Medicinal Chemistry Friendliness of Pigments from Monascus-Fermented Rice and the Molecular Docking Analysis of Their Anti-Hyperlipidemia Properties
Abstract
1. Introduction
2. Methods
2.1. Preparation of Monascus Pigments (Docking Ligand)
2.2. In Silico Evaluation of Physicochemical Properties and Drug-Likeness of Monascus Pigments
2.3. Molecular Docking
3. Results and Discussion
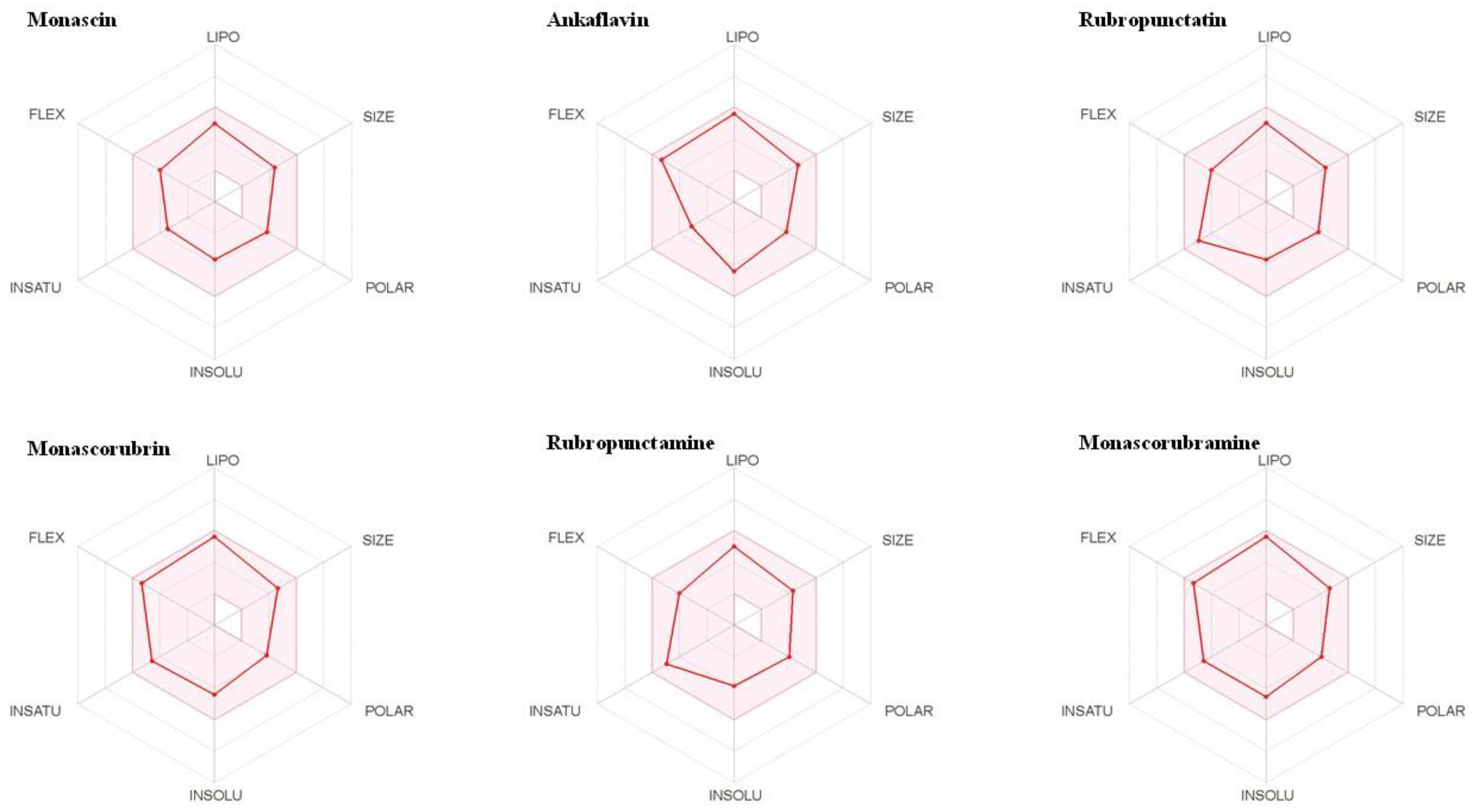
3.1. Physicochemical Properties of Monascus Pigments
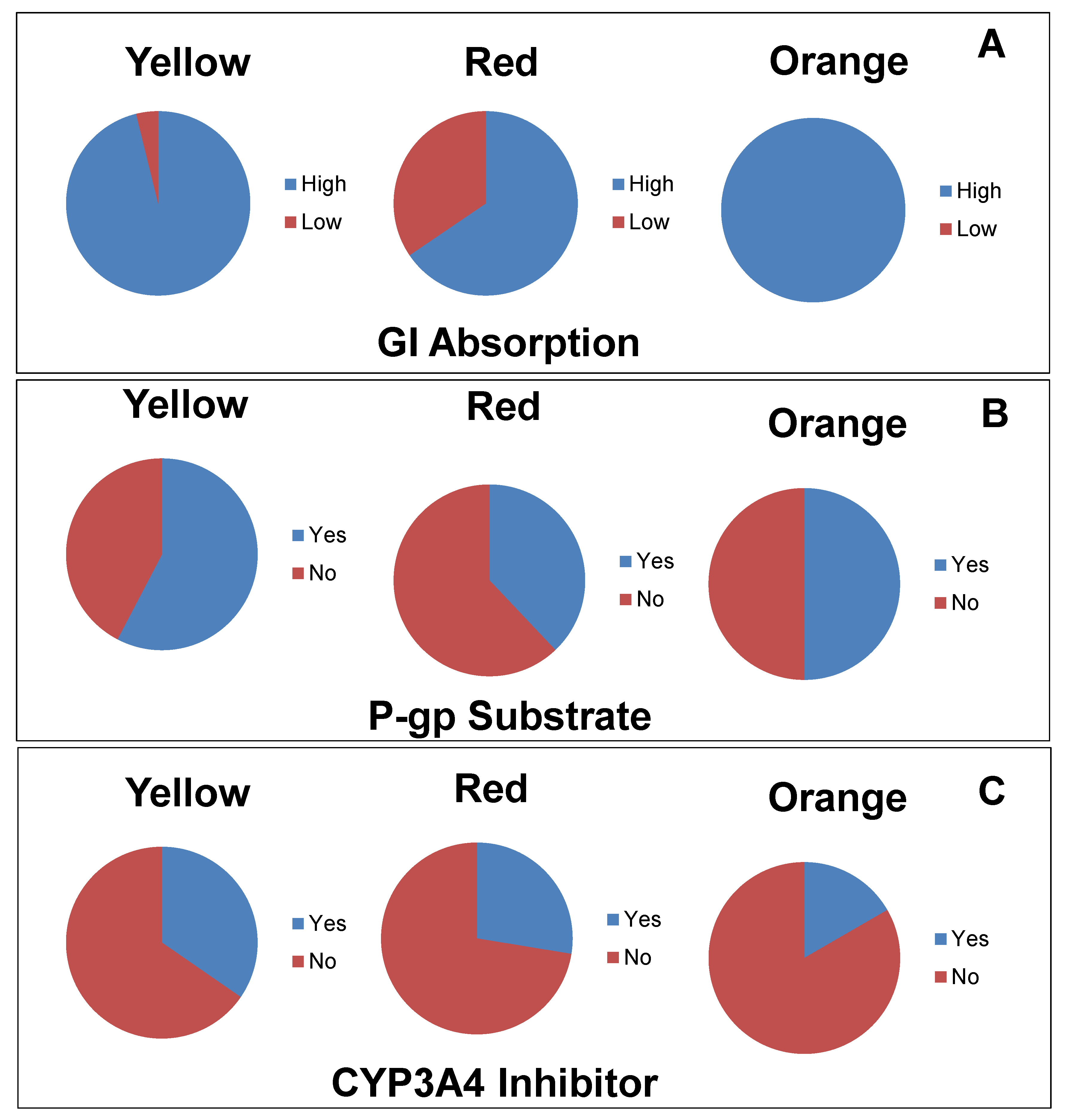
3.2. Pharmacokinetics Properties
3.3. Drug-Likeness and Bioavailability
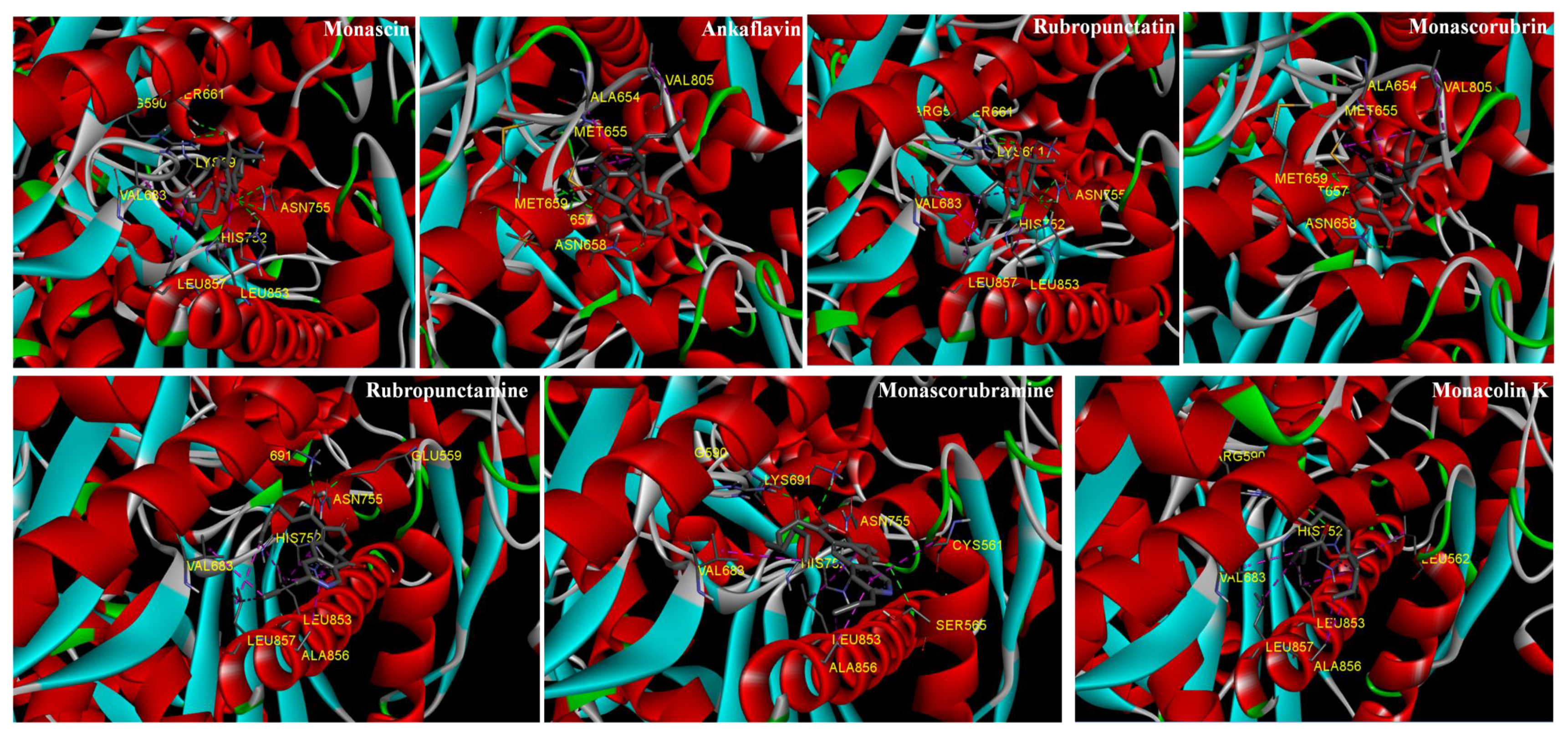
3.4. Molecular Docking of HMGR and Lipase with Monascus Pigments
3.4.1. HMGR Receptor
3.4.2. Lipase Receptor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Wang, Z.; Jin, G.; Yang, X.; Zhou, H. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017, 101, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-X.; Zhang, Q.-Q.; Huang, Y.-F.; Pang, H.-Q.; Liu, X.-G.; Gao, W.; Li, P.; Yang, H. Comprehensive chemical profiling of monascus-fermented rice product and screening of lipid-lowering compounds other than monacolins. J. Ethnopharmacol. 2019, 238, 111879. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.; Althaf, S.A.; Kumar, B.K.; Ramunaik, M.; Suvarna, C.H. A review on hyperlipidemic. Int. J. Nov. Trends Pharm. Sci. 2013, 3, 59–71. [Google Scholar]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fønnebø, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: A meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.M.; Colantonio, L.D.; Kilgore, M.L.; Mefford, M.T.; Chachappan, D.T.; Mues, K.E.; Safford, M.M.; Rosenson, R.S.; Muntner, P. Low-density lipoprotein cholesterol levels among individuals experiencing statin-associated symptoms: Data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J. Clin. Lipidol. 2020, 14, 720–729. [Google Scholar] [CrossRef]
- Bellosta, S. Safety of Statins: Focus on Clinical Pharmacokinetics and Drug Interactions. Circulation 2004, 109, III50–III57. [Google Scholar] [CrossRef]
- McGinnis, B.; Olson, K.L.; Magid, D.; Bayliss, E.; Korner, E.J.; Brand, D.W.; Steiner, J.F. Dyslipidemia: Factors related to adherence to statin therapy. Ann. Pharmacother. 2007, 41, 1805–1811. [Google Scholar] [CrossRef]
- Butalia, S.; Lee-Krueger, R.C.; McBrien, K.A.; Leung, A.A.; Anderson, T.J.; Quan, H.; Naugler, C.; Chen, G.; Campbell, D.J. Barriers and Facilitators to Using Statins: A Qualitative Study With Patients and Family Physicians. CJC Open 2020, in press. [Google Scholar] [CrossRef]
