durations. The specific compounds represented by the numbers are shown in Table S1. A, B, C, D, E, and F represent samples from different periods, respectively.
This was mutually verified with the changes in the quantity and concentration of the above compounds. The above results show that the number of compounds in stage C was the largest, and the change was the largest compared with the adjacent stage. The number of substances in stages D and E is similar, while stage F is separated from stage D and E due to the appearance of some compounds, which indicates that the determination of the mature state of daqu is scientific.
3.2.2. HCA Analysis
HCA was adopted to quickly assess the correlations between dissimilar stages of daqu (Figure 3). In this study, the Pearson (n) correlation was applied to measure the correlation and classification in line with the association between groups. The position of the line on the scale indicates the distance between the clusters. HCA identified six kinds of strong-flavor daqu in dissimilar production processes. D is the daqu with the greatest difference from other samples, indicating that the daqu in the middle stage of production is the sample with the greatest difference, which is also consistent with the PCA results. However, this is merely a preliminary analysis of strong-flavor daqu through unsupervised statistical analysis methods. It needs further analysis.
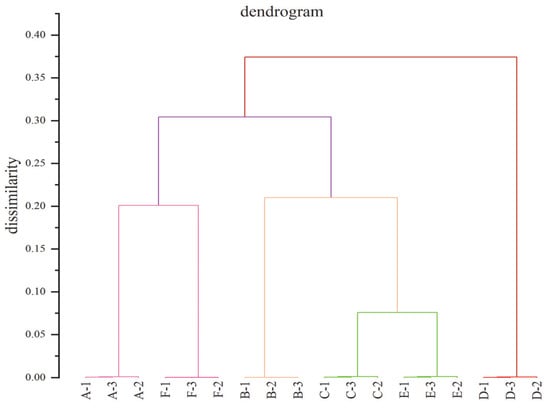
Figure 3.
Dendrograms of strong-flavor daqu. A, B, C, D, E, and F represent samples from different periods, respectively.
3.2.3. PLS-DA
In order to probe deeper into the effects of volatile compounds in daqu, the supervised multivariate statistical method PLS-DA can establish a useful statistical model of the data, which can be adopted as an analysis method to distinguish the differences between samples. This experiment uses the PLS-DA model to screen out specific volatile compounds on the basis of the volatile compounds detected in strong-flavor daqu in dissimilar production processes.
The most representative variables were identified through variable importance for the projection (VIP) value. For strong-flavor daqu, as exhibited in Figure 4a, it explains 84.5% of the total variance, R2= 99.9% and Q2 = 99.7%. Apart from D in the second quadrant and C in the third quadrant, the rest of the daqu samples are clustered in the fourth quadrant. This result is generally consistent with the PCA results. The samples in the mid-production period have the most noticeable difference compared to the samples in the early and late periods of production. Apart from that, as illustrated by the permutation plot results, the model does not overfit the data, the established PLS-DA model is valid, and the initial model is better than the predicted random arrangement model (Figure 4b). Aside from that, the permutation plot was applied to evaluate whether the PLS-DA model had a satisfactory predictive value. To put it in another way, it was used as the criteria for judging whether the model was successful. The permutation plot was compared on the basis of the fit of the data model for randomly sorted categorical variables (Y). This figure proves that the established PLS-DA model is valid, and there is no over-fitting of data because all the Q2-value blue boxes on the left are lower than the original point (standard) on the right or the blue Q2 point. The color regression line intersects the vertical axis (on the left) at or below zero, as exhibited in Figure 4b. As a result, the performance of the initial model PLS-DA is significantly better than the predicted random array model. The volatile compounds with high VIP values (VIP > 1.0) were selected in strong-flavor daqu, as exhibited in the red box in Figure 4c, namely, methacrolein, ethyl palmitate, acetic acid, methyl hexadecanoate, 9,12-octadecadienoic acid ethyl ester, 2-butenal, ethanol, ethyl oleate, ethyl acetate, phenethyl alcohol, 2-ethyl-furan, acetaldehyde, trimethyl-pyrazine, ethyl (9E)-9-octadecenoate, (R,R)-2,3-butanediol, ethyl caproate, 4-hydroxy-2-butanone, 2,3-butanediol, crotonaldehyde, 2-methyl-pyrazine, 2-methyl-2,4-hexadiene, 2-furanmethanol, 2,2,4,6,6-pentamethyl-heptane, and isobutyric acid. In order to control the entire maturation process, the present study further analyzed the substances present in the six maturation stages and summarized their concentration changes. Six key compounds were selected as indicators of the production process, namely, ethyl acetate, ethanol, phenethyl alcohol, (R,R)-2,3-butanediol, ethyl caproate, and 2,3-butanediol.
