Preparation and Antioxidant Activity In Vitro of Fermented Tremella fuciformis Extracellular Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Strains and Culture Media
2.2. Screening for High Yield Strain of Tremella Mycelium and Exopolysaccharide
2.3. Optimization of Fermentation Process
2.4. Isolation and Purification of EPS from T. fuciformis
2.4.1. Preliminary Screening of Resins
2.4.2. Separation of Polysaccharide Fractions
2.5. Antioxidant Activity Assays
2.5.1. Ferric Ion Reducing/Antioxidant Power (FRAP) Assay
2.5.2. DPPH Scavenging Activity
2.5.3. Hydroxyl Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Screening of Strains and Effect of Fermentation Conditions
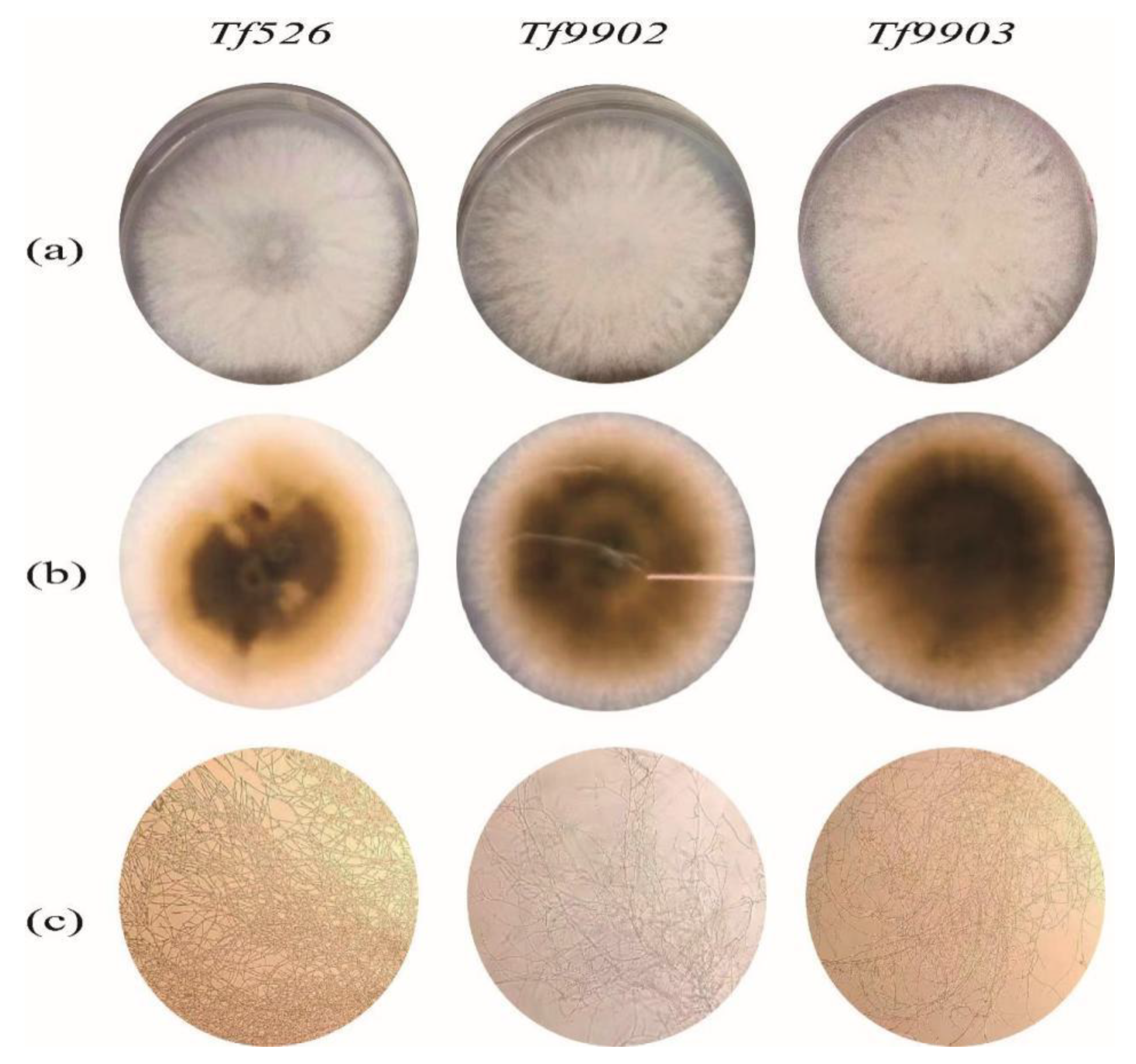
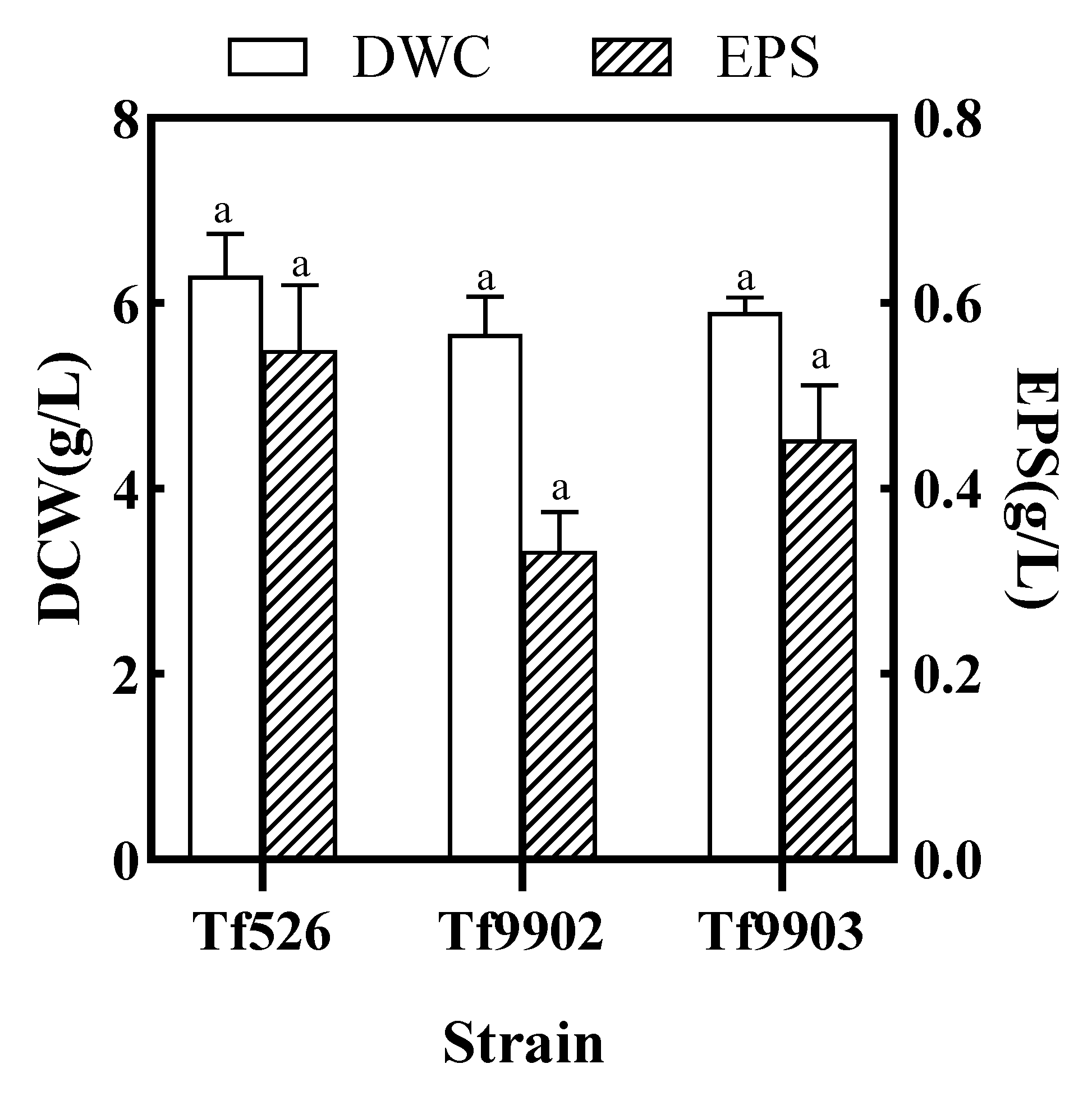
3.1.1. Preliminary Screening for High-Yield Strain
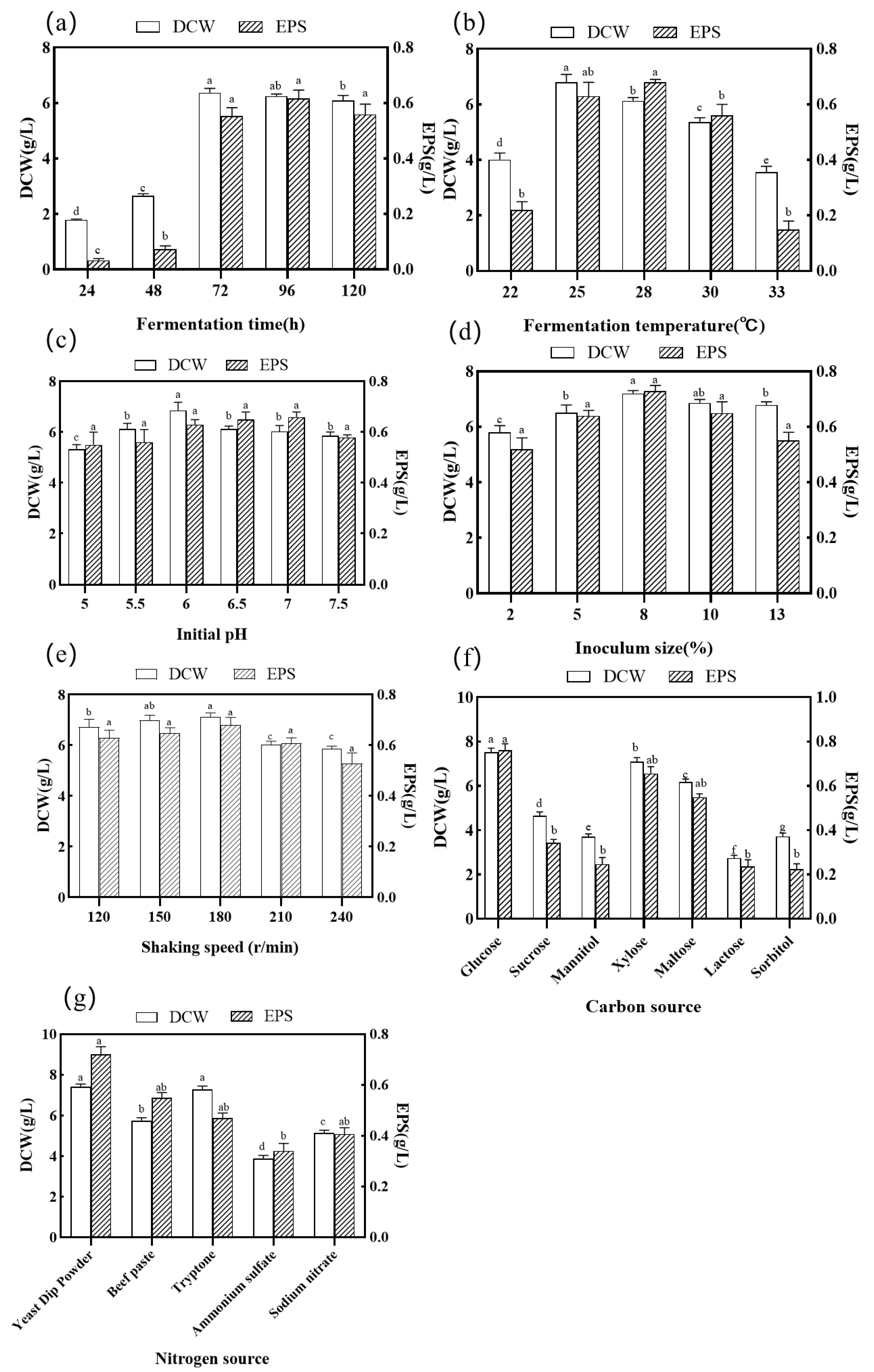
3.1.2. Effect of Fermentation Time
3.1.3. Effect of Fermentation Temperature
3.1.4. Effect of Initial pH
3.1.5. Effect of Inoculum Size
3.1.6. Effect of Shaking Speed
3.1.7. Effect of Carbon Source and Nitrogen Source
3.2. Selection of Optimal Decoloration Resin
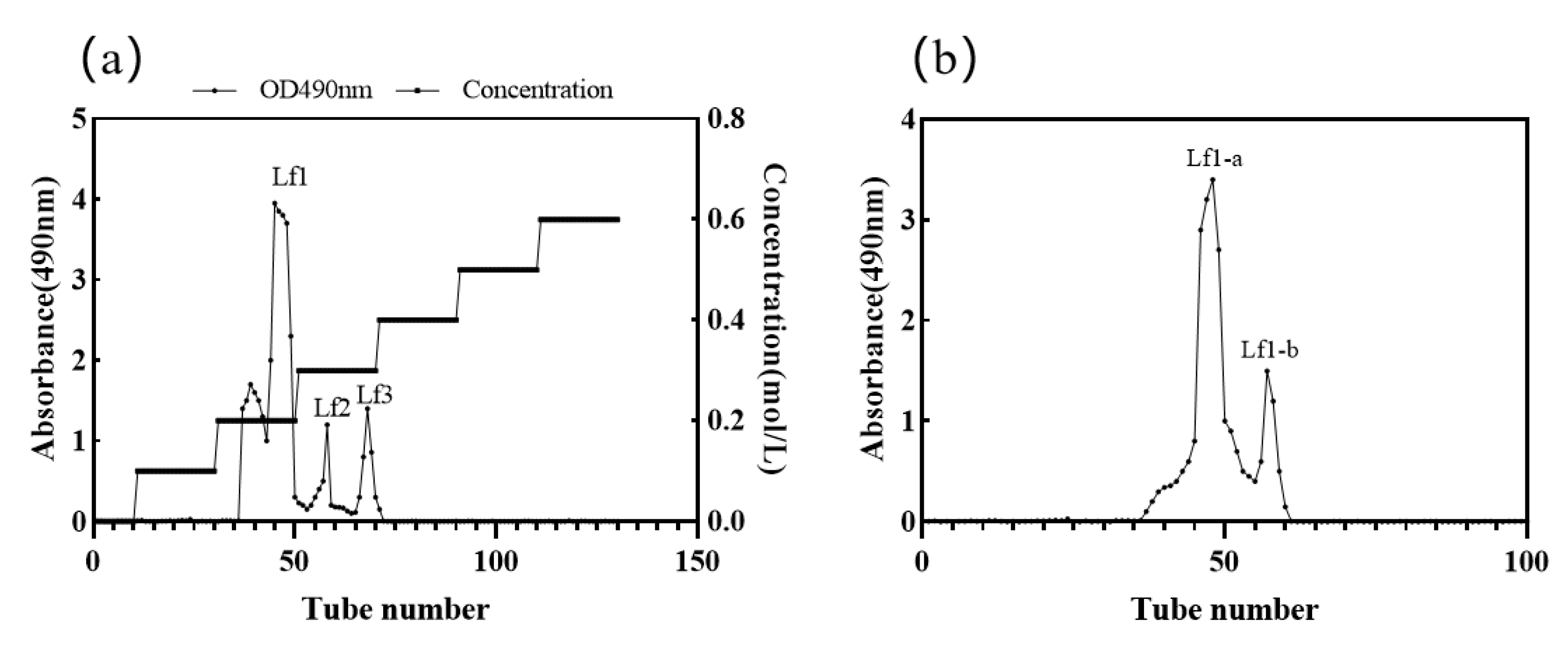
3.3. Isolation and Purification of Exopolysaccharides from T. fuciformis
3.4. Determination of Antioxidant Activity
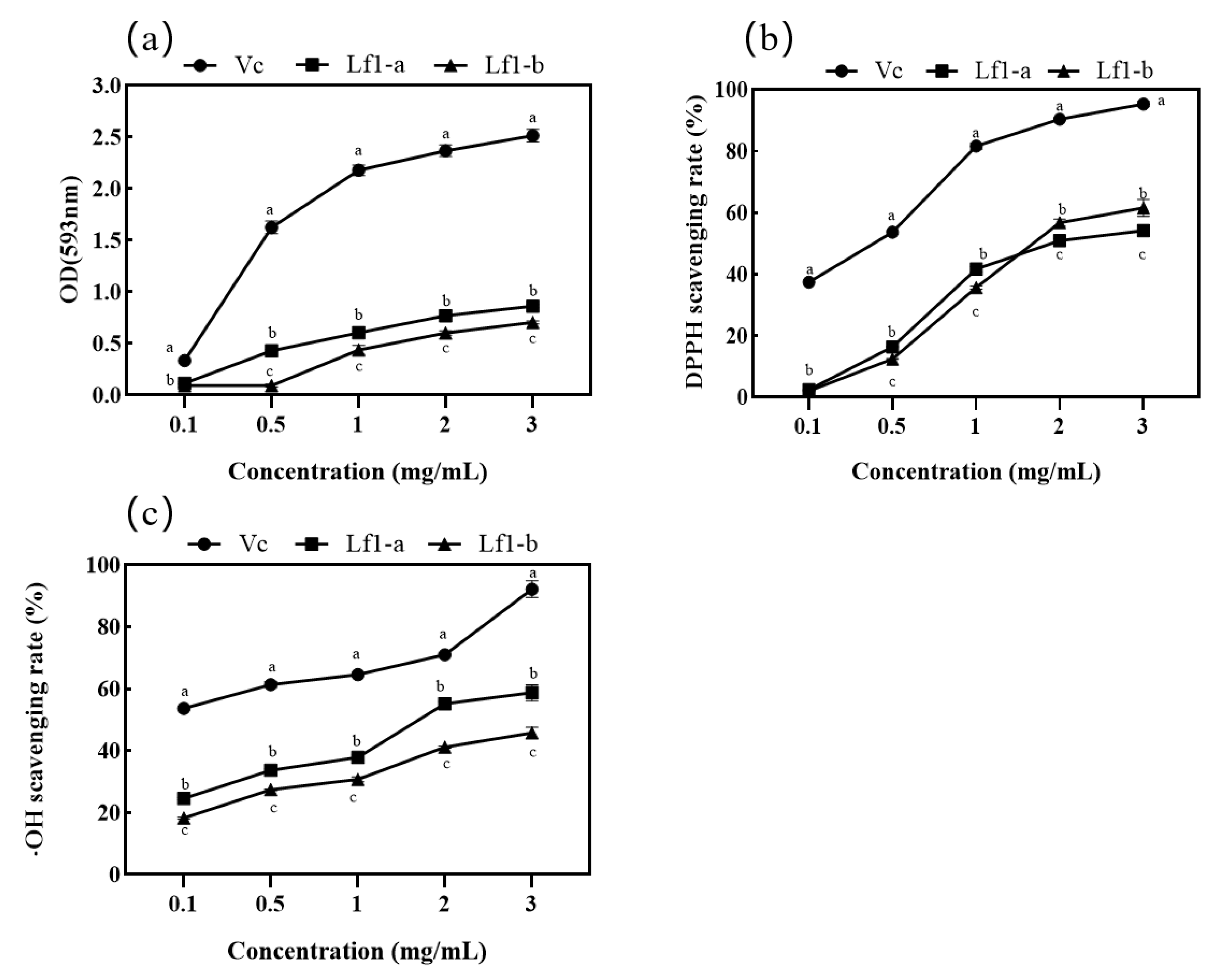
3.4.1. FRAP Scavenging Efficiency
3.4.2. DPPH Scavenging Efficiency
3.4.3. Hydroxyl Radical Scavenging Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, D.T.; Deng, Y.; Zhao, J.; Li, S.P. Molecular characterization of branched polysaccharides from Tremella fuciformis by asymmetrical flow field-flow fractionation and size exclusion chromatography. J. Sep. Sci. 2017, 40, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Oh, J.Y.; Chang, H.Y.; Yun, J.W. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol. 2006, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kwon, K.M.; Lee, S.H. Evaluation of the antioxidant activities and tyrosinase inhibitory property from mycelium culture extracts. Evid. Based Compl. Alt. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jia, B.; Peng, T. Research on chemical constituents and pharmacological action of polysaccharide in Poria cocos. Medicinal Plant 2014, 5, 51–54. [Google Scholar]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, G.W.; Lee, U.-Y.; Lee, T.S. The immuno-modulatory and antitumor effects of crude polysaccharides extracted from Tremella fuciformis. Korea J. Mycol. 2006, 34, 105–111. [Google Scholar]
