Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Fermentation Media
2.2. Inoculum Preparation and Fermentations
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Effect of Xylose Concentration on Lactic Acid Fermentation
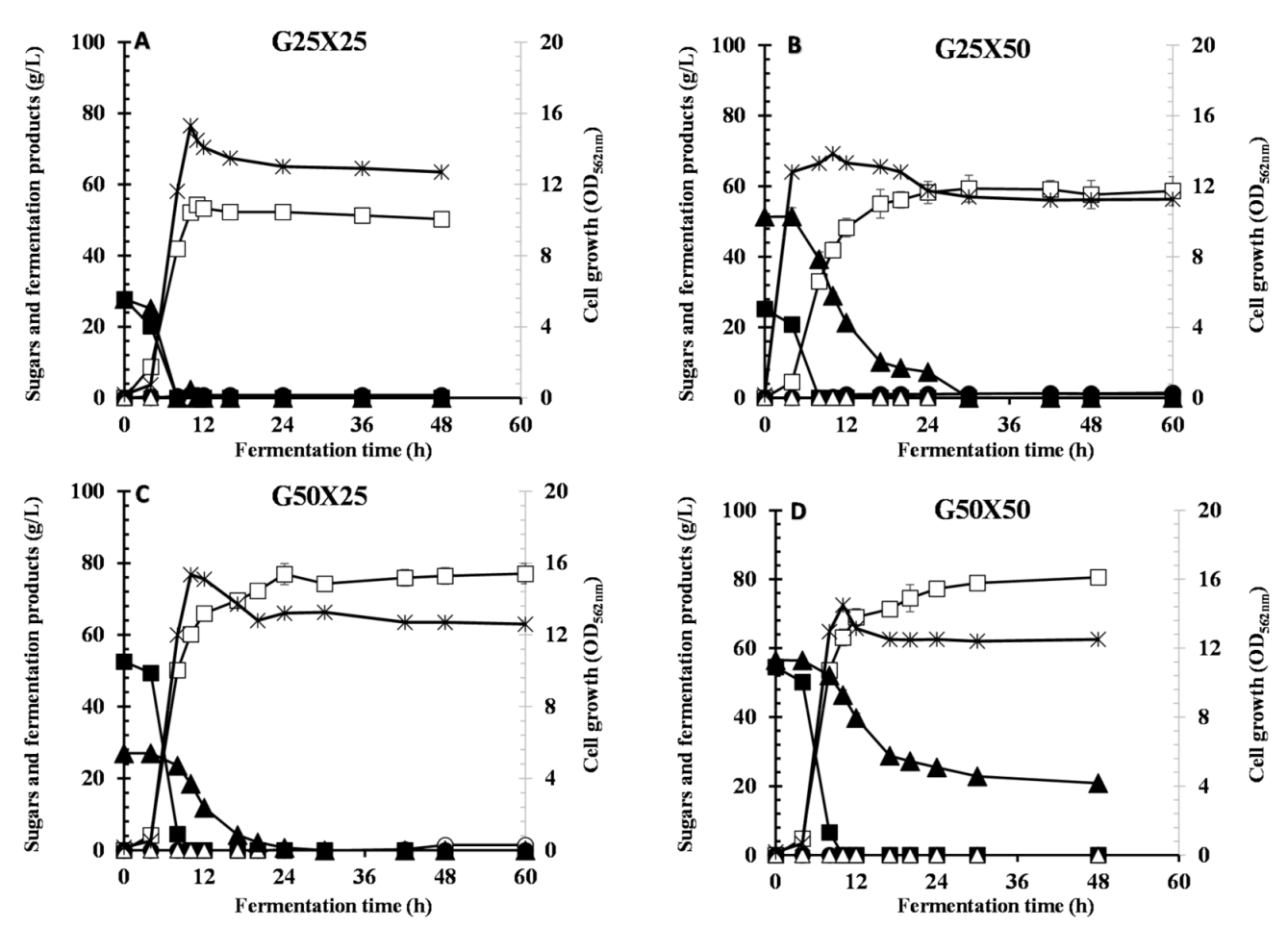
3.2. Effect of Different Ratios of Glucose and Xylose on CCR and LA Production
3.3. Effect of Different Ratios of Cellobiose and Xylose on CCR and LA Production
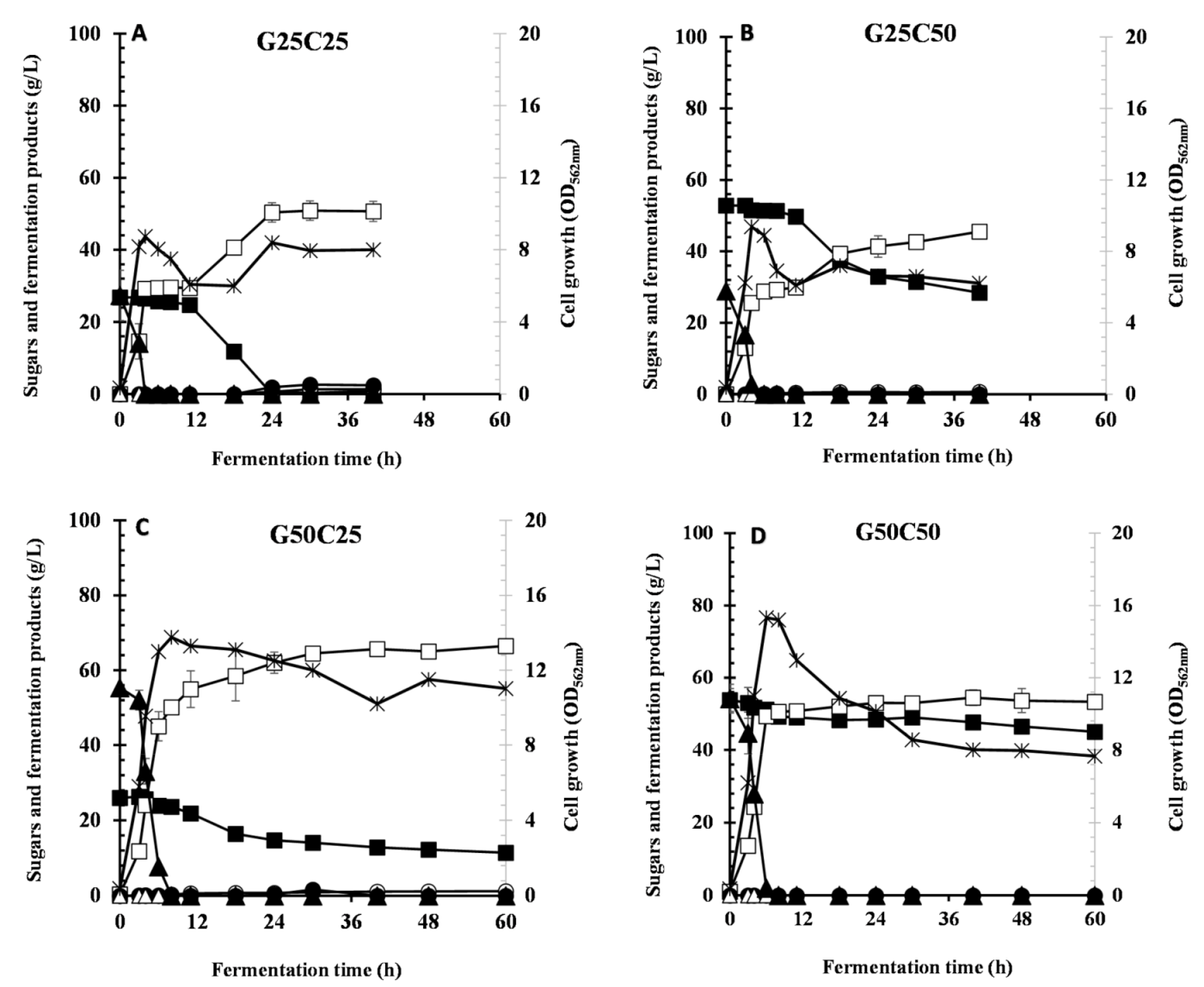
3.4. Effect of Different Ratios of Glucose and Cellobiose on CCR and LA Production
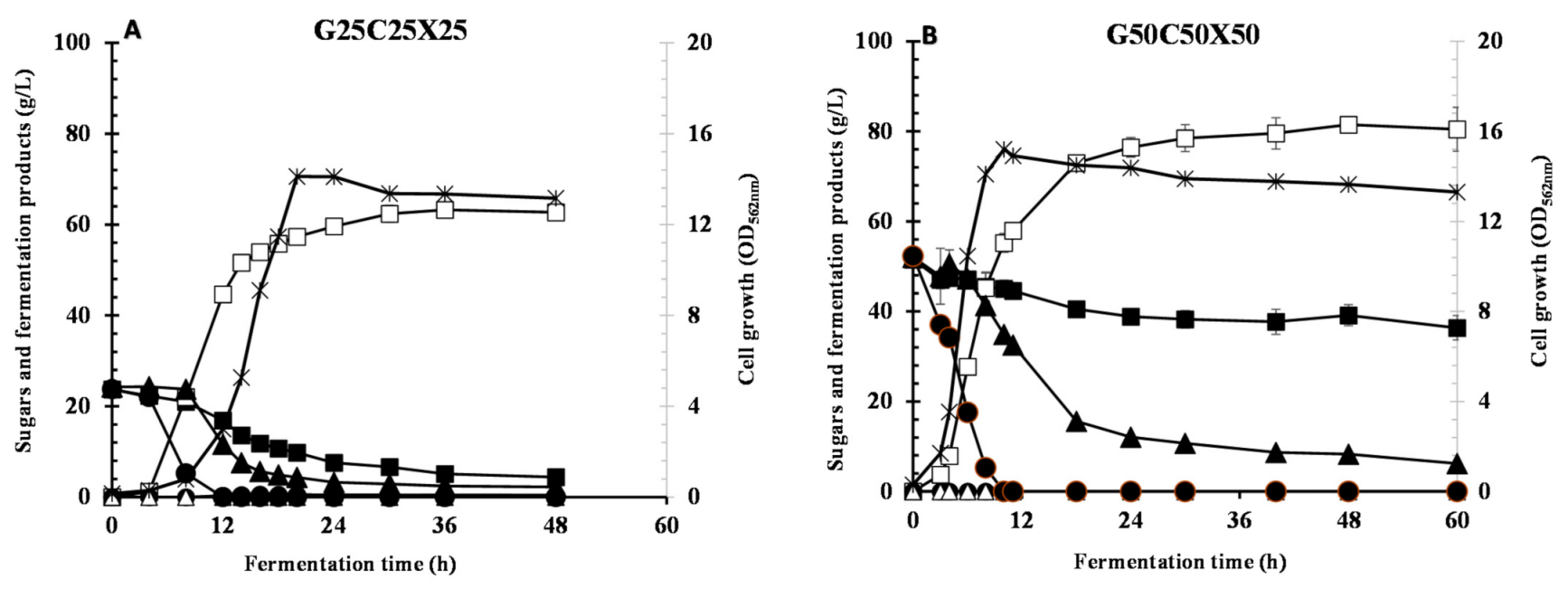
3.5. Utilization of Glucose/Xylose/Cellobiose Mixture
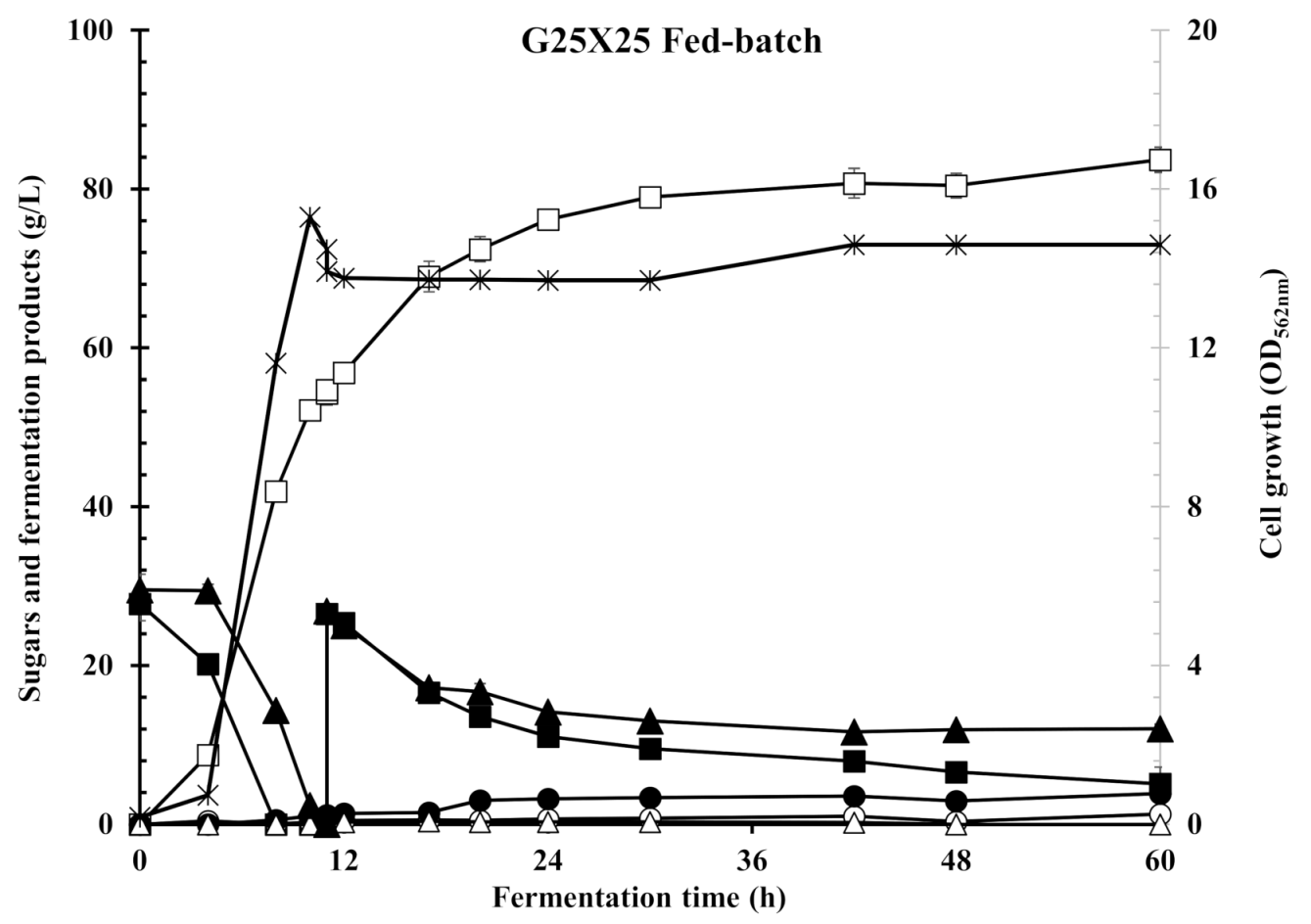
3.6. Fed-Batch Fermentation with Glucose/Xylose Mixture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaber, M.A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; Azab, M.S. Efficient biorefinery process for lactic acid production from date wastes with alleviating substrate inhibition effect using thermo-alkaline repeated batch fermentation. Biomass Convers. Biorefin. 2020, 1–14. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; Komesu, A.; Rossell, C.E.V.; Filho, R.M. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, G.; Zhu, P.; Gao, C. Recent Progress on 3D-Printed Polylactic Acid and Its Applications in Bone Repair. Adv. Eng. Mater. 2020, 22. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent devel-opments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Nwamba, M.C.; Sun, F.; Mukasekuru, M.R.; Song, G.; Harindintwali, J.D.; Boyi, S.A.; Sun, H. Trends and hassles in the mi-crobial production of lactic acid from lignocellulosic biomass. Environ. Technol. Innov. 2020, 21, 101337. [Google Scholar]
- Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Betts, W.B.; Dart, R.K.; Ball, A.S.; Pedlar, S.L. Biosynthesis and structure of lignocellulose. In Biodegradation: Natural and Synthetic Material; Betts, W.B., Ed.; Springer: London, UK, 1991; pp. 139–155. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1983. [Google Scholar] [CrossRef]
- Tan, J.; Abdel-Rahman, M.A.; Numaguchi, M.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Thermophilic Enterococcus faecium QU 50 enabled open repeated batch fermentation for l-lactictic acid production from mixed sugars without carbon catabolite repression. RSC Adv. 2017, 7, 24233–24241. [Google Scholar] [CrossRef]
- Wang, Y.; Abdel-Rahman, M.A.; Tashiro, Y.; Xiao, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. l-(+)-Lactic acid production by co-fermentation of cellobiose and xylose without carbon catabolite repression using Enterococcus mundtii QU 25. RSC Adv. 2014, 4, 22013–22021. [Google Scholar] [CrossRef]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Xiao, Y.; Tashiro, Y.; Wang, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Fed-batch fermentation for en-hanced lactic acid production from glucose/xylose mixture without carbon catabolite repression. J. Biosci. Bioeng. 2015, 119, 153–158. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shoemaker, S.P.; Mills, D.A. Relaxed control of sugar utilization in Lactobacillus brevis. Microbiology 2009, 155, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Barghoth, M.G.; Desouky, S.G. Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 17. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- He, C.-R.; Kuo, Y.-Y.; Li, S.-Y. Lignocellulosic butanol production from Napier grass using semi-simultaneous saccharification fermentation. Bioresour. Technol. 2017, 231, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef]
