Removal of Small-Molecular Byproducts from Crude Fructo-Oligosaccharide Preparations by Fermentation Using the Endospore-Forming Probiotic Bacillus coagulans
Abstract
:1. Introduction
2. Materials and Methods
2.1. FOS Synthesis
2.2. Microorganisms
2.3. Culture Media
2.4. Cultivation of B. coagulans
2.5. Determination of Sporulation Rate
2.6. Quantitation of FOS
3. Results
3.1. Growth and Sporulation of Different Strains of B. coagulans
3.2. Sporulation of B. coagulans in Basal Medium
3.3. Fermentation of B. coagulans in the Bioreactor for the Treatment of Crude FOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cfu | colony forming unit |
| DSMZ | Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (German Collection of Microorganisms and Cell Cultures) |
| HPLC | high performance liquid chromatography |
| FOS | fructo-oligosaccharides |
| FTase | fructosyltransferase |
| GF2 | 1-kestose |
| GF3 | nystose |
| GF4 | fructofranosyl nystose |
| GI | glycemic index |
| GYEA | Glucose Yeast Extract Agar |
| OD600 | optical density at λ = 600 nm |
References
- Kuo, S.-M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 2013, 4, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, Purification and Potential Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Slavin, J. Nondigestible oligosaccharides. Crit. Rev. Food Sci. Nutr. 2000, 40, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Sun, Y.; Ye, H.; Hu, B.; Zeng, X. Influences of structures of galactooligosaccharides and fructooligosaccharides on the fermentation in vitro by human intestinal microbiota. J. Funct. Foods 2015, 13, 158–168. [Google Scholar] [CrossRef]
- Gibson, G.R.; Wang, X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Molis, C.; Flourié, B.; Ouarne, F.; Gailing, M.F.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J.P. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, S. The Metabolism of Fructooligosaccharides and Fructooligosaccharide-Related Compounds in Plants. Food Rev. Int. 2010, 27, 16–50. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415S–420S. [Google Scholar] [CrossRef]
- Bornet, F.R.J.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef]
- Mutanda, T.; Mokoena, M.P.; Olaniran, A.O.; Wilhelmi, B.S.; Whiteley, C.G. Microbial enzymatic production and applications of short-chain fructooligosaccharides and inulooligosaccharides: Recent advances and current perspectives. J. Ind. Microbiol. Biotechnol. 2014, 41, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Recent trends in the microbial production, analysis and application of Fructooligosaccharides. Trends Food Sci. Technol. 2005, 16, 442–457. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic Oligosaccharides: Special Focus on Fructooligosaccharides, Its Biosynthesis and Bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Hunter, D.E. Selected Fructooligosaccharide (1-Kestose, Nystose, and 1 F -β-Fructofuranosylnystose) Composition of Foods and Feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
- Vega-Paulino, R.J.; Zúniga-Hansen, M.E. Potential application of commercial enzyme preparations for industrial production of short-chain fructooligosaccharides. J. Mol. Catal. B Enzym. 2012, 76, 44–51. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Kovacs, Z.; Quitmann, H.; Ebrahimi, M.; Czermak, P. Enzymatic production of fructo-oligosaccharides from inexpensive and abundant substrates using a membrane reactor system. Sep. Sci. Technol. 2016, 58, 1537–1545. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Lateef, A.; Gupta, U.S. Enzymatic trends of fructooligosaccharides production by microorganisms. Appl. Biochem. Biotechnol. 2014, 172, 2143–2159. [Google Scholar] [CrossRef]
- Hang, Y.D.; Woodams, E.E. Optimization of Enzymatic Production of Fructo-oligosaccharides from Sucrose. LWT 1996, 29, 578–580. [Google Scholar] [CrossRef]
- Ghazi, I.; Fernandez-Arrojo, L.; Garcia-Arellano, H.; Ferrer, M.; Ballesteros, A.; Plou, F.J. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 2007, 128, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Tanriseven, A.; Aslan, Y. Immobilization of Pectinex Ultra SP-L to produce fructooligosaccharides. Enzym. Microb. Technol. 2005, 36, 550–554. [Google Scholar] [CrossRef]
- Spohner, S.C.; Czermak, P. Heterologous expression of Aspergillus terreus fructosyltransferase in Kluyveromyces lactis. New Biotechnol. 2016, 33, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mishra, S. Enrichment and evaluation of galacto-oligosaccharides produced by whole cell treatment of sugar reaction mixture. Mol. Biol. Rep. 2019, 46, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.-L. Glycemic index of foods and glycemic control in type I diabetes. Curr. Opin. Endocrinol. Diabetes 2000, 7, 25–30. [Google Scholar] [CrossRef]
