Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Dough Production
2.2. Monitoring of Acidification and Biological Leavening
2.3. Dough Moulding, Baking and Analyses of Bread Attributes
2.4. Sample Preparation and Lysine Content Determination
2.5. Sensory Evaluation
2.6. Statistical Analyses
3. Results
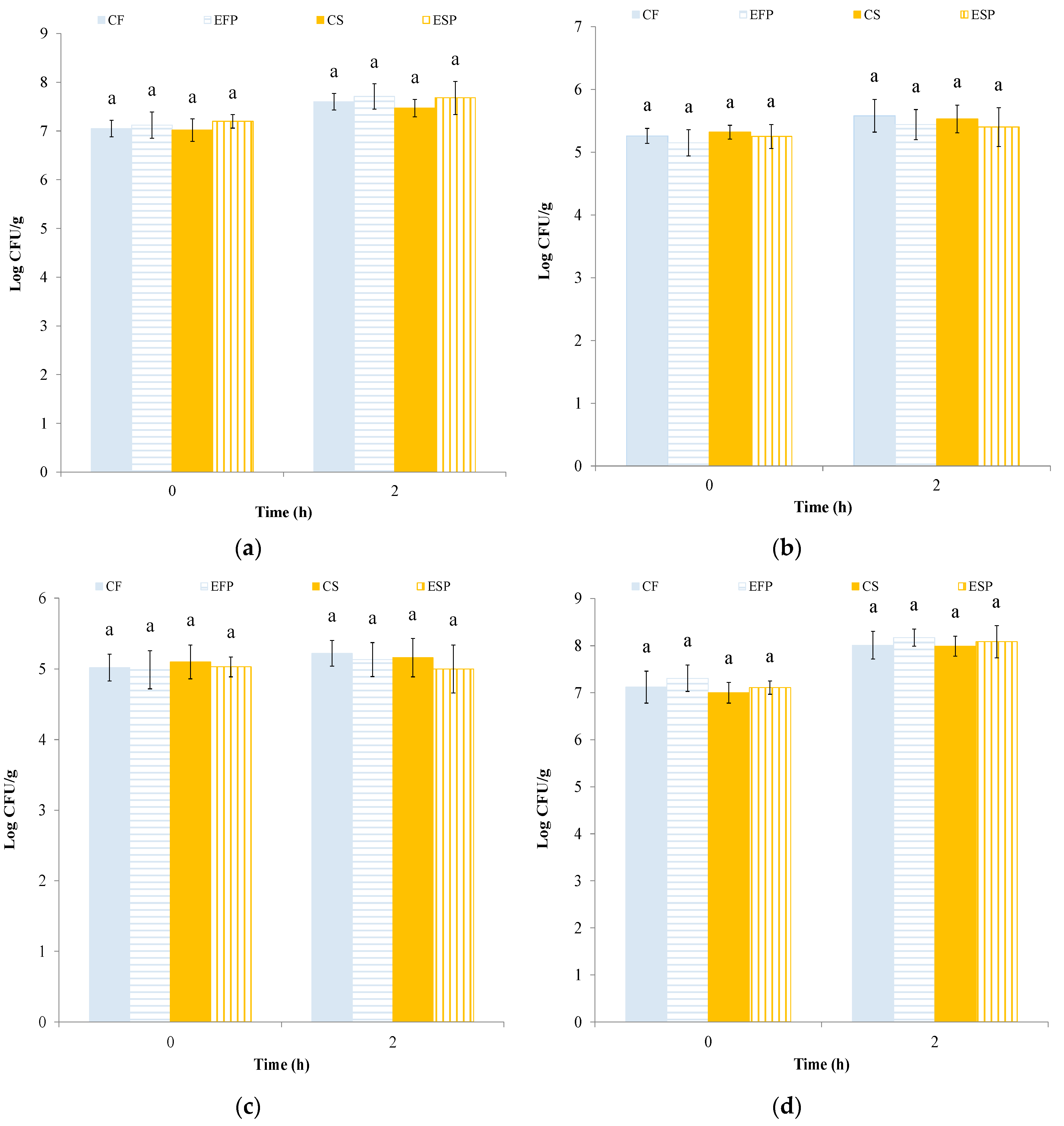
3.1. Leavening Process
3.2. Bread Attributes
3.3. Available Lysine in Bread
3.4. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siro, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Sánchez-Siles, L.M.; Siegris, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Liu, R. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Dhinda, F.; Prakash, J.; Dasappa, I. Effect of ingredients on rheological, nutritional and quality characteristics of high protein, high fibre and low carbohydrate bread. Food Bioproc. Tech. 2012, 5, 2998–3006. [Google Scholar] [CrossRef]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203–1212. [Google Scholar] [CrossRef]
- Kurek, M.A.; Wyrwisz, J.; Karp, S.; Wierzbicka, A. Effect of modified atmosphere packaging on the quality of wheat bread fortified with soy flour and oat fibre. J. Food Meas. Charact. 2019, 13, 1864–1872. [Google Scholar] [CrossRef]
- Patterson, C.A.; Maskus, H.; Bassett, C.M.C. Fortifying foods with pulses. Cereal Foods World 2010, 55, 56–62. [Google Scholar] [CrossRef]
- Kamaljit, K.; Baljeet, S.; Amarjeet, K. Preparation of bakery products by incorporating pea flours as a functional ingredient. Am. J. Food Technol. 2010, 5, 130–135. [Google Scholar] [CrossRef]
- Mohammed, I.; Ahmed, A.R.; Senge, B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind. Crops Prod. 2012, 36, 196–202. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Heydari-Dalfard, A.; Karami, F.; Fathi, M. Pseudo-cereals as a functional ingredient: Effects on bread nutritional and physiological properties-Review. Int. J. Agric. Crop Sci. 2013, 5, 1574–1580. [Google Scholar]
- Gaglio, R.; Guarcello, R.; Venturella, G.; Palazzolo, E.; Francesca, N.; Moschetti, G.; Settanni, L.; Saporita, P.; Gargano, M.L. Microbiological, chemical and sensory aspects of bread supplemented with different percentages of the culinary mushroom Pleurotus eryngii in powder form. Int. J. Food Sci. Technol. 2019, 54, 1197–1205. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M.J. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Casas-Agustench, P.; Salas-Huetos, A. Cultural and historical aspects of Mediterranean nuts with emphasis on their attributed healthy and nutritional properties. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.Z. Effect of nuts (pistachio or almonds) consumption on lipid profile of hypercholesterolemic rats. Asian J. Pharm. Clin. Res. 2012, 5, 47–53. [Google Scholar]