- Gao, K.; Boiano, S.; Marzocchella, A.; Rehmann, L. Cellulosic butanol production from alkali-pretreated switchgrass (Panicum virgatum) and phragmites (Phragmites australis). Bioresour. Technol. 2014, 174, 176–181. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Bischoff, K.M.; Liu, S.; Hughes, S.R.; Rich, J.O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010, 32, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Bin Hudari, M.S.; Zhou, X.; Zhang, D.; Li, Z.; Wu, J.C. Conversion of acid hydrolysate of oil palm empty fruit bunch to L-lactic acid by newly isolated Bacillus coagulans JI12. Appl. Microbiol. Biotechnol. 2013, 97, 4831–4838. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Qi, B.; Luo, J.; Shen, F.; Su, Y.; Khan, R.; Wan, Y. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014, 163, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, L.; Che, C.; Yang, G.; Yu, B.; Ma, Y. Bacillus sp. strain P38: An efficient producer of l-lactictate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour. Technol. 2013, 149, 169–176. [Google Scholar] [CrossRef]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of Various Pretreatment Methods on Bioethanol Production from Cotton Stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef]
- Weiss, N.D.; Felby, C.; Thygesen, L.G. Enzymatic hydrolysis is limited by biomass–water interactions at high-solids: Improved performance through substrate modifications. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tashiro, Y.; Zheng, J.; Sakai, K.; Sonomoto, K. Semi-hydrolysis with low enzyme loading leads to highly effective butanol fermentation. Bioresour. Technol. 2018, 264, 335–342. [Google Scholar] [CrossRef]