- Kim, J.H.; Ha, H.C.; Lee, M.S.; Kang, J.I.; Kim, H.S.; Lee, S.Y.; Pyun, K.H.; Shim, I. Effect of Tremella fuciformis on the neurite outgrowth of PC12h cells and the improvement of memory in rats. Biol. Pharm. Bull. 2007, 30, 708–714. [Google Scholar] [CrossRef]
- Mustafin, K.; Bisko, N.; Blieva, R.; Al-Maali, G.; Krupodorova, T.; Narmuratova, Z.; Saduyeva, Z.; Zhakipbekova, A. Antioxidant and antimicrobial potential of Ganoderma lucidum and Trametes versicolor. Turk. J. Biochem. 2022, 47, 487–489. [Google Scholar] [CrossRef]
- Aranha, G.M.; Contato, A.G.; dos Santos Salgado, J.C.; de Oliveira, T.B.; Retamiro, K.M.; Ortolan, G.G.; Crevelin, E.J.; Nakamura, C.V.; Beraldo de Moraes, L.A.; Peralta, R.M.; et al. Biochemical characterization and biological properties of mycelium extracts from Lepista sordida GMA-05 and Trametes hirsuta GMA-01: New mushroom strains isolated in Brazil. Braz J. Microbiol. 2022, 58, 349–358. [Google Scholar] [CrossRef]
- Li, T.J.; Lee, T.Y.; Lo, Y.; Lee, L.Y.; Li, I.C.; Chen, C.C.; Chang, F.C. Hericium erinaceus mycelium ameliorate anxiety induced by continuous sleep disturbance in vivo. BMC Complement. Med. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Jeong, S.C.; Koyyalamudi, S.R.; Hughes, J.M.; Khoo, C.; Bailey, T.; Marripudi, K.; Park, J.P.; Kim, J.H.; Song, C.H. Antioxidant and immunomodulating activities of exo-and endopolysaccharide fractions from submerged mycelia cultures of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2013, 15, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shim, H.S.; Ahn, Y.H.; Kim, K.S.; Park, K.J.; Choi, W.K.; Ha, H.C.; Kang, J.I.; Kim, T.S.; Yeo, I.H.; et al. Tremella fuciformis enhances the neurite outgrowth of PC12 cells and restores trimethyltin-induced impairment of memory in rats via activation of CREB transcription and cholinergic systems. Behav. Brain Res. 2012, 229, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, Q.; Lin, J.; Guo, L. Biosynthesis of Resveratrol in Blastospore of the Macrofungus Tremella fuciformis. Mol. Biotechnol. 2015, 57, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tian, B.; Liu, W.; Zhang, S.; Cao, C.; Zhang, Y.; Zou, W. A three-stage culture process for improved exopolysaccharide production by Tremella fuciformis. Bioresour. Technol. 2012, 116, 526–528. [Google Scholar] [CrossRef]