- Berent, T.; Berent, R.; Steiner, S.; Sinzinger, H. Statin-induced muscular side effects at rest and exercise—An anatomical mapping. Atheroscler. Suppl. 2019, 40, 73–78. [Google Scholar] [CrossRef]
- Rojas-Fernandez, C.; Hudani, Z.; Bittner, V. Statins and Cognitive Side Effects. Endocrinol. Metab. Clin. North Am. 2016, 45, 101–116. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, S.; Caffarelli, C.; Stolakis, K.; Cuda, C.; Giordano, N.; Nuti, R. Efficacy and Tolerability of a Nutraceutical Combination (Red Yeast Rice, Policosanols, and Berberine) in Patients with Low-Moderate Risk Hypercholesterolemia: A Double-Blind, Placebo-Controlled Study. Curr. Ther. Res. 2015, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, T. Monascus Rice Products. Adv. Food Nutr. Res. 2007, 53, 123–159. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, Y.; Wang, Y.; Zhu, J.-S.; Chang, J.; Kritchevsky, D. Monascus purpureus-fermented rice (red yeast rice): A natural food product that lowers blood cholesterol in animal models of hypercholesterolemia. Nutr. Res. 1998, 18, 71–81. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Wang, T.-H.; Lee, M.-H.; Su, N.-W. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Gordon, R.Y.; Halbert, S.C.; French, B.; Morris, P.B.; Rader, D.J. Red Yeast Rice for Dyslipidemia in Statin-Intolerant Patients. Ann. Intern. Med. 2009, 150, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Borden, W.B. Red yeast rice for dyslipidemia in statin-intolerant patients. Curr. Atheroscler. Rep. 2010, 12, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Tsai, T.-Y.; Wang, J.-J.; Pan, T.-M. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl. Microbiol. Biotechnol. 2005, 70, 533–540. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, R.; Guo, W.; Hong, J.; Li, L.; Ni, L.; Sun, J.; Liu, B.; Rao, P.; Lv, X.-C. Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high-fat diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef]
- Fang, Y.-X.; Song, H.-P.; Liang, J.-X.; Li, P.; Yang, H. Rapid screening of pancreatic lipase inhibitors from Monascus-fermented rice by ultrafiltration liquid chromatography-mass spectrometry. Anal. Methods 2017, 9, 3422–3429. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.-B.; Chen, D.; Zhang, A.-M.; Niu, S.-Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Yuliana, A.; Singgih, M.; Julianti, E.; Blanc, P.J. Derivates of azaphilone Monascus pigments. Biocatal. Agric. Biotechnol. 2017, 9, 183–194. [Google Scholar] [CrossRef]