- Moradi, F.; Amiri, H.; Soleimanian-Zad, S.; Ehsani, M.R.; Karimi, K. Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Gao, K.; Rehmann, L. ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass Bioener. 2014, 66, 110–115. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Li, F. Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett. Appl. Microbiol. 2012, 55, 240–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Zhou, Y. Transglycosylation, a new role for multifunctional cellulase in overcoming product inhibition during the cellulose hydrolysis. Bioengineered 2016, 8, 129–132. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Kuittinen, S.; Vepsäläinen, J.; Zhang, J.; Pappinen, A. Enhanced acetone-butanol-ethanol production from ligno-cellulosic hydrolysates by using starchy slurry as supplement. Bioresour. Technol. 2017, 243, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Graves, T.; Narendranath, N.V.; Dawson, K.; Power, R. Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J. Ind. Microbiol. Biotechnol. 2006, 33, 469–474. [Google Scholar] [CrossRef]
- Ye, L.; Hudari, M.S.B.; Li, Z.; Wu, J.C. Simultaneous detoxification, saccharification and co-fermentation of oil palm empty fruit bunch hydrolysate for l-lactictic acid production by Bacillus coagulans JI12. Biochem. Eng. J. 2014, 83, 16–21. [Google Scholar] [CrossRef]
- Reddy, L.V.; Park, J.H.; Wee, Y.J. Homofermentative production of optically pure l-lactictic acid from sucrose and mixed sug-ars by batch fermentation of Enterococcus faecalis RKY1. Biotechnol. Bioprocess Eng. 2015, 20, 1099–1105. [Google Scholar] [CrossRef]