- Han, C.K.; Chiang, H.C.; Lin, C.Y.; Tang, C.H.; Lee, H.; Huang, D.D.; Zeng, Y.R.; Chuang, T.N.; Huang, Y.L. Comparison of immunomodulatory and anticancer activities in different strains of Tremella fuciformis Berk. Am. J. Chin. Med. 2015, 43, 1637–1655. [Google Scholar] [CrossRef]
- Gao, Q.; Seljelid, R.; Chen, H.; Jiang, R. Characterisation of acidic heteroglycans from Tremella fuciformis Berk with cytokine stimulating activity. Carbohydr. Res. 1996, 288, 135–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, L.; Gao, L.; Huang, Q.; Qi, J. A neuritogenic compound from Tremella fuciformis. China J. Chin. Mat. Med. 2011, 36, 2358–2360. [Google Scholar]
- Du, X.J.; Zhang, J.S.; Yang, Y.; Tang, Q.J.; Jia, W.; Pan, Y.J. Purification, chemical modification and immunostimulating activity of polysaccharides from Tremella aurantialba fruit bodies. J. Zhejiang Univ. Sci. B 2010, 11, 437–442. [Google Scholar] [CrossRef]
- Kiho, T.; Kochi, M.; Usui, S.; Hirano, K.; Aizawa, K.; Inakuma, T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-H). Biol. Pharm. Bull. 2001, 24, 1400–1403. [Google Scholar] [CrossRef][Green Version]
- Jiang, R.Z.; Wang, Y.; Luo, H.M.; Cheng, Y.Q.; Chen, Y.H.; Gao, Y.; Gao, Q.P. Effect of the molecular mass of Tremella polysaccharides on accelerated recovery from cyclophosphamide-induced leucopenia in rats. Molecules 2012, 17, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Hu, Y.L.; Wang, D.Y.; Guo, L.W.; Yang, S.J.; Fan, Y.P.; Zhao, B.K.; Wang, Y.L.; Abula, S. Optimization of sulfated modification conditions of Tremella polysaccharide and effects of modifiers on cellular infectivity of NDV. Int. J. Biol. Macromol. 2011, 49, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, X.; Yang, F.; Han, Y.; Li, R.; Xue, D.; Jiang, C. Protective effect of polysaccharides isolated from Tremella fuciformis against radiation-induced damage in mice. J. Radiat. Res. 2012, 53, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hu, X.; Zhang, Y.; Liu, T. Studies on the purification of polysaccharides separated from Tremella fuciformis and their neuroprotective effect. Mol. Med. Rep. 2016, 13, 3985–3992. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Luo, D. Structure elucidation of a non-branched and entangled heteropolysaccharide from Tremella sanguinea Peng and its antioxidant activity. Carbohydr. Polym. 2016, 152, 33–40. [Google Scholar] [CrossRef]
- Kala, K.; Kryczyk-Poprawa, A.; Rzewinska, A.; Muszynska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef]