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and Biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Blanc, P.J. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Xie, J.; Li, X.; Huang, Z. Effects of some flavonoids on the mycotoxin citrinin reduction by Monascus aurantiacus Li AS3.4384 during liquid-state fermentation. AMB Express 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Liang, B.; Du, X.-J.; Li, P.; Sun, C.-C.; Wang, S. Investigation of Citrinin and Pigment Biosynthesis Mechanisms in Monascus purpureus by Transcriptomic Analysis. Front. Microbiol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Huang, Y.; Xin, Q.; Wang, Z. Diversifying of Chemical Structure of Native Monascus Pigments. Front. Microbiol. 2018, 9, 3143. [Google Scholar] [CrossRef]
- Saleh-E-In, M.; Roy, A.; Al-Mansur, M.A.; Hasan, C.M.; Rahim, M.; Sultana, N.; Ahmed, S.; Islam, R.; Amoo, S.O. Isolation and in silico prediction of potential drug-like compounds from Anethum sowa L. root extracts targeted towards cancer therapy. Comput. Biol. Chem. 2019, 78, 242–259. [Google Scholar] [CrossRef]
- Yi, F.; Li, L.; Xu, L.-J.; Meng, H.; Dong, Y.-M.; Liu, H.-B.; Xiao, P.-G. In silico approach in reveal traditional medicine plants pharmacological material basis. Chin. Med. 2018, 13, 33. [Google Scholar] [CrossRef]
- Agyei, D.; Bambarandage, E.; Udenigwe, C.C. The Role of Bioinformatics in the Discovery of Bioactive Peptides. Encycl. Food Chem. 2019, 337–344. [Google Scholar] [CrossRef]
- Ji, D.; Udenigwe, C.C.; Agyei, D. Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment. Food Sci. Hum. Wellness 2019, 8, 306–314. [Google Scholar] [CrossRef]
- Ji, D.; Xu, M.; Udenigwe, C.C.; Agyei, D. Physicochemical characterisation, molecular docking, and drug-likeness evaluation of hypotensive peptides encrypted in flaxseed proteome. Curr. Res. Food Sci. 2020, 3, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Isyaku, Y.; Uzairu, A.; Uba, S. Computational studies of a series of 2-substituted phenyl-2-oxo-, 2-hydroxyl- and 2-acylloxyethylsulfonamides as potent anti-fungal agents. Heliyon 2020, 6, e03724. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Mokhnache, K.; Madoui, S.; Khither, H.; Charef, N. Drug-likeness and pharmacokinetics of a bis-phenolic ligand: Evaluations by computational methods. Sch. J. App. Med. Sci. 2019, 1, 167–173. [Google Scholar]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 and Chemical Toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Van Waterschoot, R.A.B.; Schinkel, A.H. A Critical Analysis of the Interplay between Cytochrome P450 3A and P-Glycoprotein: Recent Insights from Knockout and Transgenic Mice. Pharmacol. Rev. 2011, 63, 390–410. [Google Scholar] [CrossRef]
