Abstract
The cultivation of Plectonema terebrans BERC10 in wastewater and integrating the wastewater-derived biomass followed by its processing for multiple products in a biorefinery could help in achieving environmental sustainability and cost effectiveness. This study evaluated the resource recovery potential of the cyanobacterium Plectonema terebrans BERC10 from urban wastewater followed by the cascading processing of the biomass into multiple bioproducts. The annual biomass productivity ranged from 0.035–0.064 gL−1d−1 and contained 40–46% lipids and 20–38% protein. The cascading processing of the biomass resulted in multiple products, including 53 mgg−1 of high-value pigments and high-quality biodiesel in accordance with American and European standards. The pigment-free and de-fatted residual biomass was used as a sole feedstock (30–70 gL−1) to produce enzymes and mycoproteins via fungal fermentation employing Aspergillus niger and Aspergillus oryzae. Interestingly, A. oryzae produced 28 UmL−1 of α-amylase and the final residues were mycoproteins after 96 h. Furthermore, the strain removed 80–90% of total phosphorous, 90–99% of total nitrogen, and significantly lowered the COD, BOD, and TDS of urban wastewater. The data demonstrated that P. terebrans has substantial potential for resource recovery and could become a candidate for a wastewater-derived algal biorefinery.
1. Introduction
Microalgae are a prospective feedstock for producing biodiesel and value-added bioproducts. One of the main advantages of utilizing algal biomass is its possessing the highest ability to fix environmental CO2 when compared to other photosynthetic systems including terrestrial plants; moreover, their cultivation does not need arable land. Additionally, microalgae could be the best option for the cleanest resource recovery of wastewater-derived nutrients and the subsequent recycling of the treated water [1]. However, despite their huge potential, there are still many hurdles in the way of achieving economic viability and application on an industrial scale [2]. The cultivation of cyanobacteria on wastewater needs rigorous knowledge about nutritional variation in the urban wastewater, which strongly affects growth and metabolite biosynthesis. Hence, detailed knowledge of the impact of nutrient variation on growth and metabolite production is needed prior to upscaling and commercialization of any potent algal strain.
In addition, achieving commercial robustness is hindered by the inefficient downstream processing which is often focused on a single product (biodiesel in most cases), where 40–60% of the biomass goes to waste along with extraction solvents, which not only compromises the cost-effectiveness, but also raises environmental concerns. Therefore, developing a sustainable, robust, and cost-effective method for downstream processing is essential. Recently, it has been proposed that complete utilization of the algal biomass in a zero-waste paradigm could be a cost-effective and viable approach to achieving the industrial and environmental targets of the microalgae biorefinery [3]. The algal biorefinery is an emerging, cost-effective, and sustainable concept for the conversion of algal biomass into various high-value products with minimal generation of waste. The concept of single-product recovery is not a cost-effective approach. Selective biorefining of biomass and cascading processing into multiple products is a crucial requirement for the commercialization of different algae-based products [4]. The cascading biorefinery concept involves the integration of different techniques to sequentially extract various major and intermediate products from biological feedstock while producing minimum waste [5].
Resource recovery from wastewater by cultivating cyanobacteria is a global interest that offers a beneficial approach to the circular bioeconomy. This concept was first proposed by Ostwald in the 1950’s when algae were used to provide dissolved oxygen for the decomposition of organic waste [6]. Nutrient removal by algae facilitates wastewater treatment by eutrophication mitigation, carbon sequestration, and production of valuable biomass. Globally, 61–2310 million m3 of wastewater is being produced on daily basis, which is becoming challenging to handle [7]; alternatively, it could be employed as a resource to produce algal biomass in a cost-efficient manner. Direct discharge of wastewater without any treatment disturbs biological diversity and ultimately disrupts the integrity of life in water bodies. Based on the available data, less than 40% of wastewater receives treatment. The percentage is higher (about 70%) in developed countries but lower in developing countries (8%) [8]. Various filamentous cyanobacterial species such as Oedogonium, Spirogyra, Klebsormidium, and Cladophora have been reported to treat wastewater. Chlorella species have shown a removal rate of 82% for NH4-N and about 70% for phosphorous. C. vulgaris showed 80% COD removal efficiency [9].
Seasonal variation causes a continued change in the chemical composition of the wastewater which resultantly affects the growth of the algal strain being employed for the wastewater treatment. Nitrogen and phosphorous are important macronutrients required by algal cells. Phosphorous is an important part of the energy-storing molecule ATP, while nitrogen accounts for 7–20% of the dry cell weight and plays a vital role in producing nucleic acids and proteins [10]. Nutrient depletion shifts the metabolic path of the cells, e.g., scarcity of macronutrients causes increased synthesis of storage lipids. For example, nitrogen starvation resulted in higher lipid and starch accumulation in the same way that the increase in involved nitrogen supports the proteins and synthesis of phycobilins. In this study, our lab isolated Plectonema terebrans BERC10 grown in wastewater throughout the year (12 months) with varying nutrient compositions, while other conditions, including the light duration, intensity, and temperature, were controlled to assess the feasibility of utilizing urban wastewater as a low-cost growth media for indoor cultivation. The impact of nutrient variation was studied on biomass and metabolite productivity. The biomass produced was processed in a multiproduct zero-waste paradigm to produce multiple products. According to our best knowledge, this is the first study that analyzed the impact of nutrient variation on growth and metabolite composition using real urban wastewater spanning over 12 months. The data showed exciting findings which could be helpful to the establishment of a wastewater-derived multiproduct biorefinery in the best technical, economical and environmental scenarios.
2. Materials and Methods
2.1. Strain Selection
A fully characterized lab strain Plectonema terebrans BERC10 was acquired from the PhycolBank at Government College University Faisalabad, Pakistan. The strain selection was based on the preliminary experiments of P. terebrans BERC10 on a lab scale that showed promising results in terms of growth kinetics, remarkable biochemical composition, and low contamination risks during cultivation. Additionally, the filamentous nature of the strain favored easy harvesting and could easily be collected by mesh, thus making the harvesting economically feasible [11]. Therefore, the strain was grown in urban wastewater (without sludge), collected from wastewater disposal throughout the year (on a monthly basis), at lab scale under controlled conditions (temperature 30–32 °C, light intensity 1700 lux) to check the impact of the seasonally varying concentrations of different nutrients on growth kinetics and metabolite composition.
2.2. Experimental Setup
Small-scale, open raceway pond-like glass boxes were used for cultivation (from October 2020 to October 2021) due to easy management and economic feasibility. The glass boxes (length = 30 cm, width, and depth = 15 cm) have a total capacity of about 7 L. A working volume of 3.5 L was used to ensure easy mixing and proper light penetration. No additional CO2 was provided during cultivation. The cultures were agitated manually 2–3 times a day to ensure even distribution of nutrients and gases.
2.3. Types and Composition of Media Used for Cultivation
Urban wastewater was used as a low-cost cultivation medium. The untreated wastewater (urban) was collected from the wastewater disposal site of Faisalabad city (93P4 + PQW, Nawaban Wala, Faisalabad, Pakistan) in different months of each season. The Modified BG11 media (MBG11) was used as a control (from here will be referred to as synthetic wastewater or MBG11) and had increased nitrogen, phosphorous, and carbon concentrations. The wastewater was analyzed before and after cultivating the Plectonema terebrans BERC10 strain, as reported previously [12] to assess the wastewater treatment potential of the strain BERC10.
2.4. Cultivation of Media Dynamics
The pH is a crucial factor for contamination control during cultivation. Moreover, a change in pH also influences the growth of cyanobacteria because each strain has its optimum pH on which it shows maximum growth. Therefore, to study the impact of wastewater-cultivated cyanobacteria on media dynamics, the pH was measured regularly at specific intervals during the cultivation period.
2.5. Growth Kinetics and CO2 Fixation Potential
The biomass was harvested, lyophilized, and stored at −20 °C for further use. The growth parameters namely biomass production, biomass productivity, specific growth rate, and doubling time were computed by using the following equations (Equations (1)–(5))
Biomass production was calculated in (gL−1), where Wy is the biomass produced (dried biomass basis) and Wx is the inoculum size (dried biomass basis).
where my and mx are the final and initial biomasses, respectively, while ty and tx are the final and initial time. Biomass productivity was calculated in gL−1d−1.
where μ is the specific growth rate (day−1)
Td is the doubling time of the cells.
The CO2 fixed (taken from the water and atmosphere) by the cyanobacterial biomass was calculated using Equation (5), where factor 1.83 means that 1 kg of algae biomass can fix 1.83 kg of CO2.
PCO2 = 1.83 × Pbiomass
2.6. Cascading Processing of the Algal Biomass
For efficient biomass processing, the cascading biorefinery approach was focused to ensure the highest resource recovery with the lowest amount of waste production. This approach involved the recovery of various products in a cascading manner. Firstly, phycobilins were extracted from wastewater-cultivated biomass. After phycobilin extraction, pigment-free biomass was processed for lipid extraction where lipids were trans-esterified to produce biodiesel. Finally, pigment-free de-oiled residual biomass was completely valorized to industrial enzymes and proteins through fungal fermentation. The following sections describe the detailed methods of the cascading biorefinery route employed in this study.
2.6.1. Extraction and Estimation of Phycobilins from the Wastewater-Cultivated Biomass
In the first step, high-value metabolites (phycobilins) were extracted. For phycobilin extraction, 50 mg of lyophilized sample was taken and treated with 0.1 M phosphate buffer, vortex, and kept for 12–24 h at room temperature under dark conditions. After the treatment, a sample was centrifuged at maximum speed. Pigment containing supernatant was separated and its absorbance was measured at wavelengths λ 652 nm, λ 620 nm, and λ 562 nm to calculate allophycocyanin, phycocyanin, and phycoerythrin concentrations, respectively (mgmL−1) [13].