- Jo, M.H.; Kim, B.; Ju, J.H.; Heo, S.Y.; Ahn, K.H.; Lee, H.J.; Yeom, H.-S.; Jang, H.; Kim, M.-S.; Kim, C.-H.; et al. Tremella fuciformis TFCUV5 mycelial culture-derived exopolysaccharide production and its anti-aging effects on skin cells. Biotechnol. Bioproc. E 2021, 26, 738–748. [Google Scholar] [CrossRef]
- Ge, X.Y.; Huang, W.W.; Xu, X.Q.; Lei, P.; Sun, D.F.; Xu, H.; Li, S. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis XY. Int. J. Biol. Macromol. 2020, 148, 173–181. [Google Scholar] [CrossRef]
- Deng, Y.F.; Huang, Q.; Hu, L.; Liu, T.; Zheng, B.S.; Lu, D.J.; Guo, C.W.; Zhou, L. Enhanced exopolysaccharide yield and antioxidant activities of Schizophyllum commune fermented products by the addition of Radix Puerariae. Rsc Adv. 2021, 11, 38219–38234. [Google Scholar] [CrossRef]
- Gusman, J.K.; Lin, C.Y.; Shih, Y.C. The Optimum submerged culture condition of the culinary-medicinal white Jelly mushroom Tremella fuciformis (Tremellomycetes) and its antioxidant properties. Int. J. Med. Mushrooms 2014, 16, 293–302. [Google Scholar] [CrossRef]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Sun, Y.; Fu, H.; Zhang, S.; Chen, J.; Xu, X. Antioxidant and immunostimulatory activities of polysaccharides extracted from Tremella aurantialba mycelia. Mol. Med. Rep. 2016, 14, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, H.; Huang, G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hong, R.; Yi, Y.; Bai, Y.; Dong, L.; Jia, X.; Zhang, R.; Wang, G.; Zhang, M.; Wu, J. In vitro digestion and human gut microbiota fermentation of longan pulp polysaccharides as affected by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2020, 147, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B 2020, 1138, 121960. [Google Scholar] [CrossRef]
- Mou, Y.; Li, J.; Zhou, K.; Yu, R.; Xu, D.; Luo, H.; Lai, D.; Zhou, L. Enhanced production of palmarumycins c12 and C13 in mycelial liquid culture of the endophytic fungus berkleasmium sp Dzf12 with in situ macroporous resin adsorption. Trop J. Pharm. Res. 2015, 14, 407–414. [Google Scholar] [CrossRef][Green Version]
