Optimization of the Enzymatic Saccharification Process of Milled Orange Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Basic Chemical Analysis of Solids
2.2.2. Determination of Enzyme Activities
2.2.3. Hydrothermal Pretreatments
2.2.4. Saccharification Experiments
2.2.5. Statistical Design of Experiments
2.2.6. Kinetic Modelling
3. Results and Discussion
3.1. Preliminary Experiments: Hydrothermal Pretreatments
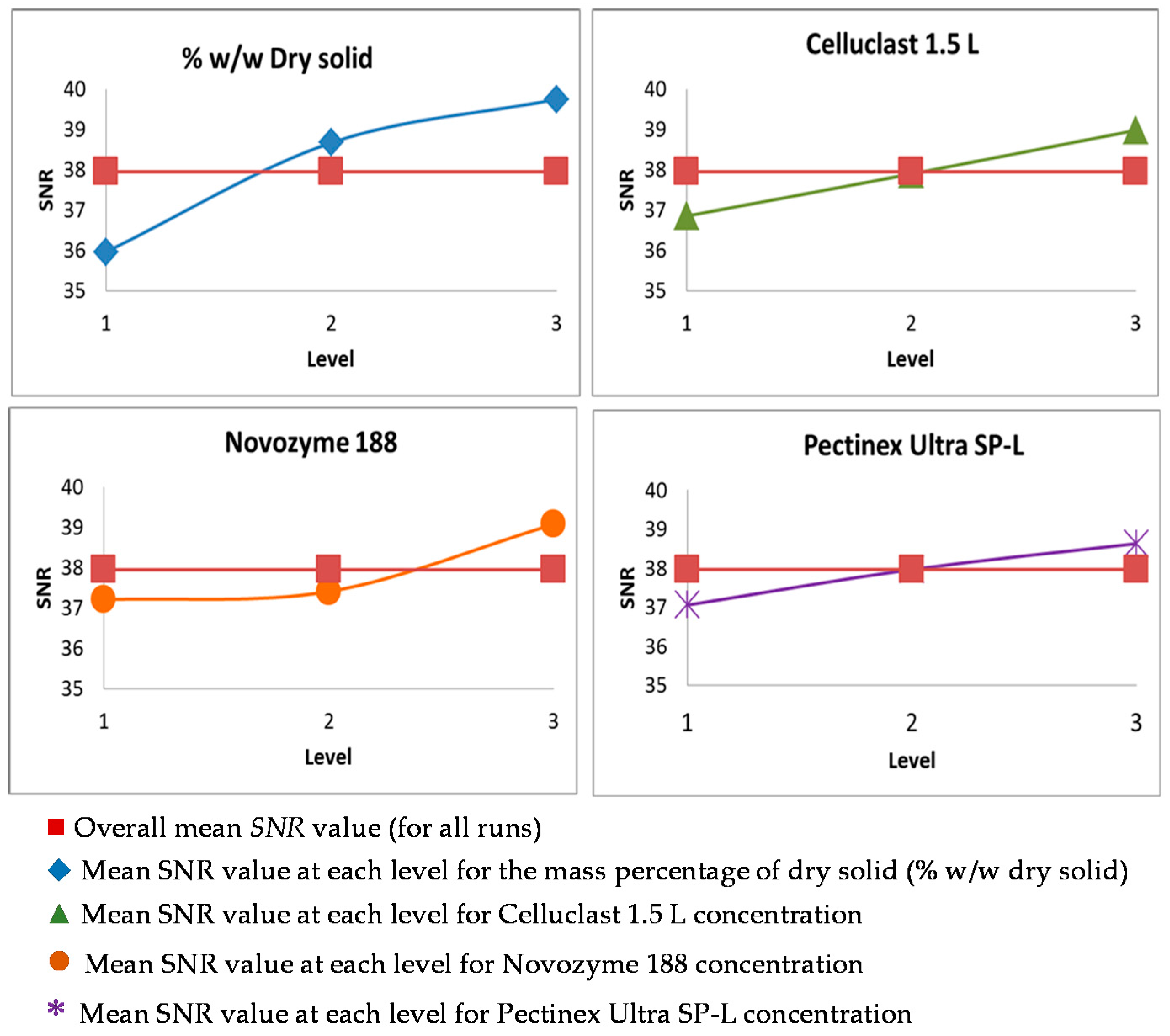
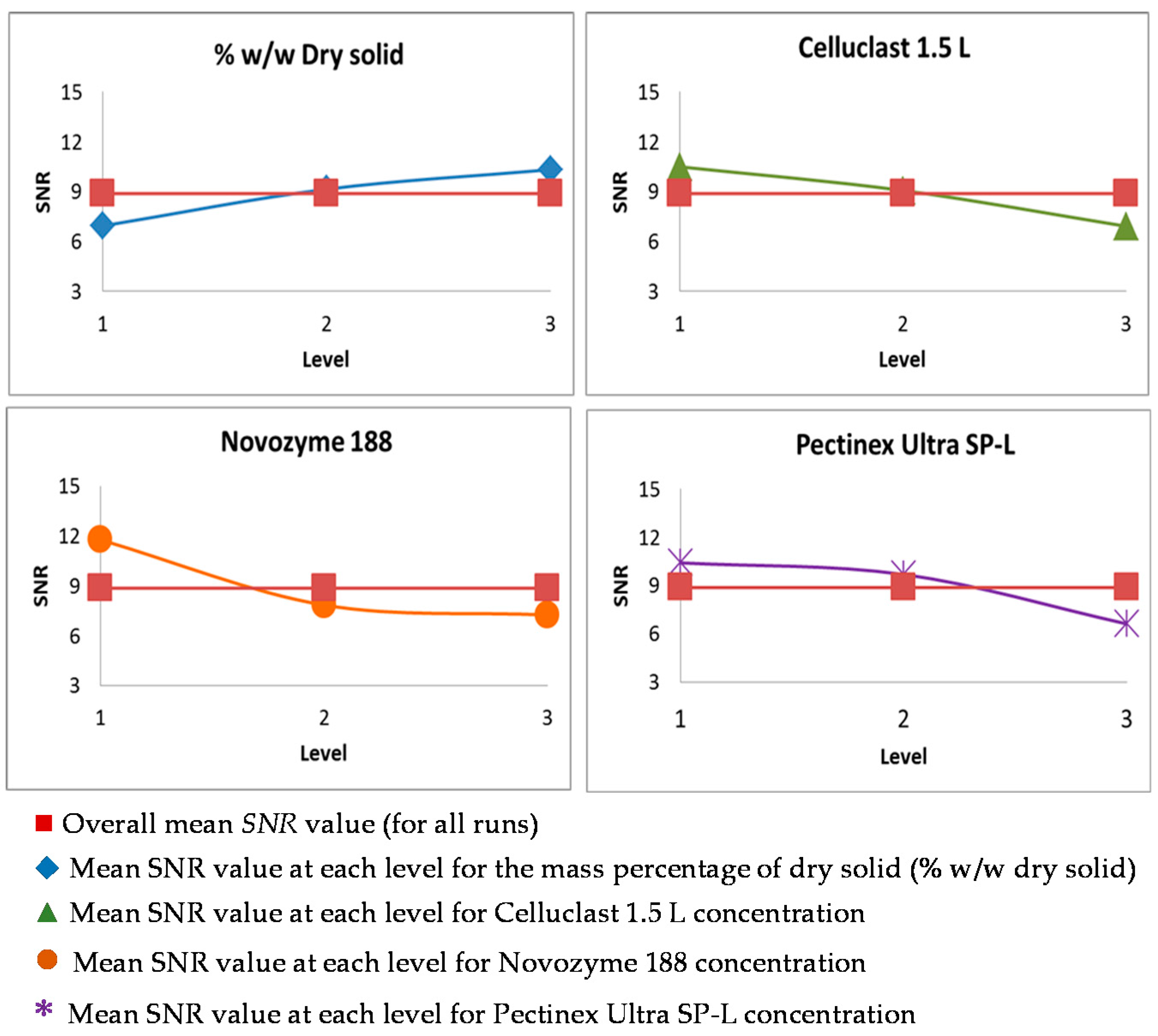
3.2. Taguchi Optimization of the Saccharification
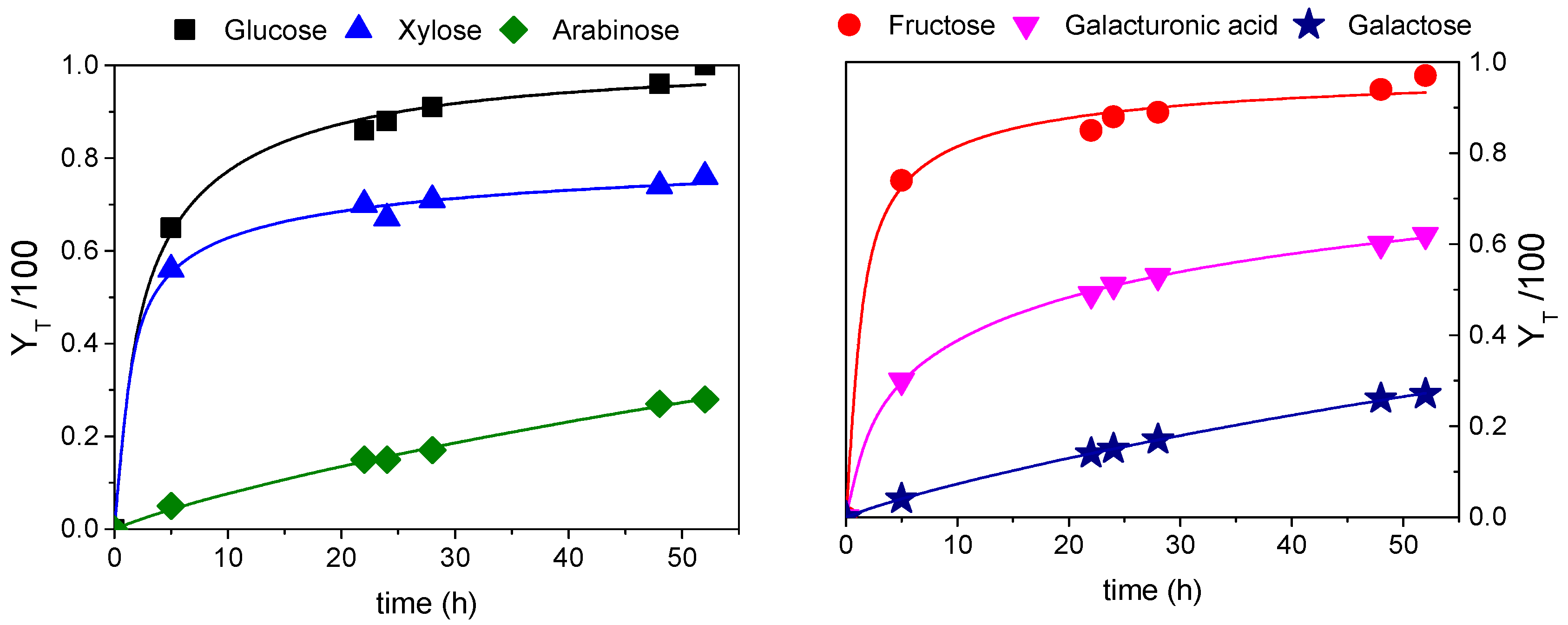
3.3. Fractal Kinetic Modelling for Best Global Yield Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Carriquiry, M.A.; Du, X.; Timilsina, G.R. Second generation biofuels: Economics and policies. Energy Pol. 2011, 39, 4222–4234. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Country Indicators, Data, Compare Data. 2014. Available online: http://www.fao.org/faostat/en/#home (accessed on 17 July 2017).