2.6.2. Extraction and Estimation of Lipid and Biodiesel Production
The pigment-free biomass was subjected to lipid extraction. Lipids were extracted using Bligh and Dyer’s method [14]. Briefly, 50 mg of the sample (oven-dried at 65 °C) was taken and mixed with 7.5 mL of chloroform:methanol (2:1) solution. The sample was kept overnight at 37 °C with continuous agitation. On the next day, it was centrifuged, the supernatant was separated in a separate tube and the lipids were re-extracted from the residual biomass. For phase separation, the solution of chloroform, methanol, and sodium chloride (1%) was added with a 1:1:0.9 ratio for 1 mL supernatant. Afterward, the solution was centrifuged and lipids containing chloroform (lower organic layer) were separated and kept at 57 °C for evaporation until constant weight. Lipids were estimated (Equation (6)) gravimetrically.
Later, the lipids were processed for FAME analysis as described previously [11].
2.6.3. Estimation of Carbohydrate Content
The carbohydrate content of pigment-free de-oiled biomass was measured by phenol-sulfuric acid [15]. Firstly, 20 mg of oven-dried biomass was treated with 1 mL of 72% H2SO4 for 10 min for the lysis of cell walls with vigorous mixing after regular intervals. The sample was centrifuged for 5 min at 13,000 rpm for the separation of sugars from the biomass. The sample was diluted (to 4% concentration) with double-distilled water. At the next step, 0.5 mL of the diluted sample was taken and 0.25 mL of 5% phenol was added for protein digestion and kept at room temperature for 3 min. In the next stage, the sample was further treated with concentrated H2SO4 and kept in a shaking incubator for 15 min at 37 °C. The formation of brown color indicated the presence of sugars. The concentration of carbohydrates was found by comparing the absorbance (at λ 490 nm) with the standard curve. The carbohydrate content was calculated by using the following formula
where D.F is the dilution factor and concentration is the amount of glucose estimated based on the standard curve.
2.6.4. Estimation of the Protein Content of the Biomass
Proteins were extracted by following the hot alkaline method while their quantification was carried out using the micro-biuret method [16,17]. Ten mg of the oven-dried sample was placed in 1 mL NaOH (0.5 N) solution. The sample was heated at 80 °C for 10 min with inverted mixing and allowed to cool at room temperature. Afterwards, the first treatment sample was centrifuged at 13,000 rpm for 5 min and the supernatant was separated into a test tube. Extraction was repeated twice under the mentioned conditions to re-extract the proteins from the residual biomass, although the final step was performed at 95 °C.
The micro-biuret method was used for the quantification of proteins, where 1 mL of CuSO4 solution (0.21% CuSO4 in 30% NaOH) was mixed with 2 mL of protein extract (supernatant). Optical density was measured by using a UV-Vis spectrophotometer (AE-S60-2U, A&E Lab, London, UK) at λ 310 nm.
Protein content was calculated (Equation (8)) by comparing it with the standard curve.
2.6.5. Fungal Fermentation of the Pigment-Free De-oiled Residual Biomass
The second stage of cascading biorefinery was focused on the utilization of residual biomass after lipid extraction. Residual biomass was subjected to fermentation using A. oryzae and A. niger as fermenting strains as described previously [3]. Both strains are considered GRAS (Generally Recognized as Safe) for human and animal consumption. Before the experiment, fungal strains were acclimatized on algal biomass for 72 h. Briefly, different concentrations of cyanobacterial biomass including 30 gL−1, 50 gL−1, and 70 gL−1 were used in the fermentation experiment. A known amount of the biomass was placed into 50 mL Erlenmeyer flasks containing 20 mL reaction volume prepared using sterilized distilled water. The inoculum of 10 μL, from acclimatized fungal strains, was added to the reaction mixture. The PDA broth and algal biomass (without fungal inoculum) served as positive and negative controls, respectively. All flasks were incubated in a shaking incubator at 30 °C with a constant shaking speed of 180 rpm. Sterilized glass beads (3–5) were added to each flask to ensure uniform fungal growth. The experiment was continued for 120 h, where 500 μL of sample from each flask was taken at 24 h, 48 h, 72 h, 96 h, and 120 h, centrifuged, and the supernatant was processed to check the enzyme activity. After 120 h, supernatant containing α-amylase was separated into a separate flask and the pellet was oven-dried and processed for biochemical analyses to track the changes in protein and carbohydrate contents before and after fermentation.
2.6.6. Enzyme Assay for the Quantification of α-Amylase Activity
The enzyme assay was used to determine the activity of enzymes, as described previously [18]. Briefly, 100 μL of the enzyme solution was incubated at 45 °C for 30 min in the presence of 1.0 mL of substrate (1% starch solution) and 1.0 mL sodium acetate buffer. The α-amylase reacts with starch and converts into dextrin, maltose, and glucose subunits. This is called a quenching reaction. In the next step, the concentration of these reducing sugars was determined using the DNS method [19]. Accordingly, 2.0 mL of DNS (3,5-dinitrosalicylic acid) solution was mixed with a 2.1 mL quenched reaction mixture. The solution was boiled for 10 min and was allowed to cool at room temperature. The blank reaction was prepared by adding 100 μL H2O, 2.0 mL of sodium acetate and 2.0 mL DNS. After cooling, absorbance was measured at λ550 nm. A glucose standard curve was used to determine the glucose standard factor. The unit of enzyme activity was calculated by using (Equation (9)).
where ΔA = OD at λ 550 nm, QRM = Quenched reaction mixture, and G.S factor = Glucose standard factor = 2.02 μmol glucose
One unit of α-amylase activity is specified as the amount of enzyme needed to release 1.0 μmol of reducing sugars.
3. Results and Discussion
3.1. Variation in the Nutrients and Their Impact on the Growth
In this study, wastewater analyses showed a significant variation in the nutrient composition of wastewater during different seasons. The variable consumption of water directly influenced the nutrient composition of wastewater during different seasons (Table 1). Nitrogen and phosphorous are the key nutrients whose optimum ratio is required for higher algal growth. Nitrogen is an essential component of different biomolecules such as nucleotides and amino acids, while phosphorous is mainly involved in the energy storage and formation of DNA and RNA subunits.

Table 1.
Physicochemical properties of the wastewater calculated in mgL−1 during different seasons.
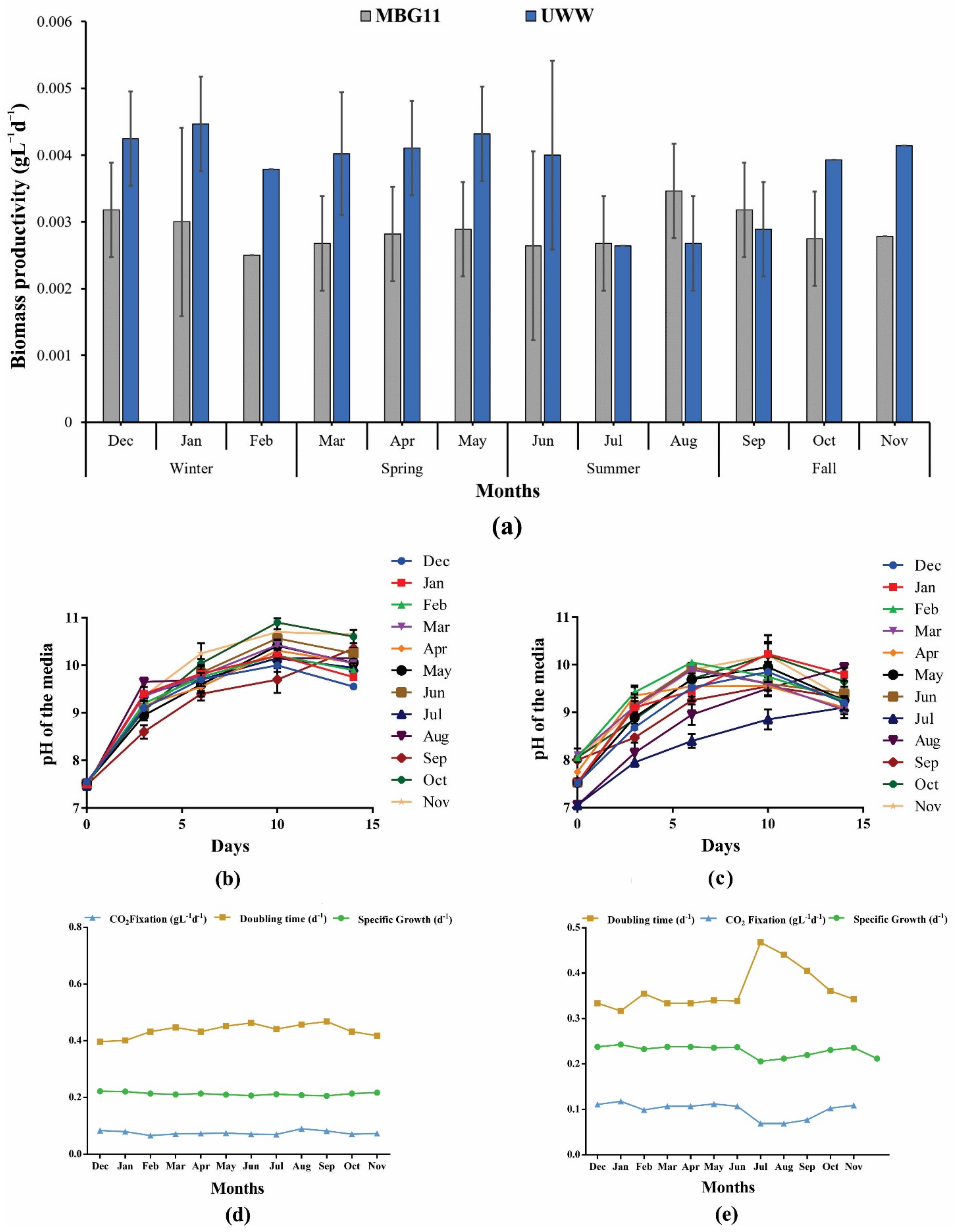
During this study, the P. terebrans BERC10 cultivated in UWW outperformed the MBG11 cultivation in terms of growth, where almost 1.3-fold higher biomass was produced in the case of UWW during the winter and spring seasons (Figure 1a). The findings from this study demonstrated that the wastewater was more suitable for cyanobacterial growth and could support significant biomass production. Maximum biomass productivity of 0.064 gL−1d−1 of BERC10 was observed in UWW while 0.048 gL−1d−1 in the case of synthetic wastewater (MBG11) (Figure 1b). Similarly, previous studies reported that algal strains performed better in wastewater when compared to the control media [20,21,22]. Previously, Spirulina platensis, a renowned cyanobacterium, grown on wastewater produced 0.058 gL−1d−1 of biomass [23], whereas BERC10 from this study produced 0.9-fold higher biomass. In contrast, Leptolyngbya sp. ISTCY101 grown in wastewater attained a relatively higher biomass productivity of 0.085 gL−1d−1 (1.32-fold higher) [20]. Interestingly, in the current research, P. terebrans BERC10 showed 2.66-fold, 2.78-fold, and 3.55-fold higher biomass productivity when compared to the biomass productivities of Chlorella pyrenoidosa (0.024 gL−1d−1), Anabaena ambigua (0.023 gL−1d−1), and Scenedesmus abundans (0.018 gL−1d−1) during phycoremediation of dairy wastewater, respectively [22]. Likewise, in this study, BERC10 showed 1.25-fold higher biomass productivity when compared to the Nostoc sp. LS04 (0.051 gL−1d−1) when cultivated in wastewater [24]. On the other hand, wastewater-cultivated Tetraselmis sp. showed 1.0-fold higher biomass productivity [25] when compared to P. terebrans BERC10.