- Rajesh, N.; Kumar, A.S.K.; Kalidhasan, S.; Rajesh, V. Trialkylamine impregnated macroporous polymeric sorbent for the effective removal of chromium from industrial wastewater. J. Chem. Eng. Data 2011, 56, 2295–2304. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical composition and bioactivities of a water-soluble polysaccharide from the endodermis of shaddock. Int. J. Biol. Macromol. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.; Wei, K.; Zhang, T.; Che, Y.; Nguyen, A.D.; Pandita, S.; Wan, X.; Cui, X.; Zhou, B.; et al. Structure of a new glycyrrhiza polysaccharide and its immunomodulatory activity. Front. Immunol. 2022, 13, 1007186. [Google Scholar] [CrossRef]
- Shi, K.; Yang, G.; He, L.; Yang, B.; Li, Q.; Yi, S. Purification, characterization, antioxidant, and antitumor activity of polysaccharides isolated from silkworm cordyceps. J. Food Biochem. 2020, 44, e13482. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Li, X.; Ma, W.; Row, K.H. Retention of large biological molecules by size-exclusion chromatography. Anal. Lett. 2017, 50, 905–915. [Google Scholar] [CrossRef]
- Tackx, P.; Tacx, J. Chain architecture of LDPE as a function of molar mass using size exclusion chromatography and multi-angle laser light scattering (SEC-MALLS). Polymer 1998, 39, 3109–3113. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Yan, Y.H.; Shan, Y.N.; Kan, J.; Jin, C.H. Structure, physical property and antioxidant activity of catechin grafted Tremella fuciformis polysaccharide. Int. J. Biol. Macromol. 2016, 82, 719–724. [Google Scholar] [CrossRef]
- Zou, Y.; Hou, X. Extraction optimization, composition analysis, and antioxidation evaluation of polysaccharides from white Jelly mushroom, Tremella fuciformis (Tremellomycetes). Int. J. Med. Mushrooms 2017, 19, 1113–1121. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzyme Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, T.; Han, Y.; Zhu, X.; Zhao, X.; Ma, X.; Jiang, D.; Zhang, Q. An efficient method for decoloration of polysaccharides from the sprouts of Toona sinensis (A. Juss.) Roem by anion exchange macroporous resins. Food Chem. 2017, 217, 461–468. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and Ion Exchange: Basic principles and their application in food processing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, S.S.; Huang, S.S.; Zhang, L.H.; Ge, Z.Z.; Sun, L.P.; Zong, W. Purification of polyphenols from Distiller’s grains by macroporous resin and analysis of the polyphenolic components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, X.; Qu, J.; Xiao, C.; Jiang, K.; Gashash, E.; Liu, D.; Song, J.; Cheng, J.; Ma, C.; et al. Diethylaminoethyl Sepharose (DEAE-Sepharose) microcolumn for enrichment of glycopeptides. Anal. Bioanal. Chem. 2017, 409, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, Z.; Sun, T.; Wang, C.; Chen, Y.Y.; Yang, Z.Y.; Du, B.; Liu, C.Y. Comparison of structural, antioxidant and immuno-stimulating activities of polysaccharides from Tremella fuciformis in two different regions of China. Int. J. Food Sci. Technol. 2018, 53, 1942–1953. [Google Scholar] [CrossRef]