- Erukainure, O.L.; Ebuehi, O.A.T.; Choudhary, M.I.; Mesaik, M.A.; Shukralla, A.; Muhammad, A.; Zaruwa, M.Z.; Elemo, G.N. Orange peel extracts: Chemical characterization, antioxidant, antioxidative burst, and phytotoxic activities. J. Diet. Suppl. 2016, 13, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Lessa, E.F.; Gularte, M.S.; Garcia, E.S.; Fajardo, A.R. Orange waste: A valuable carbohydrate source for the development of beads with enhanced adsorption properties for cationic dyes. Carbohydr. Polym. 2017, 157, 660–668. [Google Scholar] [CrossRef] [PubMed]
- De Farias Silva, C.E.; da Silva Gonçalves, A.H.; de Souza Abud, A.K. Treatment of textile industry effluents using orange waste: A proposal to reduce color and chemical oxygen demand. Water Sci. Technol. 2016, 74, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci. Total Environ. 2016, 547, 197–205. [Google Scholar] [CrossRef] [PubMed]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for D-Limonene, pectin, and bioethanol production. Int. J. Green Energy 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Jambrak, A.R.; Barba, F.; Cravotto, G.; Binello, A.; Lorenzo, J.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the residues recovery: Orange waste as raw material for new products. Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Ángel Siles López, J.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2011.
- Mussatto, S.I. Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Rivas-Cantu, R.C.; Jones, K.D.; Mills, P.L. A citrus waste-based biorefinery as a source of renewable energy: Technical advances and analysis of engineering challenges. Waste Manag. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Santi, G.; Crognale, S.; D’Annibale, A.; Petruccioli, M.; Ruzzi, M.; Valentini, R.; Moresi, M. Orange peel pretreatment in a novel lab-scale direct steam-injection apparatus for ethanol production. Biomass Bioenergy 2014, 61, 146–156. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.T.; Cherry, J. Progress and Challenges in Enzyme Development for Biomass Utilization. In Biofuels; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 95–120. [Google Scholar]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Fockink, D.H.; Urio, M.B.; Chiarello, L.M.; Sánchez, J.H.; Ramos, L.P. Principles and challenges involved in the enzymatic hydrolysis of cellulosic materials at high total solids. In Green Fuels Technology: Biofuels; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 147–173. [Google Scholar]