- Costa, J.; Silva, I.; Vicente, A.A.; Oliveira, M.B.P.; Mafra, I. Pistachio nut allergy: An updated overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 546–562. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Tabil, L.G. Pistachio (Pistacia vera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 218–247. [Google Scholar]
- López-Calleja, I.M.; de la Cruz, S.; González, I.; García, T.; Martín, R. Survey of undeclared allergenic pistachio (Pistacia vera) in commercial foods by hydrolysis probe real-time PCR. Food Control 2014, 39, 49–55. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Barbera, M.; Franciosi, E.; Francesca, N.; Moschetti, G.; Settanni, L. Persistence of a mixed lactic acid bacterial starter culture during lysine fortification of sourdough breads by addition of pistachio powder. Food Microbiol. 2020, 86, 10334. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L.; Braga, T.M.; De Fatima Silva Lopes, M.; Suzzi, G. An investigation on the bacteriocinogenic potential of lactic acid bacteria associated with wheat (Triticum durum) kernels and non-conventional flours. LWT Food Sci. Technol. 2008, 41, 1173–1182. [Google Scholar] [CrossRef]
- Kline, L.; Sugihara, T.F. Microorganisms of the San Francisco sourdough bread process. II. Isolation and characterization of un described bacterial species responsible for the souring activity. Appl. Microbiol. 1971, 21, 459–465. [Google Scholar]
- AACC. Official Methods of Analysis; American Association of Cereal Chemistry: Saint Paul, MN, USA, 2000. [Google Scholar]
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of addition of Opuntia ficus-indica mucilage on the biological leavening, physical, nutritional, antioxidant and sensory aspects of bread. J. Biosci. Bioeng. 2019, in press. [Google Scholar] [CrossRef]
- ISO 6658. Sensory Analysis—Methodology—General Guidance; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Comendador, F.J.; Cavella, S.; Di Monaco, R.; Dinnella, C.; Moneta, E.; Monteleone, E. Il pane e altri prodotti da forno. In Atlante sensoriale dei prodotti alimentari; Società Italiana di Scienze Sensoriali, Ed.; Tecniche Nuove: Milan, Italy, 2012; pp. 156–176. [Google Scholar]
- Rodrigues, Â.M.; Correia, P.M.; Guiné, R.P. Physical, chemical and sensorial properties of healthy and mixture breads in Portugal. J. Food Meas. Char. 2014, 8, 70–80. [Google Scholar] [CrossRef]
- Martins, Z.E.; Erben, M.; Gallardo, A.E.; Silva, R.; Barbosa, I.; Pinho, O. Effect of spent yeast fortification on physical parameters, volatiles and sensorial characteristics of home-made bread. Int. J. Food Sci. Technol. 2015, 50, 1855–1863. [Google Scholar] [CrossRef]
- Lopez, H.W.; Adam, A.; Leenhardt, F.; Scalbert, A.; Remesy, C. Control of the nutritional value of bread. Ind. Cereales 2001, 124, 15–20. [Google Scholar]
- Leite, S.E.; Montenegro, S.T.L.; de Oliveira, L.E. Sensitivity of spoiling and pathogen food-related bacteria to Origanum vulgare L. (Lamiaceae) essential oil. Braz. J. Microbiol. 2006, 37, 527–532. [Google Scholar]