- Jeun, J.; Jung, H.; Kim, J.H.; Kim, Y.O.; Youn, S.H.; Shin, C.S. Effect of the monascus pigment threonine derivative on regulation of the cholesterol level in mice. Food Chem. 2008, 107, 1078–1085. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; López-Vallejo, F.; Rodríguez-Morales, S.; Castillo, R.; Chamorro, G.; Tamariz, J. Molecular docking of the highly hypolipidemic agent α-asarone with the catalytic portion of hmg-coa reductase. Bioorganic Med. Chem. Lett. 2005, 15, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Ressaissi, A.; Attia, N.; Falé, P.L.; Pacheco, R.; Victor, B.L.; Machuqueiro, M.; Serralheiro, M.L. Isorhamnetin derivatives and piscidic acid for hypercholesterolemia: Cholesterol permeability, HMG-CoA reductase inhibition, and docking studies. Arch. Pharmacal Res. 2017, 40, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Aluko, R.E.; Ju, X.-R. Evaluating Molecular Mechanism of Hypotensive Peptides Interactions with Renin and Angiotensin Converting Enzyme. PLoS ONE 2014, 9, e91051. [Google Scholar] [CrossRef]
- Jimsheena, V.; Gowda, L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: Structure–activity relationship. Peptides 2010, 31, 1165–1176. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.O.; Jeun, J.; Choi, D.-Y.; Shin, C.S. L-Trp andL-Leu-OEt Derivatives of the Monascus Pigment Exert High Anti-Obesity Effects on Mice. Biosci. Biotechnol. Biochem. 2010, 74, 304–308. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wen, J.-Y.; Hsu, Y.-W.; Pan, T.-M. Monascus-Fermented Yellow Pigments Monascin and Ankaflavin Showed Antiobesity Effect via the Suppression of Differentiation and Lipogenesis in Obese Rats Fed a High-Fat Diet. J. Agric. Food Chem. 2013, 61, 1493–1500. [Google Scholar] [CrossRef]
- Liu, S.-F.; Wang, Y.-R.; Shen, Y.-C.; Chen, C.-L.; Huang, C.-N.; Pan, T.-M.; Wang, C.-K. A randomized, double-blind clinical study of the effects of Ankascin 568 plus on blood lipid regulation. J. Food Drug Anal. 2018, 26, 393–400. [Google Scholar] [CrossRef]
- Chen, C.-L.; Tseng, J.-H.; Hsiao, S.-H.; Pan, T.-M. A Randomized, Double-Blind Clinical Study on Blood Pressure Reduction and Blood Lipid Profile Amelioration on Treatment with Ankascin 568. Chin. J. Physiol. 2017, 60, 158–165. [Google Scholar] [CrossRef] [PubMed]




| No. | Color | Name | Canonical SMILES | Molecular Formula |
|---|---|---|---|---|
| 1 | yellow | monascin | CCCCCC(=O)C1C(=O)OC2(C1CC1=C(C2=O)COC(=C1)/C=C/C)C | C21H26O5 |
| 2 | yellow | ankaflavin | CCCCCCCC(=O)C1C(=O)OC2(C1CC1=C(C2=O)COC(=C1)/C=C/C)C | C23H30O5 |