- Jung, Y.H.; Park, H.M.; Kim, D.H.; Yang, J.; Kim, K.H. Fed-Batch enzymatic saccharification of high solids pretreated lignocellulose for obtaining high titers and high yields of glucose. Appl. Biochem. Biotechnol. 2017, 182, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Wojtusik, M.; Zurita, M.; Villar, J.C.; Ladero, M.; Garcia-Ochoa, F. Influence of fluid dynamic conditions on enzymatic hydrolysis of lignocellulosic biomass: Effect of mass transfer rate. Bioresour. Technol. 2016, 216, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.J.; Badino, A.C.; Cruz, A.J.G. Mixing design for enzymatic hydrolysis of sugarcane bagasse: Methodology for selection of impeller configuration. Bioprocess Biosyst. Eng. 2016, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Palhed, J.; Karimi, K.; Lundin, M.; Taherzadeh, M.J.; Zamani, A. Process optimization for citrus waste biorefinery via simultaneous pectin extraction and pretreatment. BioResources 2017, 12, 1706–1722. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Sharma, A.; Gupta, M.N. A multipurpose immobilized biocatalyst with pectinase, xylanase and cellulase activities. Chem. Central J. 2007, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of Protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wojtusik, M.; Rodríguez, A.; Ripoll, V.; Santos, V.E.; García, J.L.; García-Ochoa, F. 1,3-Propanediol production by Klebsiella oxytoca NRRL-B199 from glycerol. Medium composition and operational conditions. Biotechnol. Rep. 2015, 6, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi methodology as a statistical tool for biotechnological applications: A critical appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Goyal, D.; Goyal, A. Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energy Convers. Manag. 2017, 141, 133–144. [Google Scholar] [CrossRef]