- Yoshida, S.; Okano, K.; Tanaka, T.; Ogino, C.; Kondo, A. Homo-d-lactictic acid production from mixed sugars using xy-lose-assimilating operon-integrated Lactobacillus plantarum. Appl. Microbial. Biotechnol. 2011, 92, 67–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V.; Kumar, A.; Hardwidge, P.R.; Govind, R.; Tanaka, T.; Kondo, A. Enhanced d-lactictic acid pro-duction from renewable resources using engineered Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2011, 100, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhao, X.; Wang, Y.; Ding, X.; Wang, J.; Garza, E.; Manow, R.; Iverson, A.; Zhou, S. Enhancement of d-lactictic acid pro-duction from a mixed glucose and xylose substrate by the Escherichia coli strain JH15 devoid of the glucose effect. BMC Bio-Technol. 2016, 16, 1–10. [Google Scholar]
- Xiao, Z.; Zhang, X.; Gregg, D.J.; Saddler, J.N. Effects of Sugar Inhibition on Cellulases and β-Glucosidase during Enzymatic Hydrolysis of Softwood Substrates. Appl. Biochem. Biotechnol. 2004, 115, 1115–1126. [Google Scholar] [CrossRef]
- Patel, M.A.; Ou, M.S.; Harbrucker, R.; Aldrich, H.C.; Buszko, M.L.; Ingram, L.O.; Shanmugam, K.T. Isolation and Characterization of Acid-Tolerant, Thermophilic Bacteria for Effective Fermentation of Biomass-Derived Sugars to Lactic Acid. Appl. Environ. Microbiol. 2006, 72, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Komiyama, A.; Sonomoto, K.; Ishizaki, A.; Hall, S.; Stanbury, P. Two different pathways for d-xylose metabolism and the effect of xylose concentration on the yield coefficient of l-lactictate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl. Microbiol. Biotechnol. 2002, 60, 160–167. [Google Scholar]
- De Clerck, E.; Vanhoutte, T.; Hebb, T.; Geerinck, J.; Devos, J.; De Vos, P. Isolation, characterization, and identification of bacterial contaminants in semi-final gelatin extracts. Syst. Appl. Microbiol. 2004, 27, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhou, X.; Bin Hudari, M.S.; Li, Z.; Wu, J.C. Highly efficient production of l-lactictic acid from xylose by newly isolated Bacillus coagulans C106. Bioresour. Technol. 