- Zheng, Q.W.; He, B.L.; Wang, J.Y.; Huang, S.S.; Zou, Y.; Wei, T.; Ye, Z.W.; Guo, L.Q.; Lin, J.F. Structural analysis and antioxidant activity of extracellular polysaccharides extracted from culinary-medicinal white Jelly mushroom Tremella fuciformis (Tremellomycetes) conidium cells. Int. J. Med. Mushrooms 2020, 22, 489–500. [Google Scholar] [CrossRef]
- Porath, J.; Flodin, P. Gel filtration: A method for desalting and group separation. Nature 1959, 183, 1657–1659. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Luo, Y.; Ma, L.; Kou, X.; Huang, K. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr. Res. 2009, 344, 217–222. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, 9077. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Yang, J.H.; Mau, J.L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhang, Y.Q.; Tang, A.; Ruan, Q.Q.; Huang, G.L. Preparation and antioxidant activity of phosphorylated polysaccharide from purple sweet potato. Chem. Biol. Drug Des. 2021, 98, 828–834. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Xia, G.D.; Huang, G.L.; Huang, H.L. Extraction, chemical modification, and antioxidant activities of Daucus carota polysaccharide. Chem. Biol. Drug Des. 2021, 98, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, L.; Li, Z.; Harqin, C.; Peng, Y.; Liu, J. Physicochemical analysis, structural elucidation and bioactivities of a high-molecular-weight polysaccharide from Phellinus igniarius mycelia. Int. J. Biol. Macromol. 2018, 120, 1855–1864. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/microwave-assisted extraction. Lwt-Food Sci. Technol. 2022, 154, 112446. [Google Scholar] [CrossRef]
- Mandhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mandouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar]
- Wang, Y.; Jia, J.; Ren, X.; Li, B.; Zhang, Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.X.; Nie, S.P.; Li, C.; Xie, M.Y. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2015, 186, 231–238. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Li, E.T.; Fan, Q.; Wang, D.Y.; Li, P.; Li, X.P.; Chen, X.Y.; Qiu, S.L.; Gao, Z.Z.; et al. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhao, M.; Qi, H. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydr. Polym. 2014, 112, 578–582. [Google Scholar] [CrossRef]