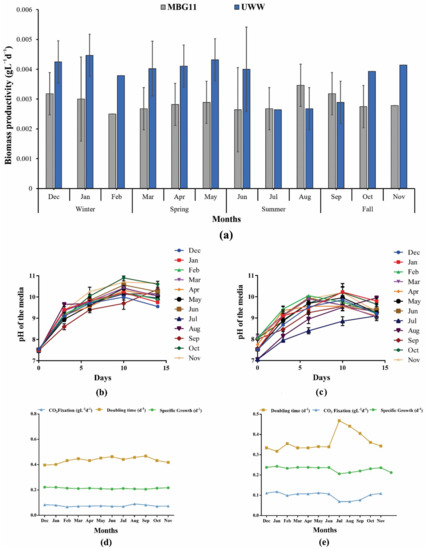
Figure 1.
Biomass productivity in response to different growth conditions, its growth kinetics, and pH variation trend: (a) biomass productivity by P. terebrans BERC10 in urban wastewater (UWW) and MBG11; (b) variation in pH in response to MBG11 cultivation; (c) variation in pH in response to UWW cultivation. Relationship of CO2 fixation, doubling time, and specific growth when the strain was cultured in (d) MBG11 media; (e) urban wastewater.
Results from the current study indicated a strong impact of nutrient composition on biomass production and variation in metabolite content. Alternatively, lower biomass production was observed in the wastewater collected during the summer seasons, which was almost half that collected in other seasons due to dilution in nutrient composition. Previously reported studies also demonstrated a decrease in biomass production due to the lower nutritional value of growth media [22,26]. Wastewater analysis endorsed the results and showed that the presence of sufficient nutrients in the wastewater collected during the winter and spring seasons positively affected biomass production, while nutrient scarcity was observed in the summer and fall seasons, which resulted in lower biomass production. The impact of nutritional variation on biomass productivity, CO2 fixation, specific growth, and doubling time are shown in Figure 1. The control used in the current study showed a consistent trend throughout the year (Figure 1d); in contrast, reduced growth and increased doubling time can be seen in the summer season (Jun–September) in the case of UWW. Additionally the strain performed better than control during the rest of the months (Figure 1e).
3.2. Growth-Dependent pH Variation of the Cultivation Media
During this study, the variation in the pH of the cultivation media was the same in both MBG11 and UWW. P. terebrans BERC10 modified the pH of the media and tended to increase with growth regardless of its initial value. There was an elevation from pH 8 to pH 10 until the 10th day and then there was a slightly decreasing trend observed until the 14th day, which indicated that BERC10 preferred to grow under alkaline conditions. Other microorganisms were unable to grow at this pH; therefore, this feature will prove helpful for contamination-free cultivation at a large scale (Figure 1b,c). The tendency of cyanobacteria to grow at alkaline pH is commonly reported [27,28]. It was also observed that enhanced photosynthetic activity is responsible for an increase in pH value [29]. The carbon concentration mechanism could also be a responsible factor for an increase in pH during the cultivation period [3,30].
3.3. Impact of Nutrition Variation on Protein Biosynthesis
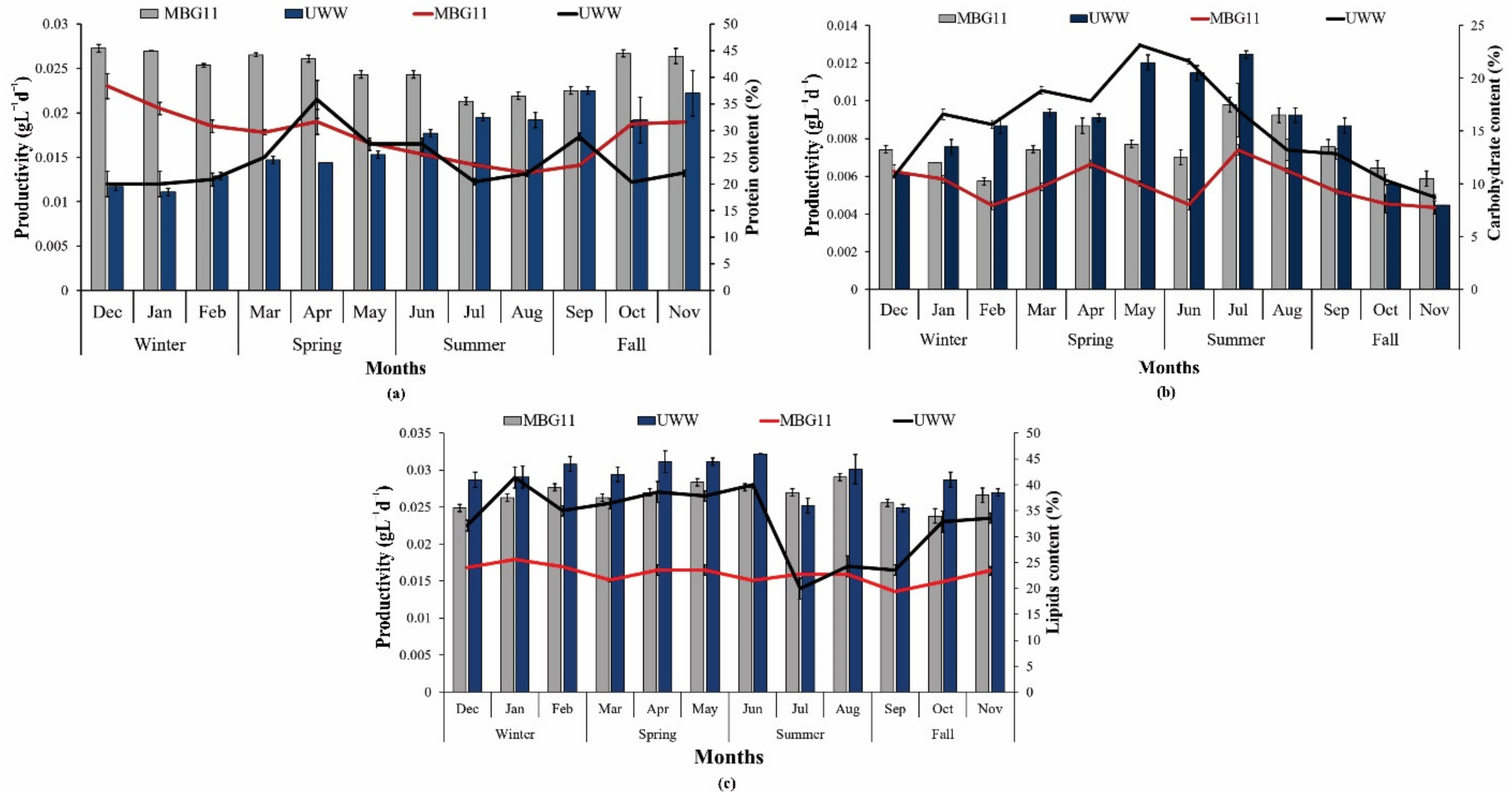
In the present study, P. terebrans BERC10 showed higher protein content (35–45%) when cultured in MBG11 throughout the year compared to the protein content produced in UWW due to the high nitrogen content in the control media. The lowest protein content (18–26%) was observed in the biomass produced in the wastewater collected during the winter and spring seasons, which contained 1.5 mgL−1 and 4.2 mgL−1 total nitrogen, respectively. Higher protein content (30–37%) was detected in the biomass produced in wastewater collected during summer and fall, which could be due to a 2-fold higher nitrogen concentration in the wastewater when compared to the wastewater collected during the winter and spring seasons (Figure 2a). Nitrogen is an important nutrient that is directly involved in protein synthesis; therefore, the protein content was low during winter and spring due to nitrogen deficiency in wastewater produced during these seasons. Another study reported the cultivation of Spirogyra sp. on wastewater and achieved a protein content of 20% [31]. Nannochloropsis sp. grown in wastewater reported a protein content of 40% [32]. Variation in nutrient composition was the major factor behind this change because wastewater analyses showed a higher nitrogen concentration (TN) (7.1 mgL−1) in summer and fall (10.9 mgL−1). Therefore, a higher concentration of nutrients favored the biosynthesis of proteins in this study, which is consistent with previous studies that reported a positive impact of nitrogen on protein biosynthesis [33]. A previously reported study also observed the direct correlation of protein synthesis with the concentration of nitrogen [34].