- Wong, J.M.W.; Jenkins, D.J.A. Carbohydrate digestibility and metabolic effects. J. Nutr. 2007, 137, 2539S–2546S. [Google Scholar] [CrossRef]
- Campos, D.; Mescua, L.; Aguilar-Galvez, A.; Chirinos, R.; Pedreschi, R. Effect of Yacon (Smallanthus sonchifolius) fructooligosaccharide purification technique using activated charcoal or ion exchange fixed bed column on recovery, purity and sugar content. Int. J. Food Sci. Technol. 2017, 52, 2637–2646. [Google Scholar] [CrossRef]
- Nobre, C.; Suvarov, P.; De Weireld, G. Evaluation of commercial resins for fructo-oligosaccharide separation. New Biotechnol. 2014, 31, 55–63. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Palacio, L.; Prádanos, P.; Hernández, A.; Filho, F.M. Selection of membranes for purification of fructooligosaccharides. Desalin. Water Treat. 2011, 27, 18–24. [Google Scholar] [CrossRef]
- Alles, M.J.L.; Tessaro, I.C.; Noreña, C.P.Z. Concentration and Purification of Yacon (Smallanthus sonchifolius) Root Fructooligosaccharides Using Membrane Technology. Food Technol. Biotechnol. 2015, 53, 190–200. [Google Scholar] [CrossRef]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. Fructo-oligosaccharides purification from a fermentative broth using an activated charcoal column. New Biotechnol. 2012, 29, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Jong, W.Y.; Seung, K.S. The production of high-content fructo-oligosaccharides from sucrose by the mixed-enzyme system of fructosyltransferase and glucose oxidase. Biotechnol. Lett. 1993, 15, 573–576. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Li, D.; Jiang, Y. Co-immobilization of glucose oxidase and catalase in silica inverse opals for glucose removal from commercial isomaltooligosaccharide. Int. J. Biol. Macromol. 2018, 107, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Fructooligosaccharides—Occurrence, preparation, and application. Enzym. Microb. Technol. 1996, 19, 107–117. [Google Scholar] [CrossRef]
- Aslan, Y.; Tanrıseven, A. Immobilization of Pectinex Ultra SP-L to produce galactooligosaccharides. J. Mol. Catal. B Enzym. 2007, 45, 73–77. [Google Scholar] [CrossRef]
- Nobre, C.; Castro, C.C.; Hantson, A.-L.; Teixeira, J.A.; De Weireld, G.; Rodrigues, L.R. Strategies for the production of high-content fructo-oligosaccharides through the removal of small saccharides by co-culture or successive fermentation with yeast. Carbohydr. Polym. 2016, 136, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, D.; Patel, Y.; Doshi, J.A.; Doshi, R.D.; Bose, A. Draft Genome Sequence of Bacillus coagulans ZB29, a Commercial Probiotic Strain. Microbiol. Resour. Announc. 2019, 8, e01125-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO and WHO: Cordoba, Argentina, 2006; Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 10 September 2019).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Aminlari, L.; Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of Probiotics Bacillus coagulans and Lactobacillus plantarum on Lipid Profile and Feces Bacteria of Rats Fed Cholesterol-Enriched Diet. In Probiotics Antimicrobial Proteins; Springer US: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Monteiro, C.R.A.V.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; Dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Li, Z.; Li, K.; Zhang, M.; Wang, C.; Luo, X.; Zhang, T. Screening, Isolation, and Identification of Bacillus coagulans C2 in Pu’er Tea. In Advances in Applied Biotechnology; Zhang, T.-C., Nakajima, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 557–562. ISBN 978-3-662-46317-8. [Google Scholar]
- Sen, R.; Babu, K.S. Modeling and optimization of the process conditions for biomass production and sporulation of a probiotic culture. Process Biochem. 2005, 40, 2531–2538. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Luo, J.; Qi, B.; Wan, Y. An efficient process for lactic acid production from wheat straw by a newly isolated Bacillus coagulans strain IPE22. Bioresour. Technol. 2014, 158, 396–399. [Google Scholar] [CrossRef]
- Weese, J.S.; Martin, H. Assessment of commercial probiotic bacterial contents and label accuracy. Can. Vet. J. 2011, 52, 43–46. [Google Scholar] [PubMed]
- Hussey, M.A.; Zayaitz, A. Endospore Stain Protocol. In Laboratory Protocol; American Society for Microbiology: Washington, DC, USA, 2007; Available online: http://www.asmscience.org/content/education/protocol/protocol.3112 (accessed on 10 May 2019).