2013, 132, 38–44. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactictic acid production from xylose. FEMS Microbiol. Lett. 2014, 362, 1–7. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Hanada, K.; Shibata, K.; Sonomoto, K. Efficient Homofermentative l-(+)-Lactic Acid Production from Xylose by a Novel Lactic Acid Bacterium, Enterococcus mundtii QU 25. Appl. Environ. Microbiol. 2010, 77, 1892–1895. [Google Scholar] [CrossRef]

| Mixture | Glucose (g/L) | Cellobiose (g/L) | Xylose (g/L) | |
|---|---|---|---|---|
| Glucose/Xylose (G/X) | G25X25 | 25 | – | 25 |
| G25X50 | 25 | – | 50 | |
| G50X25 | 50 | – | 25 | |
| G50X50 | 50 | – | 50 | |
| Cellobiose/Xylose (C/X) | C25X25 | – | 25 | 25 |
| C25X50 | – | 25 | 50 | |
| C50X25 | – | 50 | 25 | |
| C50X50 | – | 50 | 50 | |
| Glucose/Cellobiose (G/C) | G25C25 | 25 | 25 | – |
| G25C50 | 25 | 50 | – | |
| G50C25 | 50 | 25 | – | |
| G50C50 | 50 | 50 | – | |
| G10C10 | 10 | 10 | – | |
| G10C20 | 10 | 20 | – | |
| G15C15 | 15 | 15 | – | |
| G20C10 | 20 | 10 | – | |
| G20C20 | 20 | 20 | – | |
| Glucose/Cellobiose/Xylose (G/C/X) | G25C25X25 | 25 | 25 | 25 |
| G50C50X50 | 50 | 50 | 50 |
| Xylose Conc. (g/L) | Max. Biomass (OD562 nm) | Residual Xylose (g/L) | LA (g/L) | Formic Acid (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | YLA (g/g) | PLA [g/(L·h)] | Max. PLA [g/(L·h)] |
|---|---|---|---|---|---|---|---|---|---|
| 50 | 14.7 | 0.0 | 50.7 ± 1.38 | 2.60 | 0.0 | 0.0 | 0.999 | 4.22 | 5.63 |
| 75 | 17.8 | 0.0 | 76.5 ± 1.56 | 2.91 | 2.61 | 0.0 | 0.988 | 4.25 | 8.03 |
| 100 | 15.5 | 0.0 | 102.2 ± 0.661 | 2.21 | 2.50 | 0.0 | 1.0 | 3.18 | 8.55 |
| 125 | 15.7 | 31.1 | 101.2 ± 1.2 | 0.0 | 1.98 | 0.0 | 1.0 | 2.11 | 7.33 |