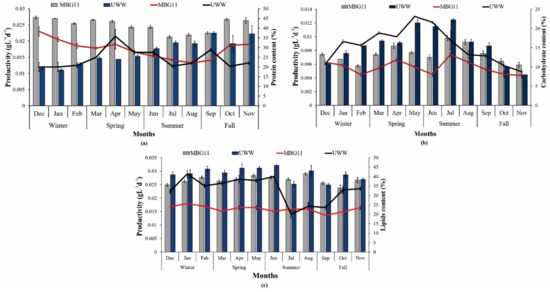
Figure 2.
Production and variations in metabolite composition: (a) protein content (%) and productivity (gL−1d−1) in response to nutrient variation in wastewater; (b) carbohydrate content (%) and productivity (gL−1d−1) in response to nutrient variation in wastewater; (c) lipid content (%) and productivity (gL−1d−1) in response to nutrient variation in wastewater. The bars show content (%) while line curves show the productivity (gL−1d−1) of metabolites.
3.3.1. Impact of Nutrition Variation on Carbohydrate Biosynthesis
Evaluation of carbohydrate synthesis of P. terebrans BERC10 in UWW and MBG11 indicated that nutrients also had a strong impact on the biosynthesis of carbohydrates. The maximum carbohydrate content of 22% was obtained in July, with a productivity of 0.013 gL−1d−1 in UWW, while the highest carbohydrate content of 18% with 0.0097 gL−1d−1 productivity was shown in the MBG11 media (Figure 2b). In most months, particularly during May, June, July and August, UWW-produced biomass had greater carbohydrate content (16–22%) and productivity when compared to the carbohydrate content (14–17%) of biomass produced in MBG11. The results from this study were in line with the previously reported study where phosphorous deprivation resulted in a higher starch content [35]. Wastewater analyses of these months indicated that phosphorus level in these months was lower due to dilution and higher consumption of water by the public. In contrast, the higher nitrogen concentration was reported in the fall and summer seasons because these seasons had relatively high temperatures, so the consumption of (drinking) water and food also increased in humans as well as in livestock animals, therefore the excretion of more urine and feces (containing nitrogenous matter) caused a higher nitrogen content in urban wastewater. The lowest carbohydrate content observed in the fall season ranged from 8–15% due to the availability of sufficient nitrogen compared to other seasons, as the availability of nitrogen had a negative impact on carbohydrate production [36,37]. The concentration of nitrogen and phosphorus was the reason behind the variations in the production of carbohydrates [37]. The lowest carbohydrate content was produced during the fall season. Another finding [38] supported the fact that nitrogen scarcity favors carbohydrate biosynthesis. Previously, wastewater-based cultivation also reported a higher carbohydrate content range from 40–50% [31]. The results from this study were according to the previously reported results, which found that Acaryochloris marina BERC03, Oscillatoria sp. BERC04, and Pleurocapsa sp. BERC06 had high carbohydrate contents of 25%, 32%, and 36% in UWW, respectively, compared to the control [11].
3.3.2. Impact of Nutrition Variation on the Biosynthesis of Lipids
Nutrient variations showed a substantial impact on lipid biosynthesis. In this study, lipids, content, and productivity of P. terebrans BERC10 were higher in UWW over the whole year except in July compared to with MBG11 media. In the case of UWW, a maximum lipid content of 46% with a productivity of 0.057 gL−1d−1 were observed in June, while the highest lipid content of 42% with 0.046 gL−1d−1 were observed in MBG11 media (Figure 2c). In the case of MBG11, the lipid content varied (36–42%) over the whole year, while UWW-cultivated biomass contained (38–46%) lipid content throughout the year. The higher lipid content in UWW involved nutrient scarcity that alters the metabolic pathways in either lipids or carbohydrate biosynthesis. In this study, nutrition starvation favored lipid synthesis. Several other studies reported higher lipid content in the wastewater-produced biomass than in the control media. For example, Chlorella sorokiniana SXAU-04 and Desmodesmus sp. PW1 achieved higher lipid content in wastewater than BG11 media [39]. Chlorella protothecoides reported 47–51% lipid content when cultivated in wastewater [40]. In another study, Cyanobacterium sp. IPPAS B-1200 produced 22% lipids when cultured in wastewater and was suitable for biodiesel production [41]. Microcystis protocystis also produced a significant amount (41.5%) of lipids in response to nutrient variations [42]. Nitrogen limitation causes three major changes: lowering of cellular content in the thylakoid membrane, stimulation of acyl hydrolase and increased hydrolysis of phospholipids. Moreover, nitrogen depletion causes the activation of diacylglycerol acyltransferase, which is involved in the synthesis of triglyceride (TAG) [43]. The lipids content of Nostoc sp. LS04 was also enhanced in response to nutrient stress [24]. Several other studies have reported that nitrogen depletion acts as an effective strategy for improved lipid biosynthesis [44,45]. However, some other findings observed a strong inverse correlation between nitrogen and lipids production [42,46]. These alternative reports indicate that the correlation of nitrogen stress with lipid biosynthesis should be carefully assessed through detailed studies involving omics in the future.
3.3.3. Biodiesel Properties of Wastewater Cultivated Biomass
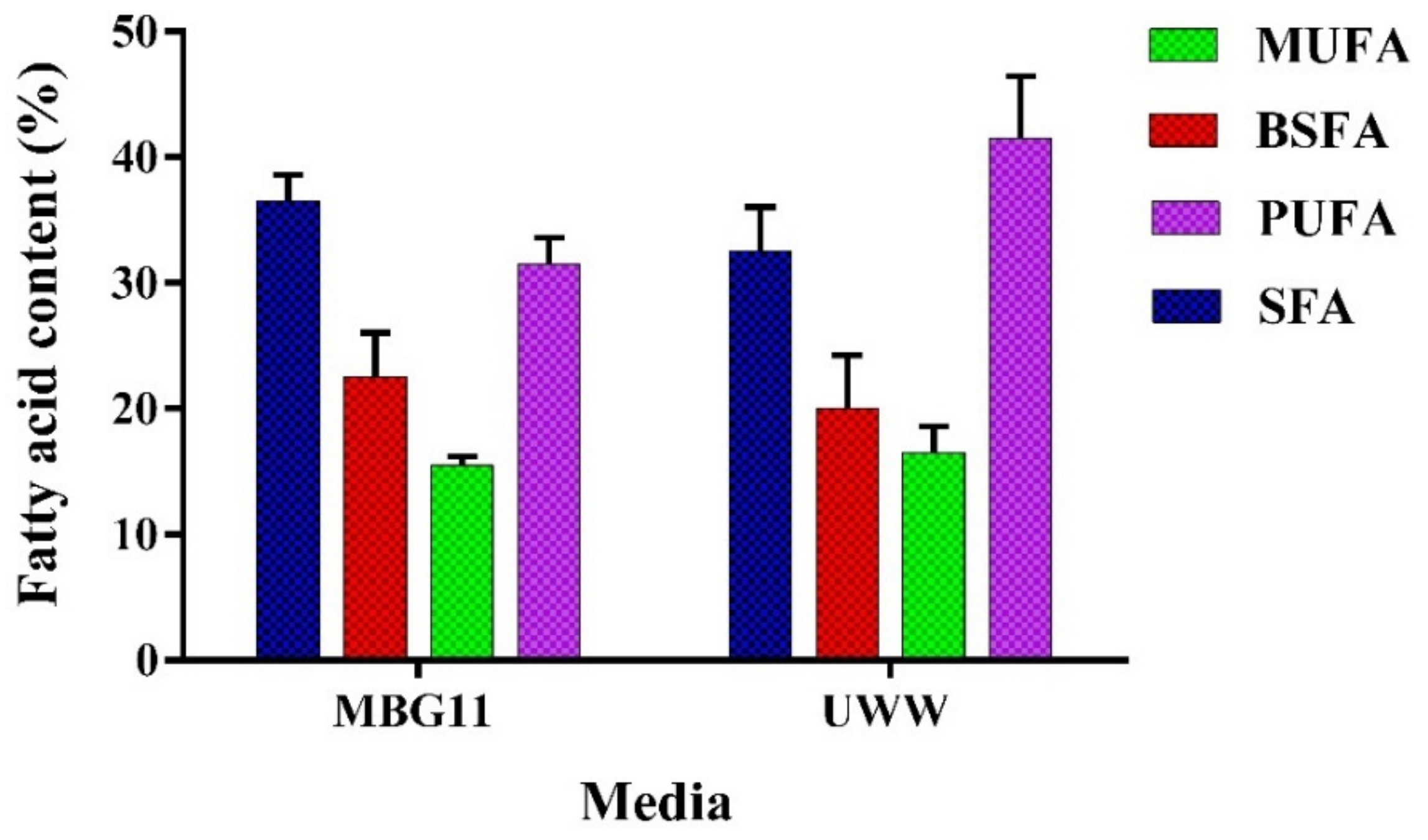
An ideal cyanobacterial strain for biodiesel production should have significant biomass production with a higher lipid content (20–40%) and should have a suitable fatty acid composition [47]. P. terebrans BERC10 performed well in the current study in terms of biomass and lipid biosynthesis; thus, the next part of the research involved the study of the fatty acid composition of the extracted lipids. The composition of fatty acid esters is an important parameter that affects biofuel properties. The most important properties include the cetane number (CN), iodine value (IV), cloud point (CP), and cold filter plugging point (CFPP). The cetane number (CN) determines the fuel quality in terms of combustion and ignition time. A high cetane number enhances the performance of the engine and ignition properties. Saturated fatty acids have a direct effect on the cetane number. The saturated fatty acids were almost the same in the biomass produced in MBG11 and UWW, measuring 38% and 35%, respectively. The iodine value (IV) is the measure of unsaturation in fuel that ultimately determines the oxidation stability of the biodiesel. Oxidation stability is inversely related to iodine value. Cloud point (CP) shows the temperature range at which the wax crystal formation begins that can cause problems in fuel lines; the lower CP range is appropriate for good-quality biofuel. The cold filter plugging point (CFPP) represents the flow performance of the fuel at a lower temperature. The concentration of unsaturated fatty acids mainly predicts the value of CFPP. The lipids from MBG11 contained 33% PUFA, while UWW contained a 40% degree of unsaturation. The balanced composition of saturated and unsaturated fatty acids is essential to ensure biodiesel properties comparable to the present biodiesel standards. A high concentration of polyunsaturated fatty acids may cause polymerization by oxidation [48]. Interestingly, the biodiesel produced from the BERC10 biomass grown in UWW had no adverse impact on biodiesel properties (Figure 3).