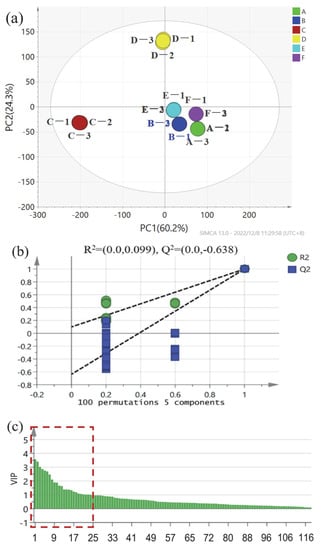
Figure 4.
Schemes follow the same formatting. PLS-DA analysis based on volatile compounds. (a) Scores, (b) permutation test, and (c) VIP plot constructed by adopting the concentrations of strong-flavor daqu’s volatile compounds. The consecutive numbers of the horizontal coordinates in (c) correspond to the different compounds, as shown in Table S2, the red boxed line indicates substances with VIP > 1. A, B, C, D, E, and F represent samples from different periods, respectively.
When the six indicator compounds are used to determine the different maturity stages of daqu and when ethyl acetate is detected in the range of 1000–1400 ng/g and ethyl caproate and 2,3-butanediol are both below 200 ng/g, the barley is still in the early stages of maturity and still needs a long time to be put into use. When the ethanol concentration is above 4000 ng/g and the concentrations of ethyl caproate as well as phenethyl alcohol are in the range of 500–2000 and 1200–2000 ng/g, respectively, it means that the daqu is in the middle stage of maturity, reaching the level of 8–12 days in this study. When the concentrations of ethyl acetate and ethyl caproate are below 600 and 160 ng/g, respectively, and the concentration of (R,R)-2,3-butanediol is around 200 ng/g, the sample is ready to produce baijiu.
4. Conclusions
In this study, a total of 115 volatile compounds were identified by HS-SPME-GC-MS in strong-flavor daqu. Subsequently, the types and concentrations of the compounds in different stages were analyzed. It was found that the volatile components change during the production processes. Aside from that, the acids and aldehydes showed a trend of increasing first and then decreasing. Moreover, all compounds changed very little in the last stage, reaching a balanced and stable system. It is worth noting that both nitrogenous and sulfur-containing compounds appeared in the later stages. Apart from that, PCA and PLS-DA analysis models were constructed to further explain the differences between daqu at dissimilar maturation stages and relate them to compounds. Finally, six indicator compounds, ethyl acetate, ethanol, phenethyl alcohol, (R,R)-2,3-butanediol, ethyl caproate, and 2,3-butanediol, were further screened by PLS-DA analysis combined with the concentration changes in compounds during the production processes, which were used for speedily determining the maturity level of the daqu. Through a systematic and in-depth analysis, this study provides a theoretical basis for the optimization of the production process and the speedy judgment of the maturity degree of strong-flavor daqu so as to improve the quality and stability of daqu and baijiu and further promote the mechanized production process of baijiu. Nevertheless, the level of aroma compounds has not been involved so far. On that account, the indicator compounds can be further verified and optimized in line with the aroma components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8120720/s1, Table S1: The numbers in the loading graphs of PCA represent the specific information of the compounds; Table S2: Compounds represented by abscissa numbers and their VIP values in VIP plot.