- Li, M.; Ma, F.; Li, R.; Ren, G.; Yan, D.; Zhang, H.; Zhu, X.; Wu, R.; Wu, J. Degradation of Tremella fuciformis polysaccharide by a combined ultrasound and hydrogen peroxide treatment: Process parameters, structural characteristics, and antioxidant activities. Int. J. Biol. Macromol. 2020, 160, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Zhao, Q.; Zhao, B.; Ouyang, J.; Mo, J.; Chen, J.; Cao, L.; Zhang, H. Molecular weight controllable degradation of Laminaria japonica polysaccharides and its antioxidant properties. J. Ocean. Univ. China 2016, 15, 637–642. [Google Scholar] [CrossRef]
- Camesano, T.A.; Wilkinson, K.J. Single molecule study of xanthan conformation using atomic force microscopy. Biomacromolecules 2001, 2, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Zhang, M.; Xie, B.J. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem. 2004, 52, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
| Resins | Particle Size (mm) | Surface Area (m2/g) | Average Pore Diameter (nm) | Polarity |
|---|---|---|---|---|
| A-722MP | 0.30–1.22 | 650–700 | 20–50 | Polar |
| DA-201 | 0.30–1.25 | ≥200 | 10–13 | Polar |
| HPD-600 | 0.30–1.25 | 550–600 | 8–9 | polar |
| AB-8 | 0.30–1.25 | 450–530 | 13–14 | Weak-polar |
| DM130 | 0.30–1.25 | 500–550 | 9–10 | Weak-polar |
| HPD-100 | 0.30–1.25 | 650–700 | 8.5–9 | Non-polar |
| XAD-1180N | 0.35–0.60 | 150–900 | 4–9 | Non-polar |
| Resin | Deproteinization Ratio (%) | Decolorization Ratio (%) | Polysaccharide Recovery Ratio (%) | ζ Value (%) |
|---|---|---|---|---|
| A-722MP | 81.72 ± 1.21 a | 62.73 ± 1.18 a | 72.73 ± 2.63 ab | 73.43 |
| AB-8 | 58.46 ± 1.83 c | 38.33 ± 1.48 cd | 69.71 ± 1.88 b | 60.06 |
| DA201 | 68.72 ± 2.21 b | 45.77 ± 2.13 bc | 58.72 ± 2.03 c | 59.13 |
| DM130 | 60.23 ± 2.13 c | 42.36 ± 2.18 c | 72.42 ± 2.98 ab | 62.75 |
| HPD100 | 33.65 ± 1.23 e | 24.86 ± 1.56 e | 75.52 ± 1.96 a | 52.83 |
| HPD600 | 65.32 ± 2.43 b | 49.21 ± 1.33 b | 53.42 ± 1.68 d | 56.15 |
| XAD1180N | 32.86 ± 2.43 e | 34.53 ± 2.14 d | 70.43 ± 1.78 b | 51.98 |
| Time/h | Deproteinization Ratio (%) | Decolorization Ratio (%) | Polysaccharide Recovery Ratio (%) | ζ Value (%) |
|---|---|---|---|---|
| 0.5 | 42.13 ± 2.13 d | 24.69 ± 1.43 d | 88.34 ± 2.18 a | 61.75 |