| 3 | orange | rubropunctatin | CCCCCC(=O)C1=C2C=C3C=C(/C=C/C)OC=C3C(=O)C2(OC1=O)C | C21H22O5 |
| 4 | orange | monascorubrin | CCCCCCCC(=O)C1=C2C=C3C=C(/C=C\C)OC=C3C(=O)C2(OC1=O)C | C23H26O5 |
| 5 | red | rubropunctamine | CCCCCC(=O)C1=C2C=C3C=C(NC=C3C(=O)C2(C)OC1=O)\C=C\C | C21H23NO4 |
| 6 | red | monascorubramine | CCCCCCCC(=O)C1=C2C=C3C=C(NC=C3C(=O)C2(C)OC1=O)\C=C\C | C23H27NO4 |
| 7 | yellow | xanthomonasin A | CCCCCC(=O)C1=C2c3oc(c(c3C[C@@]([C@]2(OC1=O)C)(O)/C=C/C)C=O)O | C21H24O7 |
| 8 | yellow | xanthomonasin B | CCCCCCCC(=O)C1=C(O)O[C@]2(C1=C1OC(=O)C(=C1C[C@@]2(O)/C=C/C)C=O)C | C23H28O7 |
| 9 | yellow | yellow Ⅱ | CCCCCCCC(=O)C1=C(O)OC2(C1=CC1=C/C(=C/C)/OCC1C2=O)C | C22H28O5 |
| 10 | yellow | monankarin A | C[C@@H]1CC(=O)C=C(O1)c1cc2c(oc1=O)cc(c(c2[C@H]([C@H](O)C)C)C)O | C20H22O6 |
| 11 | yellow | monankarin B | C[C@@H]1CC(=O)C=C(O1)c1cc2c(oc1=O)cc(c(c2[C@@H]([C@H](O)C)C)C)O | C20H22O6 |
| 12 | yellow | monankarin C | CC1CC(=O)C=C(O1)c1cc2c([C@H]([C@H](O)C)C)c(C)c(c(c2oc1=O)C)O | C21H24O6 |
| 13 | yellow | monankarin D | CC1CC(=O)C=C(O1)c1cc2c([C@@H]([C@@H](O)C)C)c(C)c(c(c2oc1=O)C)O | C21H24O6 |
| 14 | yellow | monankarin E | CC(Cc1cc(O)c(c2c1cc(C1=CC(=O)CC(O1)C)c(=O)o2)C)O | C19H20O6 |
| 15 | yellow | monankarin F | CC1CC(=O)C=C(O1)c1cc2c(CC(O)C)c(C)c(c(c2oc1=O)C)O | C20H22O6 |
| 16 | yellow | monascusone A | C[C@@H](CC1=CC2=C(CO1)C(=O)[C@]([C@H](C2)O)(C)O)O | C13H18O5 |
| 17 | yellow | monascusone B | C/C=C/C1=CC2=C(CO1)C(=O)[C@]1([C@H](C2)[C@@H](C(=O)C)C(=O)O1)C | C17H18O5 |
| 18 | yellow | FK17-P2B2 | C/C=C/C1=CC2=C(CO1)C(=O)[C@]([C@H](C2)O)(C)O | C13H16O4 |
| 19 | yellow | Y3 | CC(CC(C(CC(=O)O)(O)C)(c1c(OC(=S)CC(O)C)cc(c(c1O)C)O)O)C | C20H30O8S |
| 20 | yellow | monaphilone A | CCCCCCCC(=O)C[C@H]1CC2=C(C(=O)[C@]1(C)O)COC(=C2)/C=C/C | C22H32O4 |
| 21 | yellow | monaphilone B | CCCCCC(=O)C[C@H]1CC2=C(C(=O)[C@]1(C)O)COC(=C2)/C=C/C | C20H28O4 |
| 22 | yellow | monaphilone C | CCCCCC(=O)C[C@@H]1CC(=C(C(=O)[C@]1(C)O)C)CC(=O)CCC | C20H32O4 |
| 23 | yellow | monapurone A | CCCCCC(=O)C[C@@H]1C2=COC(=CC2=CC(=O)[C@]1(C)O)/C=C/C | C20H26O4 |
| 24 | yellow | monapurone B | CCCCC[C@@]1(OC)C[C@H]2[C@](O1)(C)C(=O)C=C1C2=COC(=C1)/C=C/C | C21H28O4 |
| 25 | yellow | monapurone C | CCCCC[C@]1(OC)C[C@H]2[C@](O1)(C)C(=O)C=C1C2=COC(=C1)/C=C/C | C21H28O4 |
| 26 | yellow | monarubrin | CCCCCC(=O)CC1C=C2C=C(/C=C/C)OC=C2C(=O)C1(C)O | C20H26O4 |
| 27 | yellow | rubropunctin | CCCCCCCC(=O)CC1C=C2C=C(/C=C/C)OC=C2C(=O)C1(C)O | C22H30O4 |
| 28 | orange | monapilol A | CCCCCCCC(=O)C1=C2C=C3C=C(/C=C/C)OC=C3[C@@H]([C@@]2(OC1=O)C)O | C23H28O5 |