- Bast, E. Mikrobiologische Methoden. Eine Einführung in Grundlegende Arbeitstechniken, 2nd ed.; Spektrum Akad.-Verl.: Heidelberg, Germany, 2010; ISBN 978-3-8274-1072-6. [Google Scholar]
- Fan, R.; Ebrahimi, M.; Czermak, P. Anaerobic Membrane Bioreactor for Continuous Lactic Acid Fermentation. Membranes 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, C.; Lyu, P.; Wang, Y.; Wang, L.; Yu, B. Contributory roles of two l-lactate dehydrogenases for l-lactic acid production in thermotolerant Bacillus coagulans. Sci. Rep. 2016, 6, 37916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Sen, R. Kinetic modeling of sporulation and product formation in stationary phase by Bacillus coagulans RK-02 vis-à-vis other Bacilli. Bioresour. Technol. 2011, 102, 9659–9667. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Vakil, B.V. Development of bioprocess for high density cultivation yield the probiotic Bacillus coagulans and its spores. J. BioSci. Biotechnol. 2016, 5, 173–181. [Google Scholar]
- Xiong, T.; Song, S.; Huang, X.; Feng, C.; Liu, G.; Huang, J.; Xie, M. Screening and identification of functional Lactobacillus specific for vegetable fermentation. J. Food Sci. 2013, 78, M84–M89. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, J.; Huang, T.; Xie, M.; Xiao, Y.; Liu, C.; Peng, Z. Fast evaluation by quantitative PCR of microbial diversity and safety of Chinese Paocai inoculated with Lactobacillus plantarum NCU116 as the culture starter. LWT 2019, 101, 201–206. [Google Scholar] [CrossRef]
- Lv, X.; Yu, B.; Tian, X.; Chen, Y.; Wang, Z.; Zhuang, Y.; Wang, Y. Effect of pH, glucoamylase, pullulanase and invertase addition on the degradation of residual sugar in L-lactic acid fermentation by Bacillus coagulans HL-5 with corn flour hydrolysate. J. Taiwan Inst. Chem. Eng. 2016, 61, 124–131. [Google Scholar] [CrossRef]