- Kopelman, R. Fractal Reaction Kinetics. Science 1988, 241, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Wojtusik, M.; Zurita, M.; Villar, J.C.; Ladero, M.; Garcia-Ochoa, F. Enzymatic saccharification of acid pretreated corn stover: Empirical and fractal kinetic modelling. Bioresour. Technol. 2016, 220, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Fockink, D.H.; Urio, M.B.; Sánchez, J.H.; Ramos, L.P. Enzymatic hydrolysis of steam-treated sugarcane bagasse: Effect of enzyme loading and substrate total solids on its fractal kinetic modeling and rheological properties. Energy Fuels 2017, 31, 6211–6220. [Google Scholar] [CrossRef]
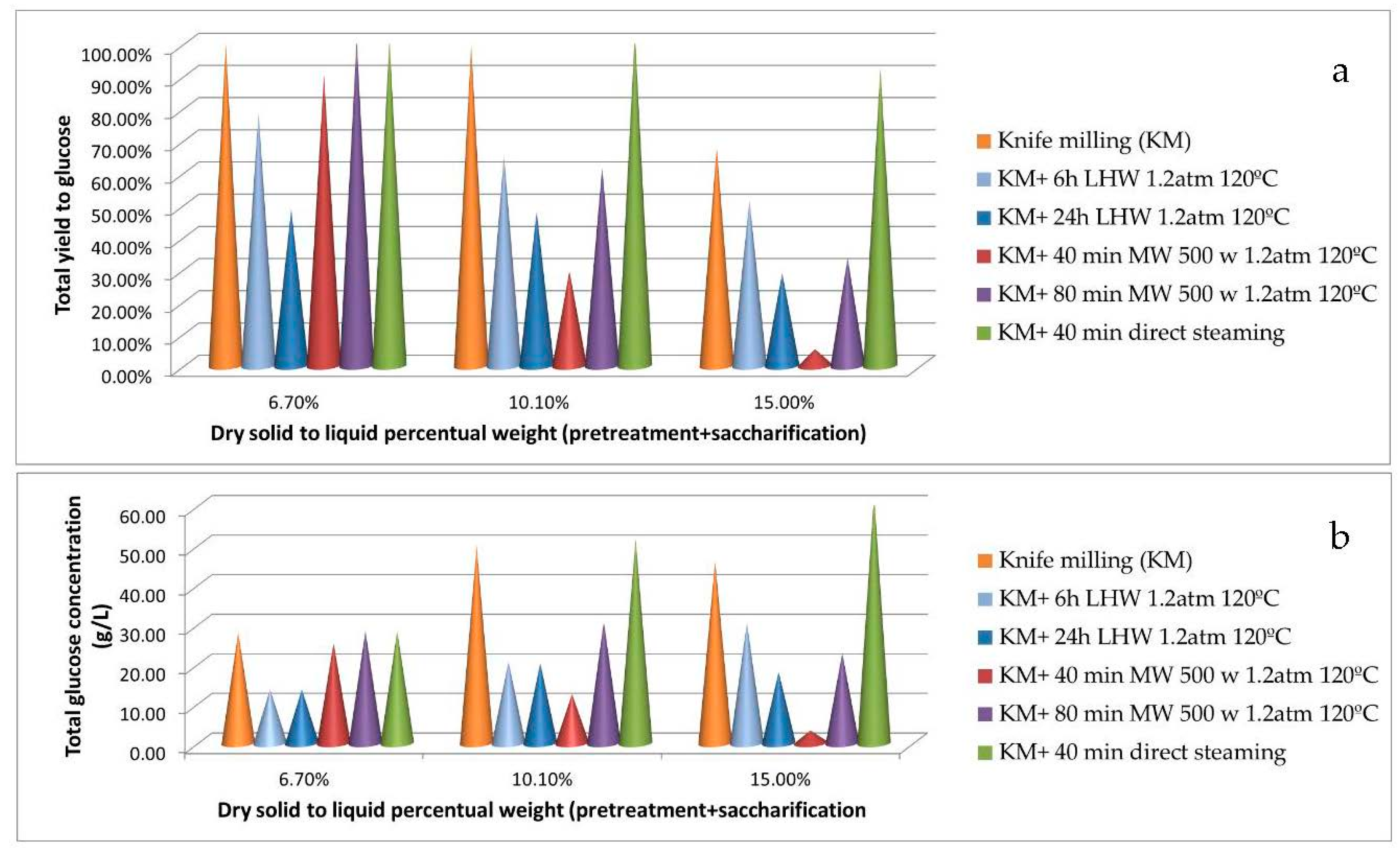
| OPW Pretreatment | Solid Dry Weight | Glucan Sol/Ins | Xylan Sol/Ins | Other Sugars Sol/Ins | Pectin Sol/Ins | Lignin/Ash |
|---|---|---|---|---|---|---|
| Knife Milling (KM) | 16.5 ± 0.2 | 5.12/12.9 | 0.04/0.72 | 1.91/13.5 | 0/18.6 | 6.47/3.72 |
| KM+ 6 h LHW 1.2 atm 120 °C | 15.7 ± 0.4 | 6.41/13.7 | 0.06/0.81 | 2.56/13.2 | 2.42/17.2 | 7.21/3.96 |
| KM+ 24 h LHW 1.2 atm 120 °C | 15.1 ± 0.2 | 6.96/14.4 | 0.06/0.84 | 4.56/12.9 | 4.56/14.2 | 7.42/4.01 |
| KM+ 40 min MW 500 w 1.2 atm 120 °C | 12.1 ± 0.3 | 7.89/15.4 | 0.45/1.12 | 7.56/8.45 | 7.84/12.2 | 8.12/4.29 |
| KM+ 80 min MW 500 w 1.2 atm 120 °C | 10.9 ± 0.1 | 8.95/17.1 | 0.89/1.04 | 10.2/4.68 | 9.41/8.56 | 8.23/4.56 |
| KM+ 40 min direct steaming | 10.2 ± 0.4 | 10.6/11.5 | 1.12/0.98 | 10.5/4.21 | 9.24/8.69 | 9.12/5.14 |
| Run | % DS | Cellulase FPU/g DS | β-Glucosidase (UI)/g DS | Polygalacturonase UI/g Pectin | Protein/Dry Solid mg/g | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-1’ | 6.1 | 1 | 6.08 | 1 | 12 | 1 | 670 | 1 | 0.29 |
| 2-2’ | 6.1 | 1 | 12.15 | 2 | 23 | 2 | 1340 | 2 | 0.58 |
| 3-3’ | 6.1 | 1 | 24.30 | 3 | 50 | 3 | 2850 | 3 | 1.16 |
| 4-4’ | 9.5 | 2 | 6.08 | 1 | 23 | 2 | 2850 | 3 | 0.40 |
| 5-5’ | 9.5 | 2 | 12.15 | 2 | 50 | 3 | 670 | 1 | 0.75 |
| 6-6’ | 9.5 | 2 | 24.30 | 3 | 12 | 1 | 1340 | 2 | 0.88 |
| 7-7’ | 11.5 | 3 | 6.08 | 1 | 50 | 3 | 670 | 1 | 0.57 |
| 8-8’ | 11.5 | 3 | 12.15 | 2 | 12 | 1 | 2850 | 3 | 0.51 |
| 9-9’ | 11.5 | 3 | 24.30 | 3 | 23 | 2 | 1340 | 2 | 0.96 |
| Run | DP ≥ 2 (g/L) | Glucose (g/L) | Fructose (g/L) | Ara + gal + xyl (g/L) | Galacturonic Acid (g/L) | YT | YS |
|---|---|---|---|---|---|---|---|
| 1-1’ | 1.30 | 14.56 | 11.56 | 1.82 | 4.52 | 50.90 | 10.72 |
| 2-2’ | 1.24 | 16.88 | 13.40 | 2.19 | 5.98 | 61.87 | 30.65 |
| 3-3’ | 1.01 | 22.64 | 18.30 | 4.27 | 9.28 | 82.95 | 69.01 |
| 4-4’ | 2.02 | 28.48 | 23.45 | 5.42 | 11.97 | 77.73 | 58.78 |