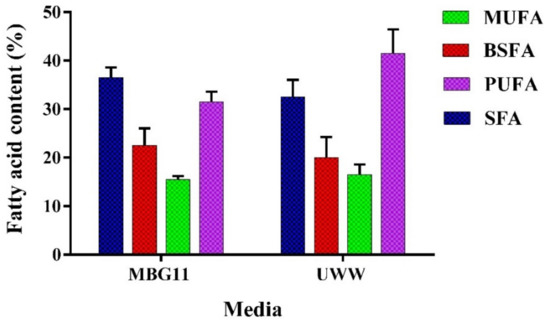
Figure 3.
FAME analysis of the biodiesel derived from the lipids extracted from P. terebrans BERC10 biomass produced in MBG11 and UWW. Mono-unsaturated Fatty Acids = MUFA, Branched Saturated Fatty Acids = BSFA, Poly Unsaturated Fatty Acids = PUFA, and Saturated Fatty Acids = SFA.
The fatty acid profile showed an elevated proportion of C16-C18 fatty acids, where 12.5 mgg−1 and 3.3 mgg−1 of palmitic acid and oleic acid, respectively, were produced in the lipids extracted from UWW-grown biomass, while a slight decrease was observed in the lipids derived from MBG11-produced biomass, which contained 10 mgg−1 and 3 mgg−1 palmitic and oleic acid, respectively in this study. The biodiesel properties of the lipids from MBG11-produced biomass were better when compared to the UWW-grown biomass due to the balanced composition of MUFA and PUFA. The MUFA showed a slight increase in the case of UWW compared to MBG11 media due to nitrogen deprivation. In the case of UWW, the estimated biodiesel properties showed a higher cetane number (68.9), appropriate oxidation stability (7.9 h), and lower iodine number (73.6 gI2/100g). Despite a small negative impact of UWW cultivation, the biodiesel properties were in line with the standards set by the European and American Society of Testing and Materials (ASTM), as shown in Table 2. The results from the current study were consistent with the previously reported studies related to the cultivation of cyanobacteria in wastewater [21,41,49].

Table 2.
Biodiesel properties of algal biomass grown in MBG11 and UWW and their comparison with standards.
3.3.4. Phycobilin Production during Different Seasons
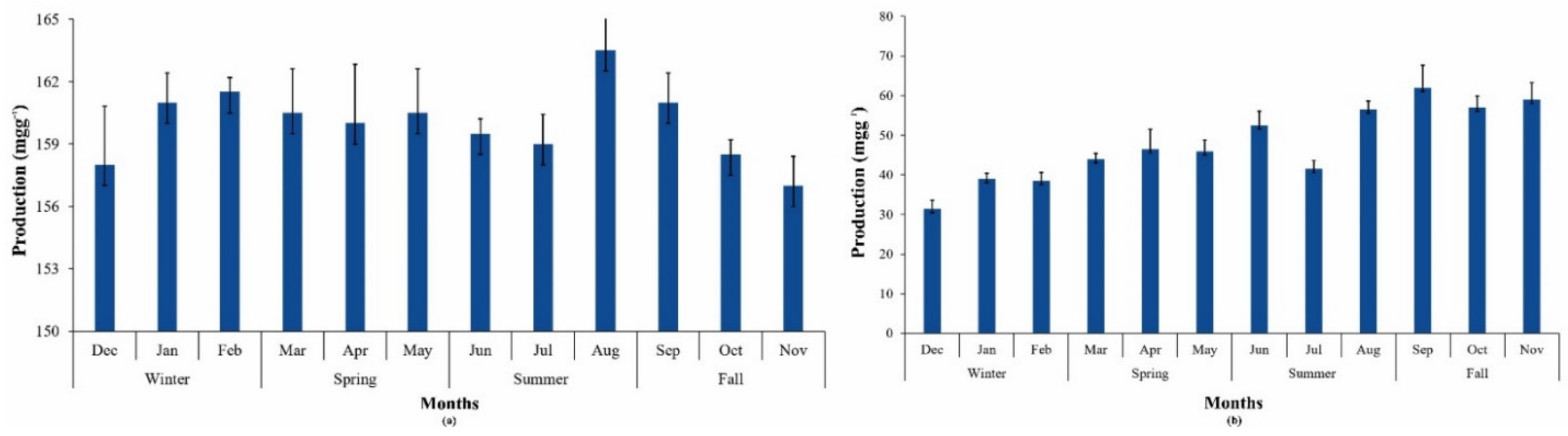
Cyanobacteria are known to produce a variety of high-value pigments (phycocyanin, phycoerythrin, and allophycocyanin) that have numerous applications in the health, food, and cosmetics industries. Phycobilin production from P.terebrans BERC10 cultivated in MBG11 media was higher than in UWW (Figure 4a). Phycobilin yield in UWW ranged from 30–62 mgg−1 throughout the year, which improved with an increasinglevel of nitrogen in wastewater during this study, while a phycobilin yield of 157-163 mgg−1 was obtained in the case of MBG11 (Figure 4a,b). Another study reported the utilization of cyanobacterial consortia for wastewater treatment, which yielded 13–18 mgg−1 of phycobilins [50]. Cultivation of Acaryochloris marina BERC03 and Oscillatoria sp. BERC04 in wastewater produced 65 mgg−1 phycobilins [11]. Phycobilin yield has a direct relationship to the availability of nutrients. Due to the deficiency of nutrients in wastewater during this study, the phycobilin production was lower than other reported strains grown in standard media. Many studies reported the negative impact of wastewater on phycobilin production. For instance the phycobilin production of Acaryochloris marina BERC03 and Oscillatoria sp. BER04 was decreased when cultivated in wastewater in comparison to standard media [11]. Moreover, cyanobacteria degrading their PBs during nutrient-depleted conditions to meet their nutrient requirements seems a possible reason for the lower production of phycobilins [51]. Production of these high-value pigments varies from species to species. Various studies have reported that supplementation with sodium bicarbonate and nitrogen significantly increases their production [52].
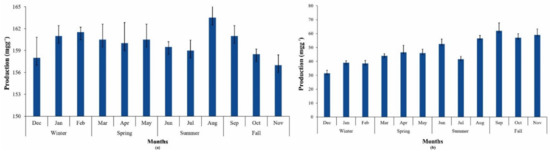
Figure 4.
Phycobilin yield during different seasons and their variations throughout the year in (a) MBG11; (b) UWW.