- Goh, Y.J.; Lee, J.-H.; Hutkins, R.W. Functional analysis of the fructooligosaccharide utilization operon in Lactobacillus paracasei 1195. Appl. Environ. Microbiol. 2007, 73, 5716–5724. [Google Scholar] [CrossRef] [Green Version]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buntin, N.; Hongpattarakere, T.; Ritari, J.; Douillard, F.P.; Paulin, L.; Boeren, S.; Shetty, S.A.; de Vos, W.M. An Inducible Operon Is Involved in Inulin Utilization in Lactobacillus plantarum Strains, as Revealed by Comparative Proteogenomics and Metabolic Profiling. Appl. Environ. Microbiol. 2017, 83, e02402–e02416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, C.C.; Nobre, C.; De Weireld, G.; Hantson, A.-L. Microbial co-culturing strategies for fructo-oligosaccharide production. New Biotechnol. 2019, 51, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobre, C.; Gonçalves, D.A.; Teixeira, J.A.; Rodrigues, L.R. One-step co-culture fermentation strategy to produce high-content fructo-oligosaccharides. Carbohydr. Polym. 2018, 201, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-L.; Wang, J.-H.; Teng, D.; Zhang, F. Preparation of high-purity fructo-oligosaccharides by Aspergillus japonicus beta-fructofuranosidase and successive cultivation with yeast. J. Agric. Food Chem. 2008, 56, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
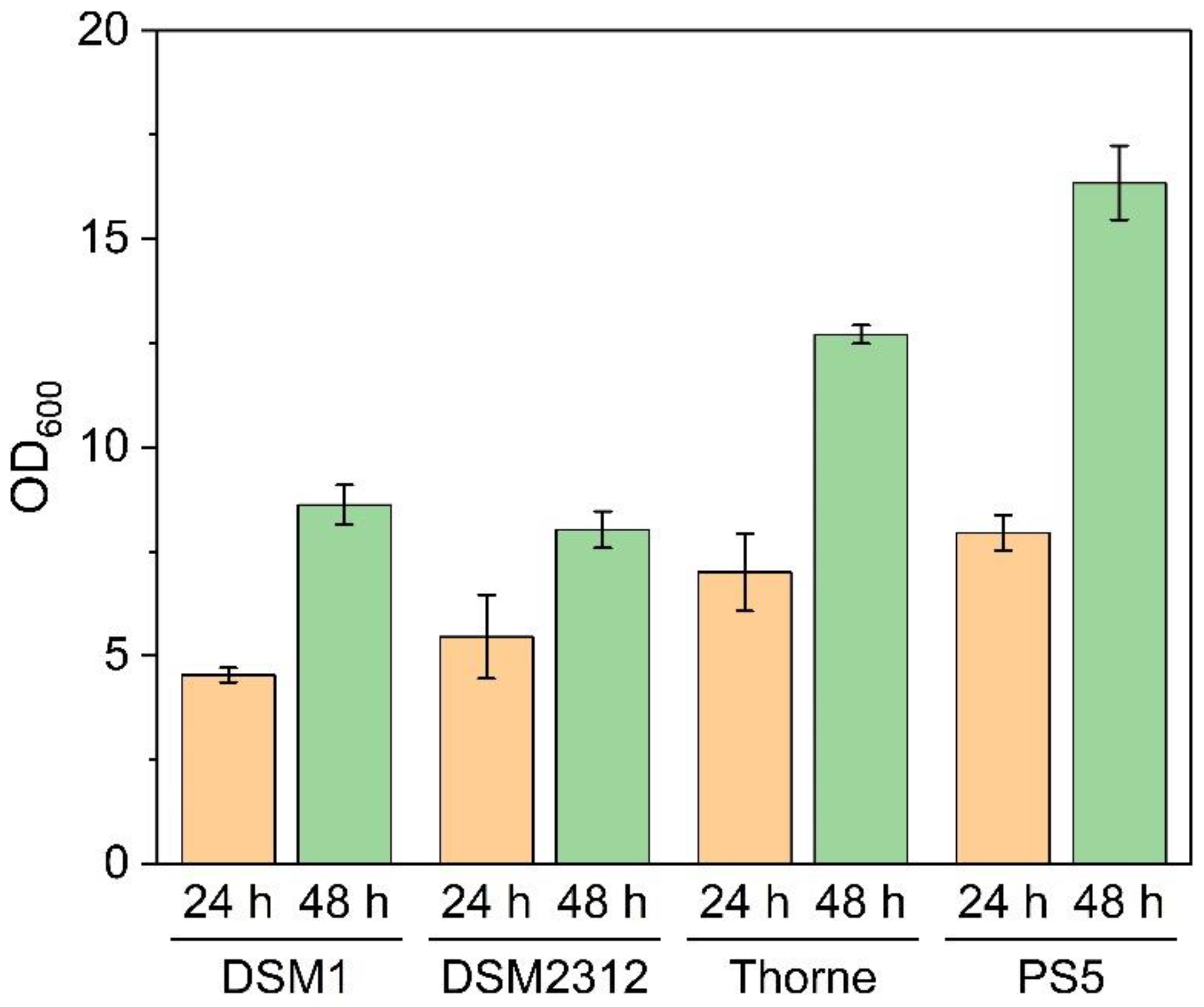
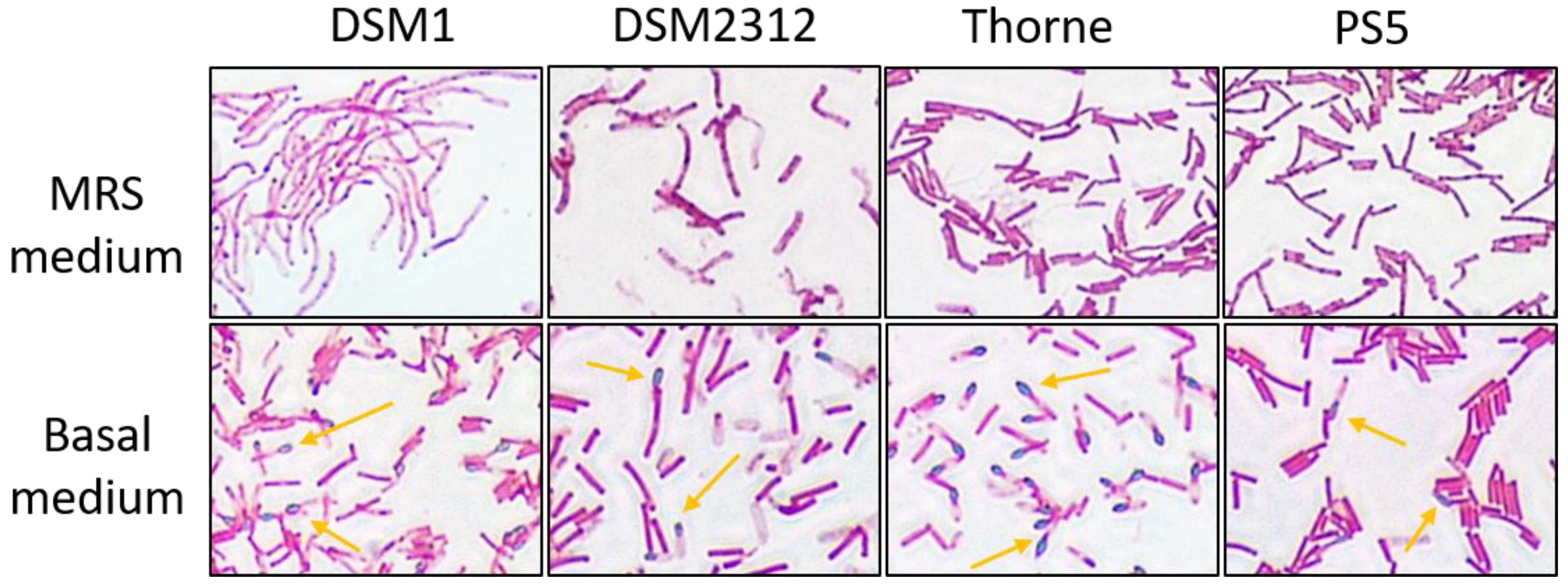
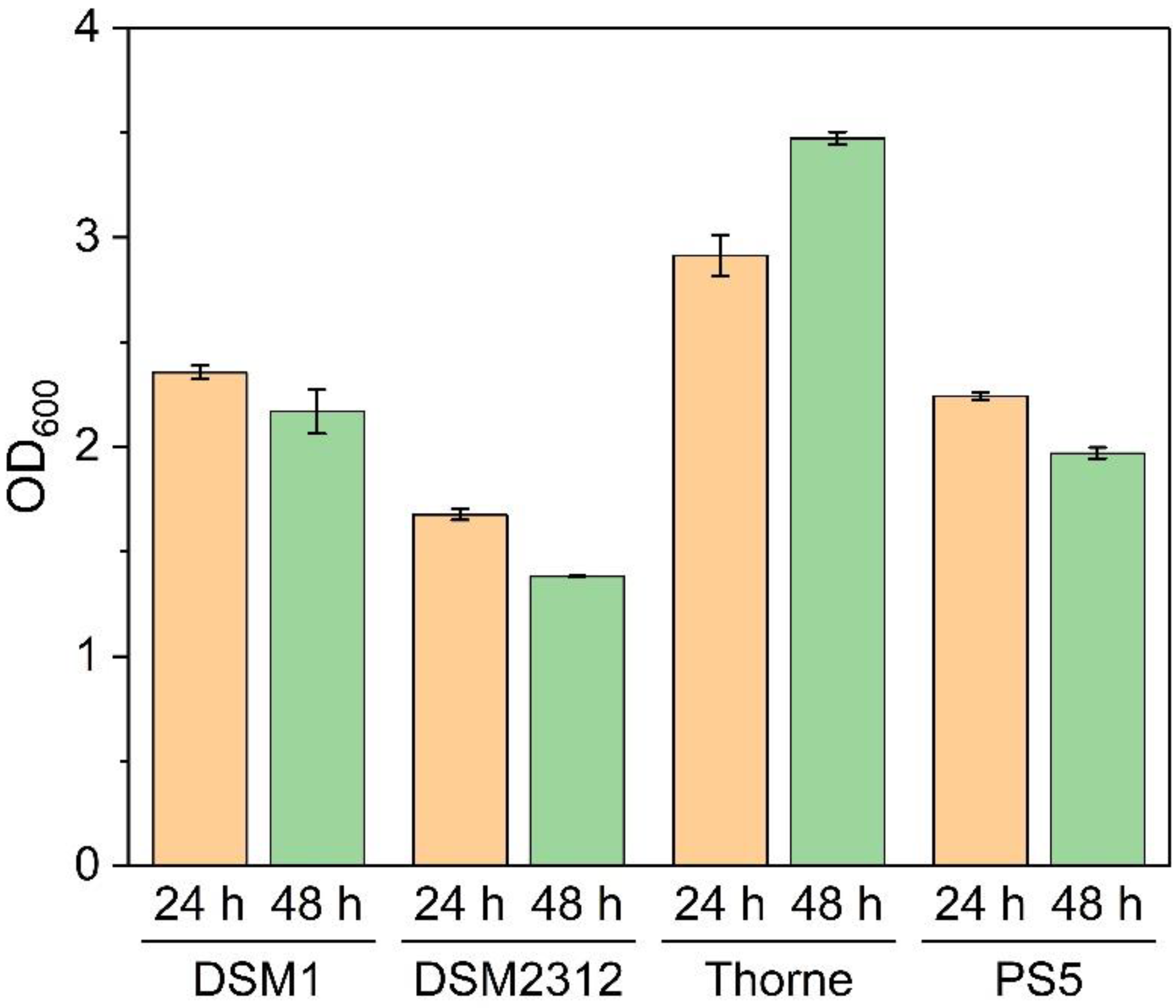
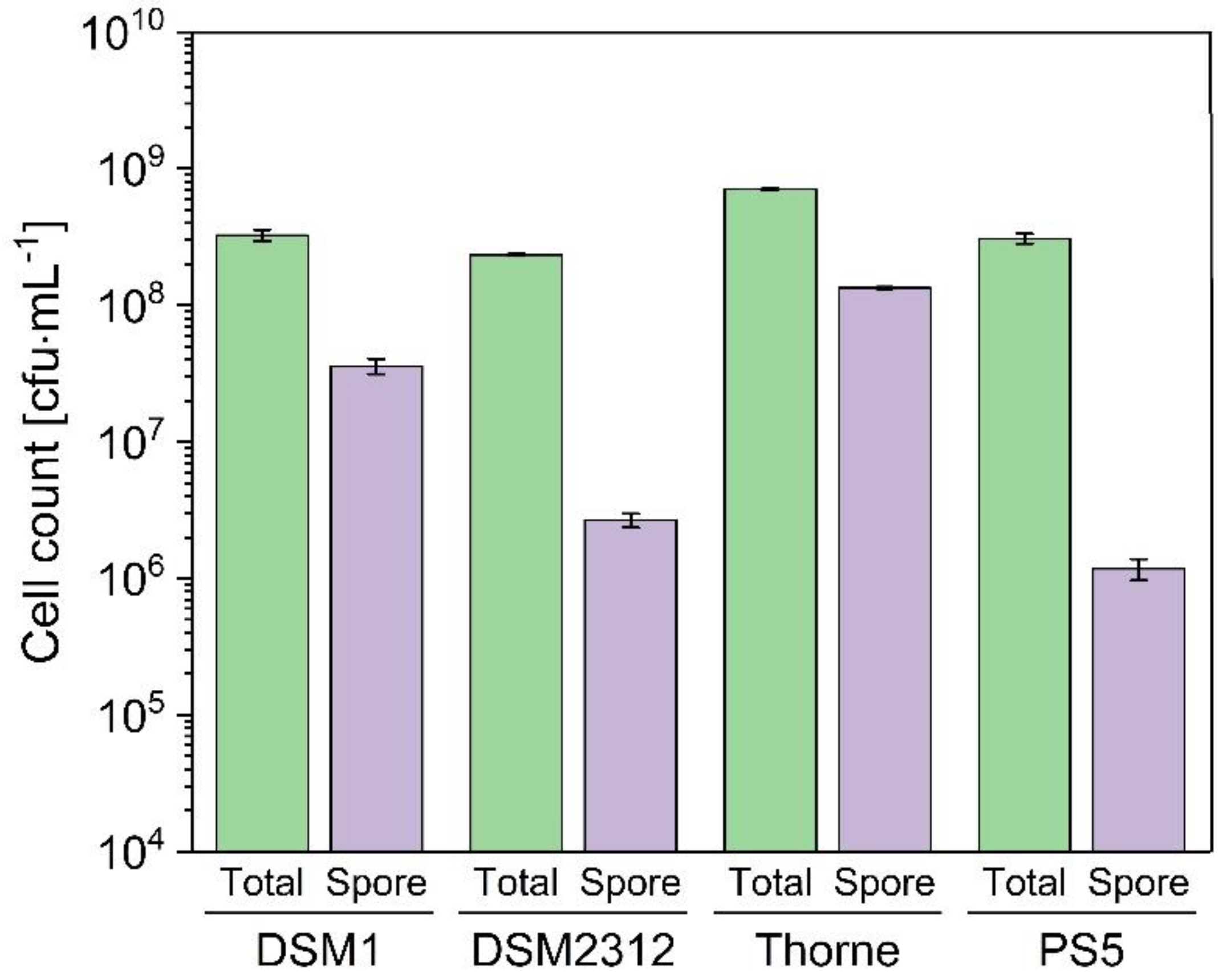
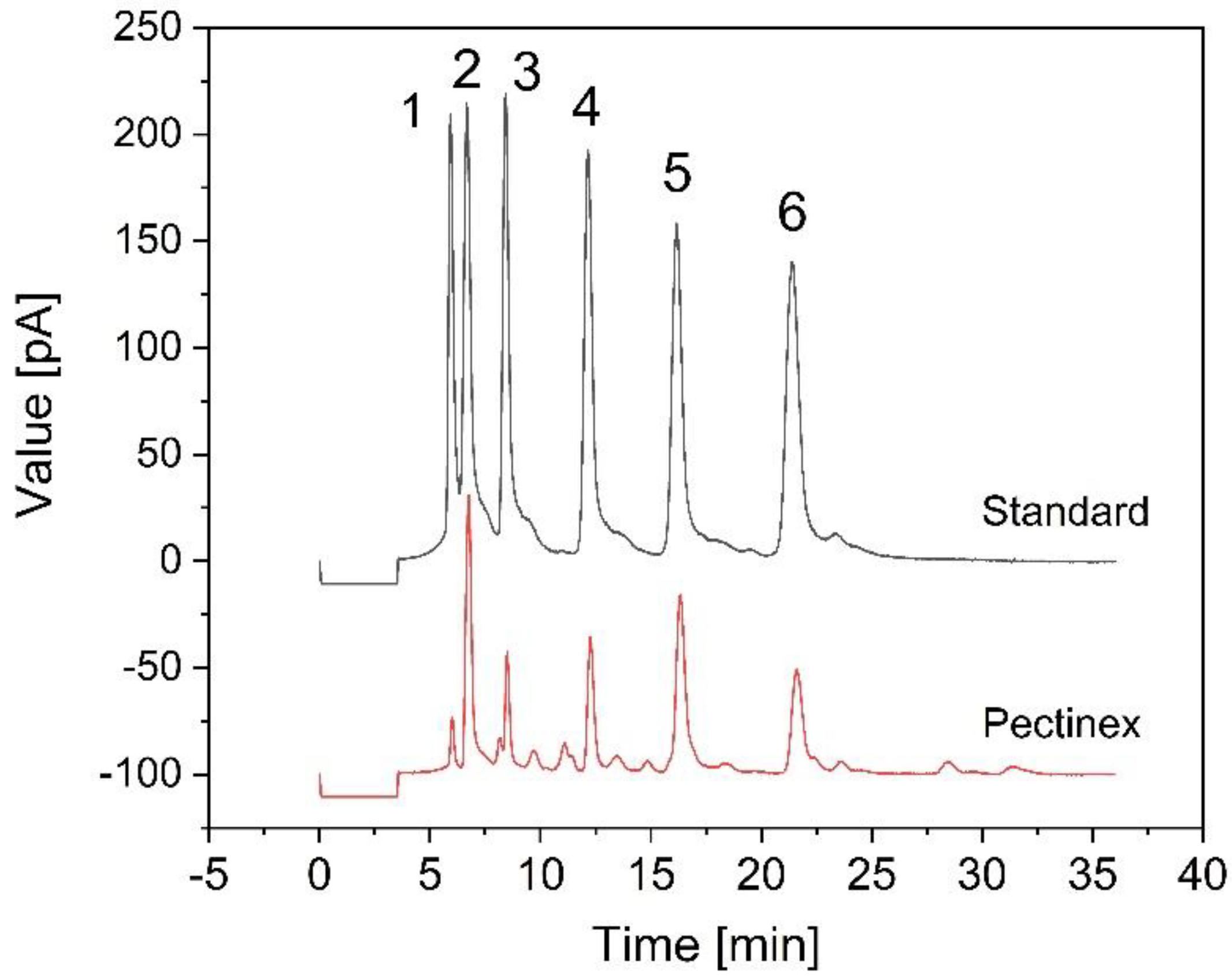



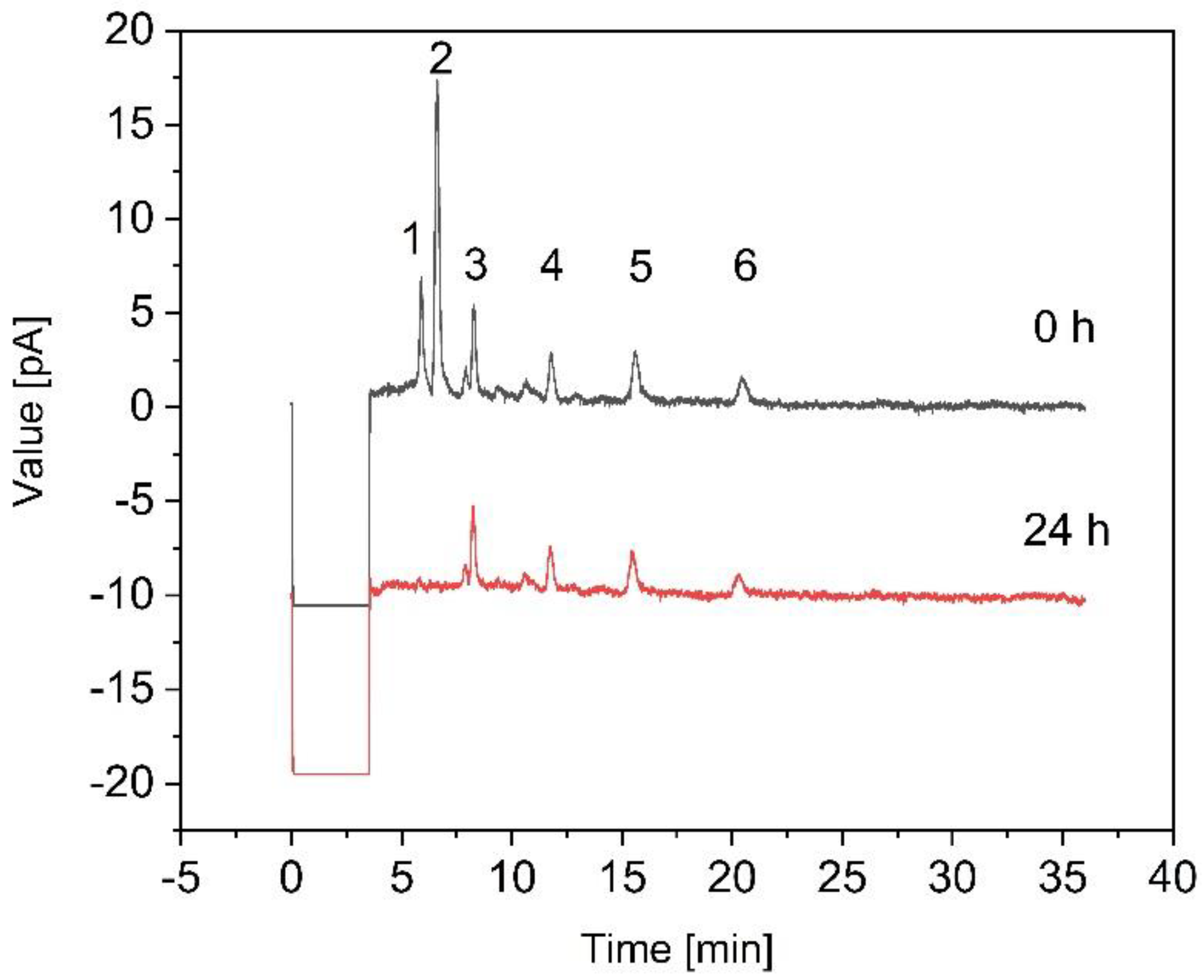
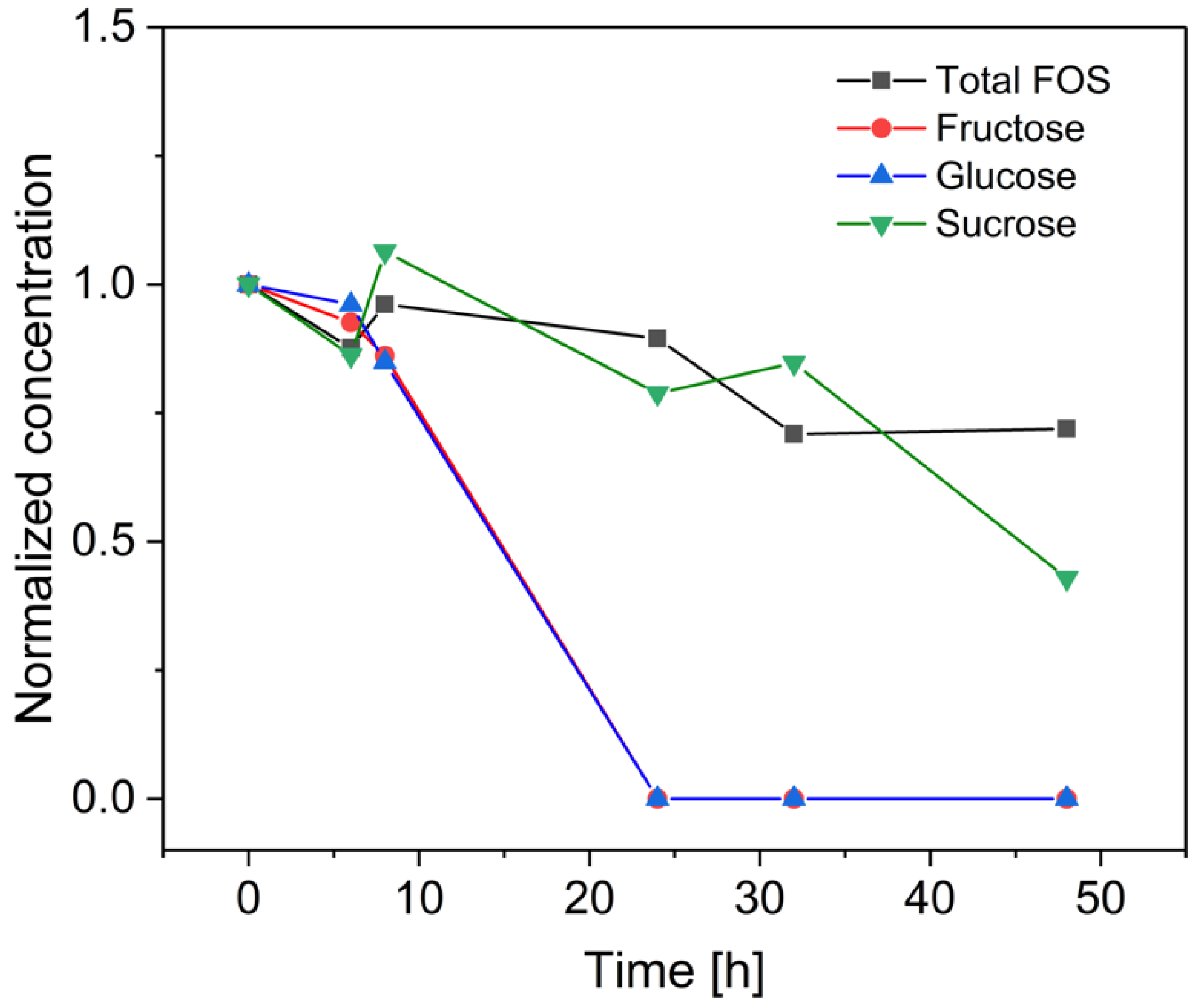
| Concentration [g·L−1] | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Time | Fructose | Glucose | Sucrose | 1-Kestose | Nystose | 1F-Nystose |