| 1 | 62.33 ± 1.23 c | 45.22 ± 1.68 c | 80.52 ± 1.78 b | 67.56 |
| 1.5 | 73.2 ± 2.52 b | 56.53 ± 2.03 b | 75.65 ± 2.32 c | 69.32 |
| 2 | 80.91 ± 1.79 a | 62.27 ± 1.89 a | 72.86 ± 2.14 c | 74.12 |
| 2.5 | 83.37 ± 1.56 a | 63.12 ± 1.32 a | 69.23 ± 1.16 d | 70.58 |
| 3 | 83.16 ± 2.23 a | 63.86 ± 2.42 a | 64.19 ± 1.45 e | 67.53 |
| Resin Dosage/g | Deproteinization Ratio % | Decolorization Ratio % | Polysaccharide Recovery Ratio % | ζ Value % |
|---|---|---|---|---|
| 1 | 62.33 ± 1.53 c | 42.12 ± 2.41 d | 89.30 ± 1.15 a | 71.76 |
| 2 | 80.61 ± 1.59 b | 63.17 ± 1.36 c | 72.86 ± 1.54 b | 73.21 |
| 3 | 86.2 ± 2.41 a | 69.55 ± 1.08 b | 60.22 ± 1.24 c | 69.71 |
| 4 | 88.93 ± 2.74 a | 72.25 ± 2.76 ab | 56.23 ± 1.13 cd | 69.24 |
| 5 | 90.23 ± 1.36 a | 75.52 ± 2.82 a | 51.6 ± 2.56 d | 67.97 |
| Temperature/°C | Deproteinization Ratio % | Decolorization Ratio % | Polysaccharide Recovery Ratio % | ζ Value % |
|---|---|---|---|---|
| 20 | 32.46 ± 1.26 c | 34.18 ± 1.36 c | 85.32 ± 1.65 a | 59.23 |
| 30 | 81.21 ± 2.13 b | 62.14 ± 2.3 a | 73.42 ± 1.96 b | 73.5 |
| 40 | 84.23 ± 2.61 b | 64.55 ± 1.73 a | 60.22 ± 2.32 c | 68.29 |
| 50 | 90.93 ± 2.74 a | 55.36 ± 2.41 b | 52.28 ± 2.18 d | 64.49 |
| TEPS Fractions (3 mg/mL) | FRAP Values | DPPH Values | Fenton System |
|---|---|---|---|
| Lf1-a | 0.9199 | 0.8984 | 0.9716 |
| Lf1-b | 0.9441 | 0.9521 | 0.9716 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Liu, Y.; Deng, Y.; Yang, B.; Guo, R.; Jin, X.; Zhou, L. Preparation and Antioxidant Activity In Vitro of Fermented Tremella fuciformis Extracellular Polysaccharides. Fermentation 2022, 8, 616. https://doi.org/10.3390/fermentation8110616
Huang Q, Liu Y, Deng Y, Yang B, Guo R, Jin X, Zhou L. Preparation and Antioxidant Activity In Vitro of Fermented Tremella fuciformis Extracellular Polysaccharides. Fermentation. 2022; 8(11):616. https://doi.org/10.3390/fermentation8110616
Chicago/Turabian StyleHuang, Qian, Yu Liu, Yongfei Deng, Bin Yang, Ruixue Guo, Xiaobao Jin, and Lin Zhou. 2022. "Preparation and Antioxidant Activity In Vitro of Fermented Tremella fuciformis Extracellular Polysaccharides" Fermentation 8, no. 11: 616. https://doi.org/10.3390/fermentation8110616
APA StyleHuang, Q., Liu, Y., Deng, Y., Yang, B., Guo, R., Jin, X., & Zhou, L. (2022). Preparation and Antioxidant Activity In Vitro of Fermented Tremella fuciformis Extracellular Polysaccharides. Fermentation, 8(11), 616. https://doi.org/10.3390/fermentation8110616