3.4. Wastewater Treatment Potential of Plectonema terebrans BERC10
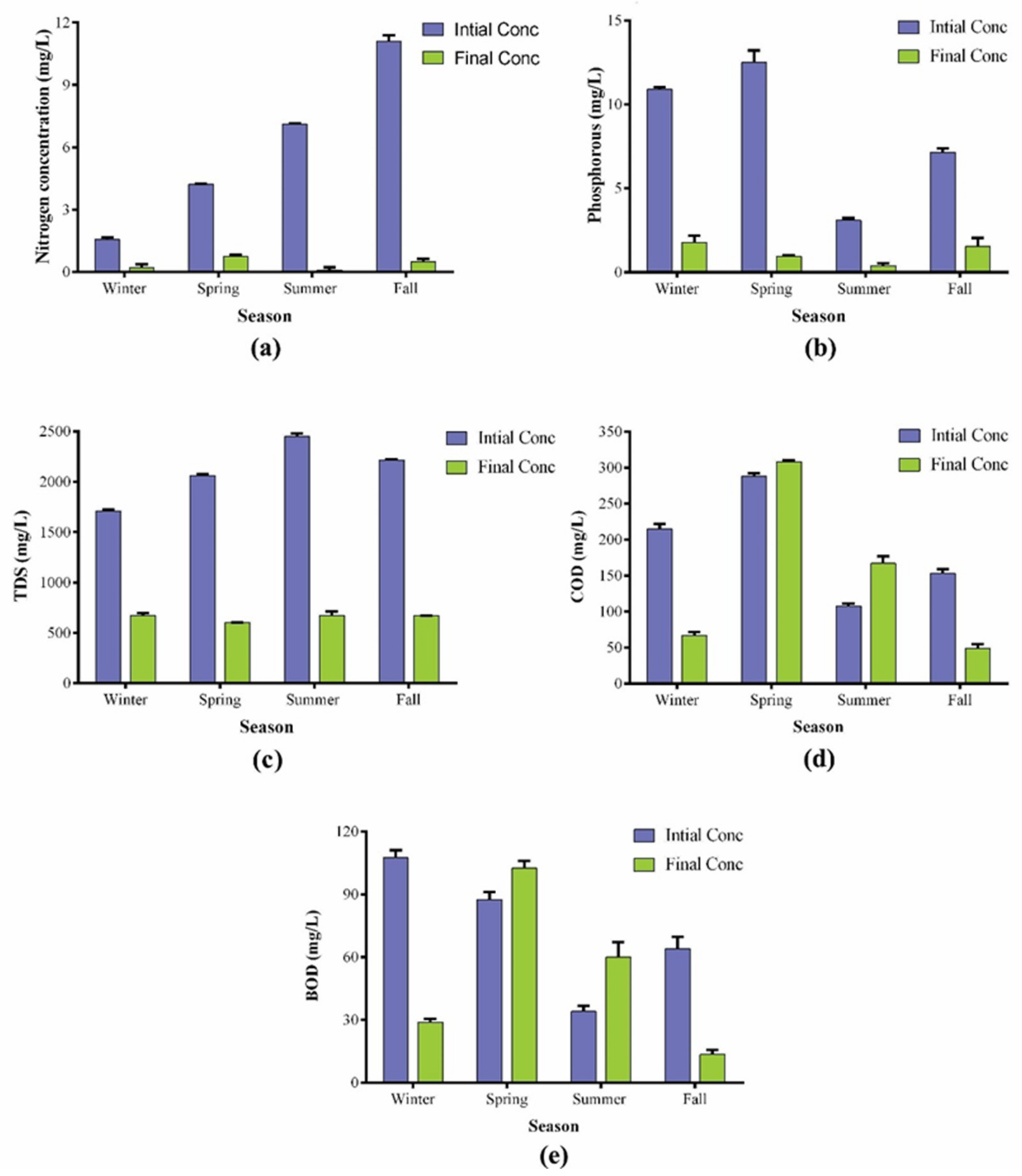
The utilization of wastewater as cultivation media offers an eco-friendly and cost-effective approach to biodiesel production and wastewater treatment. Post-cultivation wastewater analyses showed an enormous potential of the BERC10 strain to treat wastewater. Higher concentrations of N and P in waterbodies cause eutrophication, which poses serious threats to aquatic organisms. P.terebrans BERC10 in this study showed surprising results as it removed most of the nutrients during growth. The nutrient removal efficiency varied according to the variation in the nutrient composition of the wastewater. The initial concentration of total phosphorous was 11 mgL−1 in winter, 12 mgL−1 in spring, 3 mgL−1 in summer, and 7 mgL−1 in the fall season; this was reduced to 1.78 mgL−1 in winter, 0.95 mgL−1 in spring, 0.4 mgL−1 in summer and 1.55 mgL−1 in the fall season after treatment. Therefore, the removal rate of total phosphorous (TP) was shown to be 85%, 92%, 88% and 80% during winter, spring, summer, and fall respectively (Figure 5). A similar trend was observed in the case of total nitrogen (TN), where BERC10 removed 90%, 85%, 99% and 95%, which correspond to the final values of 0.2 mgL−1, 0.6 mgL−1, 0.1 mgL−1 and 0.5 mgL−1 of the total nitrogen in the treated water collected during winter, spring, summer and fall, respectively. Likewise, P. kessleri NKG021201 was shown to have a substantial potential for wastewater treatment, which removed 98% of nitrogen and 69% of phosphorous [53]. Previously, Chlorella zofingiensis cultivated in wastewater showed a nutrient removal rate of 93% of nitrogen and 90% phosphorous [54]. Oedogonium intermedium removed 36% of nitrogen and 62% of phosphorous [55]. Auxenochlorella protothecoides cultivation in wastewater resulted in a 90% reduction in COD while 60% and 80% removal of nitrogen and phosphorous, respectively [56]. In this study, the strain also performed well in the case of other parameters of wastewater analysis such as COD, BOD, and TDS. It lowered the COD and BOD of wastewater in the winter and fall seasons (by more than 70%). The initial values of COD in winter and fall seasons were 210 mgL−1 and 149 mgL−1, which were decreased to 66 mgL−1 and 49 mgL−1, respectively, while in the same way, BOD was also decreased from 110 mgL−1 and 39 mgL−1 to 29 mgL−1 and 13.5 mgL−1. On the other hand, the water collected during the spring and summer seasons showed an unexpected increase in the BOD to 102 mgL−1 and 60 mgL−1 from their initial values of 90 mgL−1 and 32 mgL−1, respectively. On the other hand, COD increased from 291 mgL−1 and 105 mgL−1 to 308 mgL−1 and 166 mgL−1 in the spring and summer seasons, respectively. Increased residence time and nutrient depletion would have resulted in the secretion of various organic compounds during these seasons, which could be the possible reason for an increase in COD and BOD in this study. Similarly, many other studies also reported that algal cultivation caused an increase in the COD and BOD of wastewater after cultivation [3,57]. Detailed studies would be required in the future to elucidate the possible causes and to find a solution to this challenge.
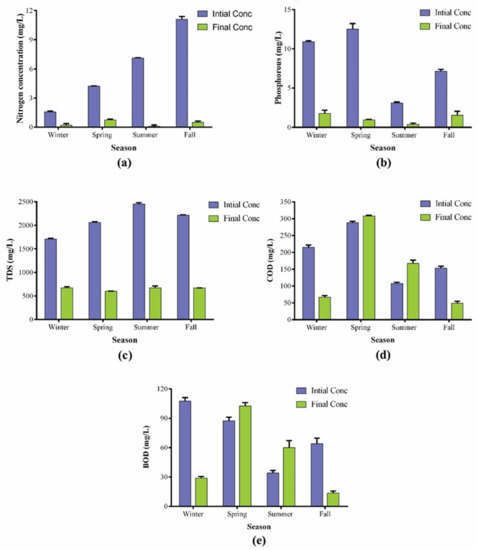
Figure 5.
Post-cultivation water analyses of the water collected during different seasons: (a) total nitrogen (TN); (b) total phosphorous (TP); (c) total dissolved solids (TDS); (d) chemical oxygen demand (COD); (e) biological oxygen demand (BOD).
Interestingly, the final concentrations of total nitrogen (TN) and total phosphorous (TP) were lower than 10 mgL−1 and 2 mgL−1, which lie within the permissible range set by the European Commission Directive 98/15/EEC for the safe discharge of treated wastewater [58]. Therefore, after the cultivation, the wastewater was suitable for irrigation purposes. The TDS of wastewater also decreased significantly because the initial concentrations of TDS were 1720 mgL−1, 2070 mgL−1, 2470 mgL−1 and 2210 mgL−1 which were decreased to 675 mgL−1, 603 mgL−1, 675 mgL−1 and 669 mgL−1 after treatment, for the wastewater collected during winter, spring, summer and fall, respectively, while the final concentration ranged from 600–700 mgL−1 during all seasons in the current study. The TDS value of the drinking water ranges from 150–250 mgL−1, so further processing would be required to make it suitable for drinking purposes.
3.5. Cascading Processing of the Biomass in Multiproduct Paradigm
After cost-effective cultivation, efficient utilization of algal biomass is imperative for the economic and environmental sustainability of the biorefinery. For effective downstream processing, the cascading biorefinery concept was applied to the synthesis of multiple bioproducts with minimal waste generation. Wastewater-grown biomass was processed in a cascading manner for maximum resource recovery.
3.5.1. Product 1: Phycobilins
The chemical composition of the biomass which was used as a feedstock is given in the previous sections. Phycobilins were selected as the first target product due to their sensitivity and economic importance. In this study, a phycobilin yield of 53 mgg−1 was obtained in biomass grown in wastewater with a 15% loss in biomass.
3.5.2. Product 2: Lipids Extraction from Pigment-Free Biomass for Biodiesel Production
As reported previously, lipids were the main constituent and comprised almost 50% of the total dry weight. At the second stage of the cascading process, lipids were extracted from depigmented and oven-dried biomass. It was interesting to see that there was not a significant difference between the lipid yield of algal feedstock and its production in depigmented biomass, where 43% of the lipid yield was obtained at the cost of a 20% loss in biomass. Biodiesel properties interpreted from FAME analysis showed that biodiesel properties were similar to the standards set by the European and American labs.