- Rosell, C.M. The science of doughs and bread quality. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 3–14. [Google Scholar]
- Dalgetty, D.D.; Baik, B.K. Fortification of bread with hulls and cotyledon fibers isolated from peas, lentils, and chickpeas. Cereal Chem. 2006, 83, 269–274. [Google Scholar] [CrossRef]
- Francesca, N.; Gaglio, R.; Alfonzo, A.; Corona, O.; Moschetti, G.; Settanni, L. Characteristics of sourdoughs and baked pizzas as affected by starter culture inoculums. Int. J. Food Microbiol. 2019, 293, 114–123. [Google Scholar] [CrossRef]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial application of selected lactic acid bacteria isolated from local semolinas for typical sourdough bread production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef]
- Vera, A.; Rigobello, V.; Demarigny, Y. Comparative study of culture media used for sourdough lactobacilli. Food Microbiol. 2009, 26, 728–733. [Google Scholar] [CrossRef]
- Alfonzo, A.; Ventimiglia, G.; Corona, O.; Di Gerlando, R.; Gaglio, R.; Francesca, N.; Moschetti, G.; Settanni, L. Diversity and technological potential of lactic acid bacteria of wheat flours. Food Microbiol. 2013, 54, 1569–1578. [Google Scholar] [CrossRef]
- Almoraie, N.M. The Effect of Walnut Flour on the Physical and Sensory Characteristics of Wheat Bread. Int. J. Food Sci. 2019, 5676205. [Google Scholar] [CrossRef]
- Chin, N.L.; Tan, L.H.; Yusof, Y.A.; Rahman, R.A. Relationship between aeration and rheology of breads. J. Texture Stud. 2009, 40, 727–738. [Google Scholar] [CrossRef]
- Rose, D.J. Bread and other baked goods. In Specialty Foods: Processing Technology, Quality and Safety, 1st ed.; Zhao, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 67–105. [Google Scholar]
- Lee, J.Y.; Lee, K.; Kwak, E.J. Fermentation characteristics of bread added with Pleurotus eryngii powder. J. Korean Soc. Food Sci. Nutr. 2009, 38, 757–765. [Google Scholar] [CrossRef]
- Das, L.; Raychaudhuri, U.; Chakraborty, R. Supplementation of common white bread by coriander leaf powder. Food Sci. Biotechnol. 2012, 21, 425–433. [Google Scholar] [CrossRef]
- Das, L.; Raychaudhuri, U.; Chakraborty, R. Herbal fortification of bread with fennel seeds. Food Technol. Biotech. 2013, 51, 434–440. [Google Scholar]
- Alfonzo, A.; Urso, V.; Corona, O.; Francesca, N.; Amato, G.; Settanni, L.; Di Miceli, G. Development of a method for the direct fermentation of semolina by selected sourdough lactic acid bacteria. Int. J. Food Microbiol. 2016, 239, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H. Hernando, I. Fiber from fruit pomace: A review of applications in cereal-based products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar]