| 150 | 10.2 | 72.5 | 75.7 ± 2.78 | 0.0 | 1.56 | 0.0 | 0.920 | 1.57 | 3.42 |
| Mixed Sugars | Max. Biomass (OD562 nm) | Residual Glucose (g/L) | Residual Xylose (g/L) | LA (g/L) | Formic Acid (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | YLA (g/g) | PLA [g/(L·h)] | Max. PLA [g/(L·h)] |
|---|---|---|---|---|---|---|---|---|---|---|
| G25X25 | 15.3 | 0.0 | 0.0 | 54.2 ± 0.898 | 0.80 | 0.420 | 0.0 | 0.956 | 4.93 | 8.29 |
| G25X50 | 13.8 | 0.0 | 0.0 | 59.3 ± 3.16 | 1.0 | 1.14 | 0.0 | 0.770 | 1.97 | 7.13 |
| G50X25 | 15.1 | 0.0 | 0.0 | 76.9 ± 2.91 | 0.0 | 0.280 | 0.0 | 0.970 | 3.20 | 11.4 |
| G50X50 | 14.5 | 0.0 | 20.8 | 80.5 ±1.45 | 0.0 | 0.0 | 0.0 | 0.890 | 1.67 | 12.2 |
| Mixed Sugars | Max. Biomass (OD562 nm) | Residual Cellobiose (g/L) | Residual Xylose (g/L) | LA (g/L) | Formic Acid (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | YLA (g/g) | PLA [g/(L·h)] | Max. PLA [g/(L·h)] |
|---|---|---|---|---|---|---|---|---|---|---|
| C25X25 | 12.6 | 0.0 | 0.0 | 51.5 ± 1.46 | 1.31 | 1.11 | 0.0 | 1.00 | 3.22 | 6.60 |
| C25X50 | 15.8 | 0.0 | 0.0 | 74.2 ± 0.438 | 2.40 | 2.36 | 0.0 | 1.20 | 3.12 | 8.68 |
| C50X25 | 14.6 | 0.0 | 0.0 | 74.5 ± 0.799 | 0.0 | 1.67 | 0.0 | 0.964 | 2.69 | 6.91 |
| C50X50 | 10.7 | 0.0 | 8.58 | 87.1 ± 1.59 | 0.0 | 1.86 | 0.0 | 1.02 | 1.81 | 7.34 |
| Mixed Sugars | Max. Biomass (OD562 nm) | Residual Glucose (g/L) | Residual Cellobiose (g/L) | LA (g/L) | Formic Acid (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | YLA (g/g) | PLA [g/(L·h)] | Max. PLA [g/(L·h)] |
|---|---|---|---|---|---|---|---|---|---|---|
| C25G25 | 8.74 | 0.0 | 0 | 50.3 ± 2.68 | 1.85 | 0.610 | 0.590 | 0.930 | 2.09 | 14.5 |
| G25C50 | 9.38 | 0.0 | 28.4 | 45.5 ± 1.31 | 0.0 | 0.662 | 0.0 | 0.853 | 1.13 | 12.6 |