3.5.3. Product 3: Fermentation of the Pigment-Free Defatted Residual Biomass
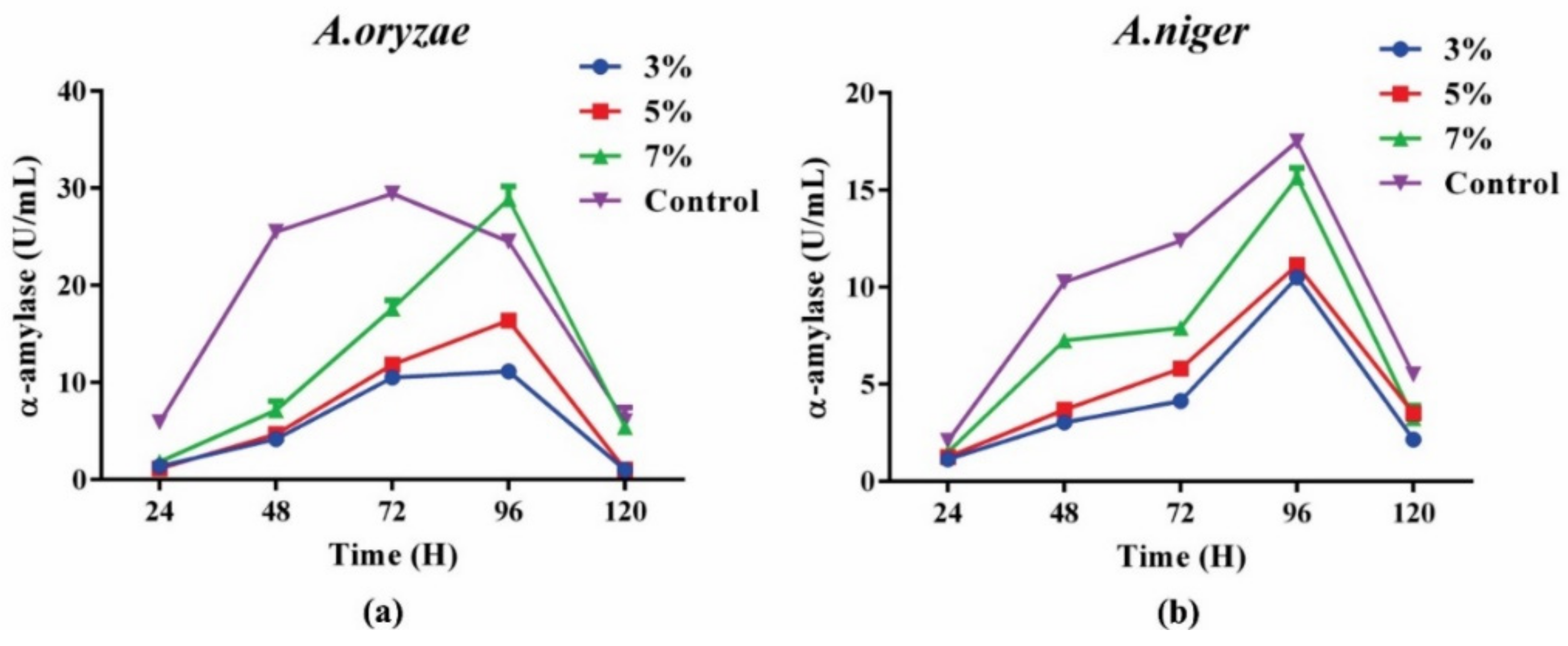
Adequate exploitation of residual biomass is important via efficient downstream processing in an algal biorefinery in order to achieve cost-effectiveness. The fate and possible conversion route of residual biomass depend upon its metabolite composition. After pigment and lipid extraction, the compositional analyses showed that 17% of the carbohydrate content was in the biomass. Residual biomass had enough carbohydrates to utilize in an efficient way. Fermentation of the carbohydrate-rich residual biomass showed that A. oryzae outperformed A. niger in terms of α-amylase production. In the case of A. oryzae, an enzyme production of 28 UmL−1 was achieved with a concentration of 70 gL−1 when compared to A. niger, which produced almost 15 UmL−1 after 96, which indicated that A. niger produced 1.86-fold higher enzyme units compared to the A. oryzae (Figure 6). However, both strains performed better in the control group (PDA media) when compared to their enzyme production in the residual biomass. It was shown that both strains attained maximum enzyme production at 96 h, after that a decreasing trend was observed that showed the efficient utilization of carbon source for fungal growth during 1st 96 h. The compositional analysis after fermentation showed a decreasing trend of carbohydrate levels, while an increasing trend of proteins was observed. The increase in protein content indicated the biotransformation of algal carbohydrates into fungal proteins. Another study reported 4.6-fold higher α-amylase production (131 UmL−1) by using the residual algal biomass as a sole substrate [3], which indicates that this part may need further optimization in the future.
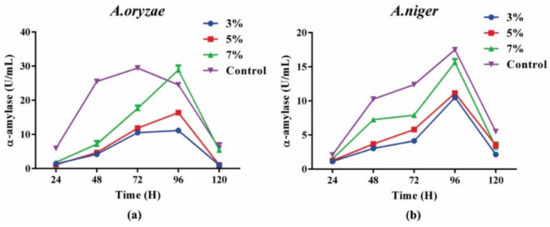
Figure 6.
α-amylase enzyme production through fungal fermentation of pigment free de-oiled biomass: (a) by A. oryzae; (b) by A. niger.
A similar observation regarding the utilization of wastewater-cultivated biomass for multiple applications has been recorded where the de-oiled biomass of Nostoc sp. LS04 was used as a bio-stimulant for plant growth [24]. Many other studies reported α-amylase production through the utilization of different feedstocks. For instance, cassava was reported to be a substrate for the production of α-amylase where 14 UmL−1 enzymes were achieved [59]. Various other studies reported the use of meat and brewery wastewater as a substrate for α-amylase production [60]. The cascading approach for biomass processing was applied in a study where dairy wastewater-grown biomass (with 22% lipids and 38% carbohydrates) was subjected to lipid extraction at the first step. At the second step, de-oiled biomass was acid-hydrolyzed to release the reducing sugars. These reducing sugars were used for bioethanol production via fermentation by using Saccharomyces cerevisiae [61].
4. Conclusions
The strain Plectonema terebrans BERC10 showed a great potential to grow in varying nutrient-starved conditions along with maximum resource recovery from urban wastewater. Additionally, it achieved a remarkable metabolite composition with 46% lipid content, which indicated the suitability of the strain to become feedstock for biodiesel. The successful integration of the cascading biorefinery concept resulted in the efficient recovery of multiple products with minimal waste production. The complete biotransformation of the BERC10 biomass into several bioproducts, including high-value pigments (53 mgg−1); lipid-derived, better quality biodiesel (40–46%); and industrially important α-amylase enzyme (28 U mL−1) using urban wastewater as a sole growth media, may help to cope with the restrictions imposed by the high cost involved in the production and downstream processing of the algal biomass. The wastewater treatment technology by microalgae and complete valorization of biomass in the biorefinery concept still have many challenges that include cost, higher contamination risk, and inefficient downstream processing. So, the ability of microalgae to grow in harsh outdoor conditions and its potential to remove wastewater pollutants should be further studied. Moreover, the potential of genetically modified microalgae may also be investigated to analyze their adaptation under unfavorable conditions and removal potential of different pollutants. In addition, techno-economic and life cycle assessment-based studies will be conducted in the future for the pilot-scale studies in collaboration with governmental and non-governmental wastewater treatment agencies to assess the economic and environmental feasibility of employing P. terebrans BERC10 as a candidate for a wastewater-derived multiproduct biorefinery.