| 29 | orange | monapilol B | CCCCCC(=O)C1=C2C=C3C=C(/C=C/C)OC=C3[C@@H]([C@@]2(OC1=O)C)O | C21H24O5 |
| 30 | orange | monapilol C | CCCCCCCC(=O)C1=C2C=C3C=C(/C=C/C)OC=C3[C@]([C@@]2(OC1=O)C)(O)CC(=O)C | C26H32O6 |
| 31 | orange | monapilol D | CCCCCC(=O)C1=C2C=C3C=C(/C=C/C)OC=C3[C@]([C@@]2(OC1=O)C)(O)CC(=O)C | C24H28O6 |
| 32 | red | N-glucosylrubropunctamine | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H]1OC(CO)[C@@H]([C@H](C1O)O)O | C27H33NO9 |
| 33 | red | N-glucosylmonascorubramine | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H]1OC(CO)[C@@H]([C@H](C1O)O)O | C29H37NO9 |
| 34 | red | N-glutarylrubropunctamine | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)C2(OC1=O)C)C(C(=O)O)CCC(=O)O | C26H29NO8 |
| 35 | red | N-glutarylmonascorubramine | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)C2(OC1=O)C)C(C(=O)O)CCC(=O)O | C28H33NO8 |
| 36 | red | Red Derivat 1 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H](C(=O)O)C | C26H31NO6 |
| 37 | red | Red Derivat 2 | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H](C(=O)O)C | C24H27NO6 |
| 38 | red | Red Derivat 3 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H](C(=O)O)CC(=O)O | C27H31NO8 |
| 39 | red | Red Derivat 4 | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@H](C(=O)O)CC(=O)O | C25H27NO8 |
| 40 | red | Red Derivat 5 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@@H](C(=O)O)C | C26H31NO6 |
| 41 | red | Red Derivat 6 | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@@H](C(=O)O)C | C24H27NO6 |
| 42 | red | Red Derivat 7 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@@H](C(=O)O)CC(=O)O | C27H31NO8 |
| 43 | red | Red Derivat 8 | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)[C@@]2(OC1=O)C)[C@@H](C(=O)O)CC(=O)O | C25H27NO8 |
| 44 | red | R3 | CCCCCC(=O)C1C(=O)OC2(C1C1=COC(=CC1=CC2=O)CC(O)C)C | C21H26O6 |
| 45 | red | Unamed | C/C=C/C1=CC2=CC3=[O+]NOC3(C(C2CN1C(C(=O)O)CCCCN)O)C | C19H28N3O5 |
| 46 | red | PP-V | CCCCCCCC(=O)C1=C2C=C3C=C(/C=C\C(=O)[O-])[NH2+]C=C3C(=O)C2(OC1=O)C | C23H25NO6 |
| 47 | red | New Red Pigment | CCCCCC(C1C(=O)OC2(C1c1c[nH]c(cc1=CC2=O)CC(O)C)C)O | C21H29NO5 |
| 48 | red | Isolate MPs 1 | CCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)C2(OC1=O)C)C(C(=O)O)CCCNC(=N)N | C27H34N4O6 |
| 49 | red | Isolate MPs 2 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)C2(OC1=O)C)C(C(=O)O)CCCNC(=N)N | C29H38N4O6 |
| 50 | red | Isolate MPs 3 | CCCCCCCC(=O)C1=C2C=c3cc(/C=C/C)n(cc3C(=O)C2(OC1=O)C)CC(=O)O | C25H29NO6 |
| 51 | red | glycyl-rubropunctatin | CCCCCC(=O)C1=C2CC3=C(C(=O)[C@@]2(OC1=O)C)CN(C(=C3)/C=C/C)CC(=O)O | C23H27NO6 |