Author Contributions
Conceptualization and writing, methodology, S.Y.; software and visualization, J.D.; visualization, S.L.; visualization, L.X.; conceptualization, writing, review, editing, and funding acquisition & supervision, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32172340), Postgraduate research ability Improvement Program of Beijing Technology and Business University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Sun, B. Effect of fermentation processing on the flavor of baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Sun, B.; Sun, X.; Sun, J.; Zheng, F.; Huang, M. The occurrence of propyl lactate in Chinese Baijius (chinese liquors) detected by direct injection coupled with gas chromatography-mass spectrometry. Molecules 2015, 20, 19002–19013. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, B.; Liu, S.; Zou, N.; Yang, J.; Zhong, Z.; Zhang, X.; Song, L.; Qin, Y.; Pan, C. Residue levels of five grain-storage-use insecticides during the production process of sorghum distilled spirits. Food Chem. 2016, 206, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, B. Baijiu, Chinese liquor, History, classification and manufacture. J. Ethn. Foods 2016, 3, 19–25. [Google Scholar] [CrossRef]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Luo, L. Environmental factors affecting microbiota dynamics during traditional solid-state fermentation of Chinese Daqu starter. Front. Microbiol. 2016, 7, 1237. [Google Scholar] [CrossRef]
- He, G.; Huang, J.; Wu, C.; Jin, Y.; Zhou, R. Bioturbation effect of fortified Daqu on microbial community and flavor metabolite in Chinese strong-flavor liquor brewing microecosystem. Food Res. Int. 2020, 129, 108851. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Fan, Y.; Huang, X.; Han, B. Biochemical characterisation and dominance of different hydrolases in different types of Daqu—A Chinese industrial fermentation starter. J. Sci. Food Agric. 2018, 98, 113–121. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Tan, Y.; Wang, H.; Yang, F.; Chen, L.; Hao, F.; Lv, X.; Du, H.; Xu, Y. Effect of Pichia on shaping the fermentation microbial community of sauce-flavor Baijiu. Int. J. Food Microbiol. 2021, 336, 108898. [Google Scholar] [CrossRef]
- Yang, L.; Fan, W.; Xu, Y. Metaproteomics insights into traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2506–2529. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.; Fu, Z.; Wang, W.; Xu, Y.; Teng, C.; Zhang, C.; Yang, R.; Sun, B.; Li, X. Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism. Food Res. Int. 2020, 129, 108837. [Google Scholar] [CrossRef]
- Jin, Y.; Li, D.; Ai, M.; Tang, Q.; Huang, J.; Ding, X.; Wu, C.; Zhou, R. Correlation between volatile profiles and microbial communities, A metabonomic approach to study Jiang-flavor liquor Daqu. Food Res. Int. 2019, 121, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Cates, V.; Meloan, C. Separation of sulfones by gas chromatography. J. Chromatogr. 1963, 11, 472–478. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Duan, J.; Zhao, J.; Li, H.; Sun, J.; Huang, M.; Sun, B. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. 2021, 365, 130430. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, S.; Zhang, G.; Xu, L.; Li, H.; Sun, J.; Huang, M.; Zheng, F.; Sun, B. Exploration of key aroma active compounds in strong flavor Baijiu during the distillation by modern instrument detection technology combined with multivariate statistical analysis methods. J. Food Compos. Anal. 2022, 110, 104577. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Xu, J.; Huang, M.; Wang, J.; Chao, J.; Wu, J.; Sun, H.; Ding, H.; Ye, H. Characterization of key odorants in Langyatai Baijiu with Jian flavour by sensory-directed analysis. Food Chem. 2021, 352, 104577. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, H.; Lu, H.; Wu, M.; Lin, M.; Zhang, C.; Zhao, Z.; Li, W.; Zhang, C.; Li, X.; et al. Characterization of an aspergillus niger for efficient fatty acid ethyl ester synthesis in aqueous phase and the molecular mechanism. Front. Aquat. Microbiol. 2022, 12, 820380. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liu, X.; Li, X.; Zhang, C.; Li, W.; Sun, X.; Wang, W.; Sun, B. Discovery and development of a novel short-chain fatty acid ester synthetic biocatalyst under aqueous phase from Monascus purpureus isolated from Baijiu. Food Chem. 2021, 338, 128025. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Zhang, Y. Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J. Agric. Food Chem. 2007, 55, 9956–9962. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, C.; Li, X.; Sun, B.; Eldin, A.; Jia, Y. A combinational optimization method for efficient synthesis of tetramethylpyrazine by the recombinant Escherichia coli. Biochem. Eng. J. 2018, 129, 33–43. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, P.; Sun, J.; Jia, Y.; Wan, C.; Zhou, Q.; Huang, F. Investigation of volatile thiol contributions to rapeseed oil by odor active value measurement and perceptual interactions. Food Chem. 2022, 373, 131607. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Li, Q.; Wang, Y.; Ren, G. Metabolic Pathway of main alcohols and aldehydes in baijiu and its relationship with health drinking. Liquor-Mak. Sci. Technol. 2021, 88–90. [Google Scholar] [CrossRef]
- Guo, X.; Fan, E.; Ma, B.; Li, Z.; Zhang, Y.; Zhang, Z.; Chen, Y.; Xiao, D. Recent progress in micro components of Chinese Baijiu. Food Sci. 2020, 41, 267–276. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).