- Rajeswari, H.; Jagadeesh, S.L.; Suresh, G.J. Physicochemical and sensory qualities of bread fortified with banana, aonla and sapota powders. J. Nutr. Health Food Eng. 2018, 8, 487–492. [Google Scholar]
- Wang, Y.Y.D.; Fields, M.L. Feasibility of home fermentation to improve the amino acid balance of corn meal. J. Food Sci. 1978, 43, 1104. [Google Scholar] [CrossRef]
- Karovičová, Z.K.J.; Kohajdova, J. Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51–57. [Google Scholar]


| Samples | pH | TTA | ||
|---|---|---|---|---|
| 0 h | 2 h | 0 h | 2 h | |
| CF | 6.00 ± 0.05 a | 5.66 ± 0.02 a | 1.00 ± 0.10 a | 1.40 ± 0.10 a |
| EFP | 5.97 ± 0.03 a | 5.67 ± 0.03 a | 0.90 ± 0.20 a | 1.40 ± 0.10 a |
| CS | 5.98 ± 0.04 a | 5.57 ± 0.02 a | 1.00 ± 0.00 a | 1.70 ± 0.10 a |
| ESP | 5.95 ± 0.02 a | 5.58 ± 0.04 a | 1.00 ± 0.10 a | 1.70 ± 0.20 a |
| p value | 0.456 | 0.244 | 0.693 | 0.156 |
| Trials | Height (mm) | Crust Color | Crumb Color | Firmness Value (N) | Void Fraction (%) | Cell Density (n.cm−2) | Mean Cell Area (mm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||||||
| CF | 41.00 ± 1.44 a | 68.98 ± 1.26 ab | 1.56 ± 0.91 b | 23.96 ± 2.80 b | 72.01 ± 3.44 a | −1.63 ± 0.08 a | 14.21 ± 0.47 c | 15.33 ± 0.04 c | 2.31 ± 0.25 c | 0.16 ± 0.02 b | 0.30 ± 0.07 ab |
| EFP | 36.00 ± 1.02 ac | 64.33 ± 1.74 c | 3.79 ± 1.60 a | 27.04 ± 2.11 b | 64.89 ± 1.50 b | −1.81 ± 0.30 a | 15.30 ± 0.41 c | 19.01 ± 0.07 a | 0.93 ± 0.02 d | 0.24 ± 0.10 b | 0.04 ± 0.00 c |
| CS | 38.00 ± 1.41 ab | 70.76 ± 0.73 a | 1.42 ± 0.37 b | 35.06 ± 1.99 a | 71.82 ± 3.35 a | −3.84 ± 0.14 b | 22.01 ± 0.62 a | 18.21 ± 0.08 b | 4.30 ± 0.10 b | 0.36 ± 0.05 b | 0.40 ± 0.02 a |
| ESP | 33.00 ± 1.51 c | 65.62 ± 2.14 bc | 0.23 ± 0.93 b | 34.49 ± 1.89 a | 67.08 ± 0.44 ab | −3.20 ± 0.33 c | 20.35 ± 0.26 b | 19.17 ± 0.11 a | 5.37 ± 0.01 a | 1.10 ± 0.34 a | 0.20 ± 0.03 b |
| p value | 0.001 | 0.003 | 0.004 | 0.001 | 0.02 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.001 | 0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonzo, A.; Gaglio, R.; Barbera, M.; Francesca, N.; Moschetti, G.; Settanni, L. Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads. Fermentation 2020, 6, 2. https://doi.org/10.3390/fermentation6010002
Alfonzo A, Gaglio R, Barbera M, Francesca N, Moschetti G, Settanni L. Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads. Fermentation. 2020; 6(1):2. https://doi.org/10.3390/fermentation6010002
Chicago/Turabian StyleAlfonzo, Antonio, Raimondo Gaglio, Marcella Barbera, Nicola Francesca, Giancarlo Moschetti, and Luca Settanni. 2020. "Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads" Fermentation 6, no. 1: 2. https://doi.org/10.3390/fermentation6010002
APA StyleAlfonzo, A., Gaglio, R., Barbera, M., Francesca, N., Moschetti, G., & Settanni, L. (2020). Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads. Fermentation, 6(1), 2. https://doi.org/10.3390/fermentation6010002