| 52 | red | Isolate MPs 4 | CCCCCCCC(=O)C1=C2CC3=C(C(=O)[C@@]2(OC1=O)C)CN(C(=C3)/C=C/C)CC(=O)O | C25H31NO6 |
| 53 | red | Monascopyridine A | CCCCCC(=O)[C@@H]1C(=O)O[C@]2([C@H]1Cc1cc(/C=C/C)ncc1C2=O)C | C21H25NO4 |
| 54 | red | Monascopyridine B | CCCCCCCC(=O)[C@@H]1C(=O)O[C@]2([C@H]1Cc1cc(/C=C/C)ncc1C2=O)C | C23H29NO4 |
| 55 | red | Monascopyridine C | CCCCCC(=O)C[C@@H]1Cc2cc(/C=C/C)ncc2C(=O)[C@@]1(C)O | C20H27NO3 |
| 56 | red | Monascopyridine D | CCCCCCCC(=O)C[C@@H]1Cc2cc(/C=C/C)ncc2C(=O)[C@@]1(C)O | C22H31NO3 |
| 57 | yellow | Monasfluore A | CCCCCC(=O)C1C(=O)OC2(C1C1=COC(=CC1=CC2=O)/C=C/C)C | C21H24O5 |
| 58 | yellow | Monasfluore B | CCCCCCCC(=O)C1C(=O)OC2(C1C1=COC(=CC1=CC2=O)/C=C/C)C | C23H28O5 |
| 59 | yellow | purpureusone | CCCCCCCC(=O)[C@H]1C(=O)O[C@@]2(C1CC(=C(C2=O)C)CC(=O)CCC)C | C23H34O5 |
| 60 | red | Red Shandong 1 | C=CCCCC(C1=CC2=C/C(=C/C=C)/NCC2C(C1O)O)O | C18H25NO3 |
| 61 | red | Red Shandong 2 | C=CCCCCCC(C1=CC2=C/C(=C/C=C)/NCC2C(C1O)O)O | C20H29NO3 |
| Ligand | Affinity Energy (kJ/mol) | Ki (μM) |
|---|---|---|
| Monascin | −28.88 | 8.66 |
| Ankaflavin | −28.88 | 8.66 |
| Rubropunctatin | −28.89 | 8.63 |
| Monascorubrin | −28.89 | 8.63 |
| Rubropunctamine | −28.05 | 12.11 |
| Monascorubramine | −26.79 | 20.14 |
| Monacolin K | −30.98 | 3.71 |
| Monascin | Ankaflavin | Rubropunctatin | Monascorubrin | Rubropunctamine | Monascorubramine | Monacolin K | |
|---|---|---|---|---|---|---|---|
| GLU559 | ▲ | ||||||
| CYS561 | ● | ||||||
| LEU562 | ● | ||||||
| SER565 | ▲ | ||||||
| ARG590 | ▲ | ▲ | ▲ | ▲ | |||
| ALA654 | ● | ● | |||||
| MET655 | ● | ● | |||||
| MET657 | ▲ | ▲ | |||||
| ASN658 | ▲ | ▲ | |||||
| MET659 | ▲ | ▲ | |||||
| SER661 | ▲ | ▲ | |||||
| VAL683 | ● | ● | ● | ● | ● | ||
| LYS691 | ▲ | ▲ | ▲ | ▲ | |||
| LYS735 | |||||||
| HIS752 | ▲ | ▲ | ● | ▲ | ● | ||
| ASN755 | ▲ | ▲ | ▲ | ▲ | |||
| VAL805 | ● | ● | |||||
| GLY806 | |||||||
| GLY807 | |||||||
| GLY808 | |||||||
| LEU853 | ● | ● | ● | ● | ● | ||
| ALA856 | ● | ● | ● | ||||
| LEU857 | ● | ● | ● | ● |
| HMGR Residues in H-Bonding | Number of H-Bonds and Their Corresponding Distance (Å) | ||||||
|---|---|---|---|---|---|---|---|
| Monascin | Ankaflavin | Rubropunctatin | Monascorubrin | Rubropunctamine | Monascorubramine | Monacolin K | |
| GLU559:OE2 | 1(1.98) | ||||||
| ARG590:HH11 | 1(2.03) | ||||||
| ARG590:HH21 | 1(1.83) | 1(3.01) | |||||
| ARG590:HH22 | 1(2.31) | 1(2.40) | |||||
| MET657:HN | 1(2.77) | 1(2.73) | |||||
| MET659:HN | 1(2.23) | 1(2.41) | |||||
| ASN658:HD21 | 1(2.03) | 1(2.14) | |||||
| ASN658:HN | 2(2.16, 2.23) | 2(2.38, 2.16) | |||||
| SER661:HG | 1(2.34) | 1(2.58) | |||||