| G50C25 | 13.7 | 0.0 | 11.4 | 65.6 ± 0.459 | 0.0 | 1.08 | 0.0 | 0.959 | 1.64 | 12.7 |
| G50C50 | 15.3 | 0.0 | 45.0 | 53.3 ± 1.96 | 0.0 | 0.0 | 0.0 | 0.846 | 0.889 | 12.4 |
| G10C10 | 4.58 | 0.0 | 0.0 | 23.7 ± 1.34 | 0.0 | 0.62 | 0.0 | 1.04 | 1.31 | 9.98 |
| G10C20 | 5.80 | 0.0 | 0.0 | 32.8 ± 0.212 | 0.0 | 1.34 | 0.0 | 1.05 | 1.82 | 10.3 |
| G15C15 | 8.02 | 0.0 | 0.0 | 31.1 ± 1.69 | 1.65 | 0.960 | 0.671 | 1.05 | 1.72 | 10.1 |
| G20C10 | 8.50 | 0.0 | 0.0 | 32.1 ± 0.141 | 2.20 | 1.06 | 0.820 | 1.03 | 1.78 | 11.6 |
| G20C20 | 7.76 | 0.0 | 0.0 | 40.5 ± 0.212 | 0.658 | 0.560 | 0.611 | 1.02 | 2.25 | 9.85 |
| Mixed Sugars | Max. Biomass (OD562 nm) | Residual Glucose(g/L) | Residual Cellobiose (g/L) | Residual Xylose (g/L) | LA (g/L) | Formic Acid (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | YLA (g/g) | PLA [g/(L·h)] | Max. PLA [g/(L·h)] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G25X25C25 | 14.1 | 0.0 | 4.39 | 2.22 | 62.7 ± 0.424 | 0.414 | 0.431 | 0.0 | 0.962 | 1.30 | 5.65 |
| G50X50C50 | 15.2 | 0.0 | 36.3 | 6.22 | 80.4 ± 4.87 | 0.0 | 0.058 | 0.0 | 0.710 | 1.34 | 9.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. https://doi.org/10.3390/fermentation7010028
Abdel-Rahman MA, Hassan SE-D, Alrefaey HMA, Elsakhawy T. Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation. 2021; 7(1):28. https://doi.org/10.3390/fermentation7010028
Chicago/Turabian StyleAbdel-Rahman, Mohamed Ali, Saad El-Din Hassan, Hassan M.A. Alrefaey, and Tamer Elsakhawy. 2021. "Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10" Fermentation 7, no. 1: 28. https://doi.org/10.3390/fermentation7010028
APA StyleAbdel-Rahman, M. A., Hassan, S. E.-D., Alrefaey, H. M. A., & Elsakhawy, T. (2021). Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation, 7(1), 28. https://doi.org/10.3390/fermentation7010028