Author Contributions
Conceptualization, M.A.M.; methodology, M.N.H., D.B. and S.Y.A.Q.; software, M.G.; validation, M.G.; formal analysis, C.-G.L. and D.B.; investigation, M.N.H.; re-sources, T.A.T., C.-G.L., D.B., P.-L.S., S.Y.A.Q. and M.G.; data curation, S.Y.A.Q.; writing—original draft preparation, M.N.H.; writing—review and editing, M.A.M., T.A.T., C.-G.L. and P.-L.S.; visualization, P.-L.S.; supervision, M.A.M.; project administration, M.A.M.; funding acquisition, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Higher Education Commission of Pakistan via grant No. NRPU 7300.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the participation of Aqib Zafar Khan, Muhammad Usman, and Muhammad Adnan-ul-Haq in helping to collect wastewater during different seasons, and Sana Malik for helping with experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, B.R.; Mathimani, T.; Sudhakar, M.; Rajendran, K.; Nizami, A.-S.; Brindhadevi, K.; Pugazhendhi, A. A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew. Sust. Energ. Rev. 2021, 138, 110649. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource recovery through bioremediation of wastewaters and waste carbon by microalgae: A circular bioeconomy approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.-G.; Mehmood, M.A. A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; De Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J.; Gotaas, H.; Ludwig, H.F.; Lynch, V. Algae symbiosis in oxidation ponds: III. Photosynthetic oxygenation. Sewage Ind. Wastes 1953, 25, 692–705. [Google Scholar]
- Renuka, N.; Ratha, S.K.; Kader, F.; Rawat, I.; Bux, F. Insights into the potential impact of algae-mediated wastewater beneficiation for the circular bioeconomy: A global perspective. J. Environ. Manag. 2021, 297, 113257. [Google Scholar] [CrossRef]
- Batool, M.; Shahzad, L. An analytical study on municipal wastewater to energy generation, current trends, and future prospects in South Asian developing countries (an update on Pakistan scenario). Environ. Sci. Pollut. Res. 2021, 28, 32075–32094. [Google Scholar] [CrossRef]
- Liu, J.; Pemberton, B.; Lewis, J.; Scales, P.J.; Martin, G.J. Wastewater treatment using filamentous algae—A review. Bioresour. Technol. 2020, 298, 122556. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Mishra, S. Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 179, 565–572. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Liu, C.-G.; Musharraf, S.G.; Siddiqui, A.J.; Khan, F.; Tarbiah, N.I.; Gull, M.; Rashid, U.; Mehmood, M.A. Characterization of a newly isolated cyanobacterium Plectonema terebrans for biotransformation of the wastewater-derived nutrients to biofuel and high-value bioproducts. J. Water Process. Eng. 2021, 39, 101702. [Google Scholar] [CrossRef]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rausch, T. The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 1981, 78, 237–251. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D. A micro-biuret method for estimating proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef]
- Aleem, B.; Rashid, M.H.; Zeb, N.; Saqib, A.; Ihsan, A.; Iqbal, M.; Ali, H. Random mutagenesis of super Koji (Aspergillus oryzae): Improvement in production and thermal stability of α-amylases for maltose syrup production. BMC Microbiol. 2018, 18, 200. [Google Scholar] [CrossRef]
- Tayyaba, H.; Muhammad, H.R.; Muhammad, R.J.; Asma, A. Gamma ray mediated mutagenesis of Phialocephala humicola: Effect on kinetics and thermodynamics of a-amylase production. Afr. J. Microbiol. Res. 2012, 6, 4639–4646. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, I.S. Evaluation of cyanobacterial endolith Leptolyngbya sp. ISTCY101, for integrated wastewater treatment and biodiesel production: A toxicological perspective. Algal Res. 2015, 11, 294–303. [Google Scholar] [CrossRef]
- Shahid, A.; Usman, M.; Atta, Z.; Musharraf, S.G.; Malik, S.; Elkamel, A.; Shahid, M.; Alkhattabi, N.A.; Gull, M.; Mehmood, M.A. Impact of wastewater cultivation on pollutant removal, biomass production, metabolite biosynthesis, and carbon dioxide fixation of newly isolated cyanobacteria in a multiproduct biorefinery paradigm. Bioresour. Technol. 2021, 333, 125194. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Pareek, N. Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.; Gao, Y.; Zhao, H. Nutrients removal and recovery from saline wastewater by Spirulina platensis. Bioresour. Technol. 2017, 245, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Kamaraj, B.; Chi, N.T.L.; Cornejo, P. Cultivation of Nostoc sp. LS04 in municipal wastewater for biodiesel production and their deoiled biomass cellular extracts as biostimulants for Lactuca sativa growth improvement. Chemosphere 2021, 280, 130644. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.; Agustini, C.B.; Trierweiler, L.F.; Gutterres, M. Influence of period light on cultivation of microalgae consortium for the treatment of tannery wastewaters from leather finishing stage. J. Clean. Prod. 2020, 263, 121618. [Google Scholar] [CrossRef]
- Zayadan, B.K.; Sadvakasova, A.K.; Usserbayeva, A.A.; Bolatkhan, K.; Baizhigitova, A.M.; Akmukhanova, N.R.; Sidorov, R.A.; Sinetova, M.A.; Los, D.A. Waste-free technology of wastewater treatment to obtain microalgal biomass for biodiesel production. Int. J. Hydrog. Energy. 2017, 42, 8586–8591. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, D. Biomass and lipid productivities of cyanobacteria-Leptolyngbya foveolarum HNBGU001. BioEnergy Res. 2021, 14, 278–291. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Dailianis, S.; Charalampous, N.; Stefanidou, N.; Moustaka-Gouni, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Brewery wastewater treatment using cyanobacterial-bacterial settleable aggregates. Algal Res. 2020, 49, 101957. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G.; Vayenas, D.V. Two-step treatment of brewery wastewater using electrocoagulation and cyanobacteria-based cultivation. J. Environ. Manag. 2020, 265, 110543. [Google Scholar] [CrossRef]
- Larsdotter, K. Wastewater treatment with microalgae—A literature review. Vatten 2006, 62, 31. [Google Scholar]
- Ge, S.; Madill, M.; Champagne, P. Use of freshwater macroalgae Spirogyra sp. for the treatment of municipal wastewaters and biomass production for biofuel applications. Biomass Bioenerg. 2018, 111, 213–223. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Productivity and community response along an ammonia gradient in cultured wild marine microalgae, using wastewater-derived nutrients for cost-effective feedstock production. J. Appl. Phycol. 2021, 33, 2933–2945l. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Ma, Y.; Chen, P.; Zheng, H.; Doan, Y.T.; Liu, H.; Chen, C. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour. Technol. 2016, 201, 33–40. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Azimi, Y.; Yu, D.; Wu, Y.-H.; Hu, H.-Y. Effects of nitrogen and phosphorus concentrations on the growth of microalgae Scenedesmus. LX1 in suspended-solid phase photobioreactors (ssPBR). Biomass Bioenerg. 2018, 109, 47–53. [Google Scholar] [CrossRef]
- Yao, C.-H.; Ai, J.-N.; Cao, X.-P.; Xue, S. Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl. Microbiol. Biotechnol. 2013, 97, 6099–6110. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: Effect of nutrients limitation and photoperiods. New Biotechnol. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Kushwaha, D.; Upadhyay, S.; Mishra, P.K. Growth of cyanobacteria: Optimization for increased carbohydrate content. Appl. Biochem. Biotechnol. 2018, 184, 1247–1262. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García, J. Assessing the potential of soil cyanobacteria for simultaneous wastewater treatment and carbohydrate-enriched biomass production. Algal Res. 2020, 51, 102042. [Google Scholar] [CrossRef]
- Cui, H.; Ma, H.; Chen, S.; Yu, J.; Xu, W.; Zhu, X.; Gujar, A.; Ji, C.; Xue, J.; Zhang, C. Mitigating excessive ammonia nitrogen in chicken farm flushing wastewater by mixing strategy for nutrient removal and lipid accumulation in the green alga Chlorella sorokiniana. Bioresour. Technol. 2020, 303, 122940. [Google Scholar] [CrossRef]
- Feng, X.; Walker, T.H.; Bridges, W.C.; Thornton, C.; Gopalakrishnan, K. Biomass and lipid production of Chlorella protothecoides under heterotrophic cultivation on a mixed waste substrate of brewer fermentation and crude glycerol. Bioresour. Technol. 2014, 166, 17–23. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Sadvakasova, A.K.; Zayadan, B.K.; Kakimova, A.B.; Sarsekeyeva, F.K.; Kossalbayev, B.D.; Bozieva, A.M.; Alwasel, S.; Allakhverdiev, S.I. Prospects for the creation of a waste-free technology for wastewater treatment and utilization of carbon dioxide based on cyanobacteria for biodiesel production. J. Biotechnol. 2020, 324, 162–170. [Google Scholar] [CrossRef]
- Cordeiro, R.S.; Vaz, I.C.; Magalhaes, S.; Barbosa, F.A. Effects of nutritional conditions on lipid production by cyanobacteria. An. Acad. Bras. Cienc. 2017, 89, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, & Komárek by using modified nutrient media. Aquac. Int. 2020, 28, 1371–1388. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Antonopoulou, G.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. A Leptolyngbya-based microbial consortium for agro-industrial wastewaters treatment and biodiesel production. Environ. Sci. Pollut. Res. 2018, 25, 17957–17966. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Ferrer, I.; Paániker, C.C.; Goómez-Pinchetti, J.L.; Rousseau, D.P.; Van Hulle, S.W.; Garfí, M. Natural pigments and biogas recovery from microalgae grown in wastewater. ACS Sustain. Chem. Eng. 2020, 8, 10691–10701. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef]
- Mogany, T.; Swalaha, F.M.; Kumari, S.; Bux, F. Elucidating the role of nutrients in C-phycocyanin production by the halophilic cyanobacterium Euhalothece sp. J. Appl. Phycol. 2018, 30, 2259–2271. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xu, J.; Ma, L. Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J. Biosci. Bioeng. 2018, 126, 644–648. [Google Scholar] [CrossRef]
- Cole, A.J.; Neveux, N.; Whelan, A.; Morton, J.; Vis, M.; de Nys, R.; Paul, N.A. Adding value to the treatment of municipal wastewater through the intensive production of freshwater macroalgae. Algal res. 2016, 20, 100–109. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Zhang, H.; Ma, X.; Li, L.; Cheng, Y.; Chen, P.; Ruan, R. Growing wastewater-born microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production. App. Energy 2012, 98, 433–440. [Google Scholar] [CrossRef]
- Gentili, F.G. Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Bioresour. Technol. 2014, 169, 27–32. [Google Scholar] [CrossRef]
- EUR-Lex. Commission directive 98/15/EC of 27 February 1998 amending council directive 91/271/EEC with respect to certain requirements established in annex I thereof. Off. J. Eur. Comm. 1998, 67, 29–30. Available online: http://data.europa.eu/eli/dir/1998/15/oj (accessed on 10 September 2022).
- Kamaraj, M.; Subramaniam, D. Amylase production by Aspergillus niger in submerged cultivation using cassava. J. Appl. Biol. 2020, 8, 82–87. [Google Scholar] [CrossRef]
- Hernández, M.S.; Rodríguez, M.R.; Guerra, N.P.; Rosés, R.P. Amylase production by Aspergillus niger in submerged cultivation on two wastes from food industries. J. Food Eng. 2006, 73, 93–100. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).