| LYS691:HZ3 | 1(2.40) | 1(2.31) | 1(2.62) | 1(2.40) | |||
| HIS752:HD1 | 1(2.75) | 1(2.50) | 1(2.60) | ||||
| ASN755:HD21 | 1(2.82) | 1(2.68) | 1(2.53) | ||||
| ASN755:HD22 | 1(2.71) | 1(2.84) | 1(2.21) | ||||
| Total | 6 | 5 | 6 | 5 | 3 | 4 | 2 |
| Ligand | Affinity Energy (kJ/mol) | Ki (μM) |
|---|---|---|
| Monascin | −28.03 | 12.19 |
| Ankaflavin | −28.87 | 8.70 |
| Rubropunctatin | −25.52 | 33.59 |
| Monascorubrin | −29.29 | 7.35 |
| Rubropunctamine | −29.29 | 7.35 |
| Monascorubramine | −24.69 | 47.08 |
| Oleic acid | −19.66 | 357.23 |
| Monascin | Ankaflavin | Rubropunctatin | Monascorubrin | Rubropunctamine | Monascorubramine | Oleic Acid | |
|---|---|---|---|---|---|---|---|
| ASN89 | ▲ | ▲ | ▲ | ||||
| PRO236 | ● | ||||||
| ILE249 | ● | ● | ● | ||||
| LYS269 | ● | ● | ● | ● | ▲ | ||
| ARG266 | ▲ | ||||||
| ALA272 | ● | ● | ● | ||||
| ASP273 | ■ | ■ | ■ | ||||
| TYR289 | ●▲ | ||||||
| ASN329 | ▲ | ||||||
| ALA333 | ● | ● | |||||
| PHE336 | ● | ● | ● | ● | |||
| ARG338 | ●▲ |
| Lipase Residues in H-Bonding | Number of H-Bonds and Their Corresponding Distance (Å) | ||||||
|---|---|---|---|---|---|---|---|
| Monascin | Ankaflavin | Rubropunctatin | Monascorubrin | Rubropunctamine | Monascorubramine | Oleic Acid | |
| ASN89:HD21 | 1(2.92) | ||||||
| ASN89:HD22 | 1(2.32) | 2(2.43, 3.05) | |||||
| ARG266:HH21 | 1(2.72) | ||||||
| LYS269:CA | 1(3.43) | ||||||
| TYR289:HH | 1(2.54) | ||||||
| ASN329:OD1 | 1(3.30) | ||||||
| ARG338:HH11 | 1(2.77) | ||||||
| Total | 1 | 1 | 3 | 2 | 0 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Agyei, D.; Ji, D. Medicinal Chemistry Friendliness of Pigments from Monascus-Fermented Rice and the Molecular Docking Analysis of Their Anti-Hyperlipidemia Properties. Fermentation 2020, 6, 111. https://doi.org/10.3390/fermentation6040111
Sun N, Agyei D, Ji D. Medicinal Chemistry Friendliness of Pigments from Monascus-Fermented Rice and the Molecular Docking Analysis of Their Anti-Hyperlipidemia Properties. Fermentation. 2020; 6(4):111. https://doi.org/10.3390/fermentation6040111
Chicago/Turabian StyleSun, Nina, Dominic Agyei, and Dawei Ji. 2020. "Medicinal Chemistry Friendliness of Pigments from Monascus-Fermented Rice and the Molecular Docking Analysis of Their Anti-Hyperlipidemia Properties" Fermentation 6, no. 4: 111. https://doi.org/10.3390/fermentation6040111
APA StyleSun, N., Agyei, D., & Ji, D. (2020). Medicinal Chemistry Friendliness of Pigments from Monascus-Fermented Rice and the Molecular Docking Analysis of Their Anti-Hyperlipidemia Properties. Fermentation, 6(4), 111. https://doi.org/10.3390/fermentation6040111