| DSM1 | 0 h | 5.4 | 25.6 | 4.4 | 4.4 | 4.5 | 3.9 |
| 24 h | 0.4 | 0.6 | 3.3 | 3.6 | 3.1 | 3.7 | |
| 48 h | 0.0 | 0.0 | 2.6 | 3.8 | 3.5 | 3.6 | |
| Thorne | 0 h | 6.1 | 21.2 | 5.6 | 4.0 | 6.1 | 1.9 |
| 24 h | 0.0 | 0.0 | 4.4 | 3.9 | 4.9 | 2.0 | |
| 48 h | 0.0 | 0.0 | 2.4 | 2.7 | 3.7 | 2.2 | |
| Microbiological Feature | DSM1 | DSM2312 | Thorne | PS5 |
|---|---|---|---|---|
| Biomass accumulation 1 | + | + | ++ | +++ |
| Biomass accumulation 2 | ||||
| Total viable cell count 2 | +++ | + | ++++ | ++ |
| Spore count 2 | +++ | ++ | ++++ | + |
| Sporulation rate 2 | +++ | ++ | ++++ | + |
| Nutrient requirements | – | – – | – | – – – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Burghardt, J.P.; Xiong, T.; Czermak, P. Removal of Small-Molecular Byproducts from Crude Fructo-Oligosaccharide Preparations by Fermentation Using the Endospore-Forming Probiotic Bacillus coagulans. Fermentation 2020, 6, 6. https://doi.org/10.3390/fermentation6010006
Fan R, Burghardt JP, Xiong T, Czermak P. Removal of Small-Molecular Byproducts from Crude Fructo-Oligosaccharide Preparations by Fermentation Using the Endospore-Forming Probiotic Bacillus coagulans. Fermentation. 2020; 6(1):6. https://doi.org/10.3390/fermentation6010006
Chicago/Turabian StyleFan, Rong, Jan P. Burghardt, Tao Xiong, and Peter Czermak. 2020. "Removal of Small-Molecular Byproducts from Crude Fructo-Oligosaccharide Preparations by Fermentation Using the Endospore-Forming Probiotic Bacillus coagulans" Fermentation 6, no. 1: 6. https://doi.org/10.3390/fermentation6010006
APA StyleFan, R., Burghardt, J. P., Xiong, T., & Czermak, P. (2020). Removal of Small-Molecular Byproducts from Crude Fructo-Oligosaccharide Preparations by Fermentation Using the Endospore-Forming Probiotic Bacillus coagulans. Fermentation, 6(1), 6. https://doi.org/10.3390/fermentation6010006