| 5-5’ | 0.90 | 33.11 | 24.74 | 3.68 | 9.40 | 89.89 | 81.62 |
| 6-6’ | 1.46 | 34.06 | 26.41 | 4.03 | 12.58 | 92.48 | 86.33 |
| 7-7’ | 1.35 | 45.02 | 32.12 | 5.32 | 12.94 | 99.68 | 99.42 |
| 8-8’ | 1.12 | 45.27 | 36.21 | 5.64 | 17.70 | 99.70 | 99.46 |
| 9-9’ | 2.70 | 41.87 | 32.73 | 4.89 | 7.89 | 92.75 | 86.82 |
| Run | Enzyme Cost per Run | Glucose (g/L) | Fructose (g/L) | glu+fru (kg/10 L) | Enzyme Cost (€/kg glu+fru) | |
|---|---|---|---|---|---|---|
| 1-1′ | 0.0468 | 14.56 | 11.56 | 0.2612 | 0.44 | 0.18 |
| 2-2′ | 0.1285 | 16.88 | 13.4 | 0.3028 | 0.44 | 0.42 |
| 3-3′ | 0.2570 | 22.64 | 18.3 | 0.4094 | 0.45 | 0.63 |
| 4-4′ | 0.2177 | 28.48 | 23.45 | 0.5193 | 0.45 | 0.42 |
| 5-5′ | 0.1894 | 33.11 | 24.74 | 0.5785 | 0.43 | 0.33 |
| 6-6′ | 0.1732 | 34.06 | 26.41 | 0.6047 | 0.44 | 0.29 |
| 7-7′ | 0.2445 | 45.02 | 32.12 | 0.7714 | 0.42 | 0.32 |
| 8-8′ | 0.2975 | 45.27 | 36.21 | 0.8148 | 0.44 | 0.37 |
| 9-9′ | 0.1691 | 41.87 | 32.73 | 0.746 | 0.44 | 0.23 |
| Parameter | Glucose | Fructose | Xylose | Galacturonic Acid | Arabinose | Galactose |
|---|---|---|---|---|---|---|
| k′ | 0.32 ± 0.03 | 0.47 ± 0.05 | 0.327± 0.021 | 0.116± 0.003 | 0.0098± 0.001 | 0.0092 ± 0.0003 |
| h | 0.62 ± 0.07 | 0.91 ± 0.09 | 1.11 ± 0.05 | 0.72 ± 0.01 | 0.15 ± 0.04 | 0.14 ± 0.01 |
| SQR | 0.0027 | 0.00112 | 0.00112 | 0.000096 | 0.00018 | 0.000016 |
| RMSE | 0.0198 | 0.021 | 0.0011 | 0.0037 | 0.005 | 0.0015 |
| F-value | 4294 | 3835 | 6420 | 41,795 | 3195 | 32,769 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, D.; Senit, J.J.; De la Torre, I.; Santos, T.M.; Yustos, P.; Santos, V.E.; Ladero, M. Optimization of the Enzymatic Saccharification Process of Milled Orange Wastes. Fermentation 2017, 3, 37. https://doi.org/10.3390/fermentation3030037
Velasco D, Senit JJ, De la Torre I, Santos TM, Yustos P, Santos VE, Ladero M. Optimization of the Enzymatic Saccharification Process of Milled Orange Wastes. Fermentation. 2017; 3(3):37. https://doi.org/10.3390/fermentation3030037
Chicago/Turabian StyleVelasco, Daniel, Juan J. Senit, Isabel De la Torre, Tamara M. Santos, Pedro Yustos, Victoria E. Santos, and Miguel Ladero. 2017. "Optimization of the Enzymatic Saccharification Process of Milled Orange Wastes" Fermentation 3, no. 3: 37. https://doi.org/10.3390/fermentation3030037