Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region
Abstract
1. Introduction
2. Materials and Methods
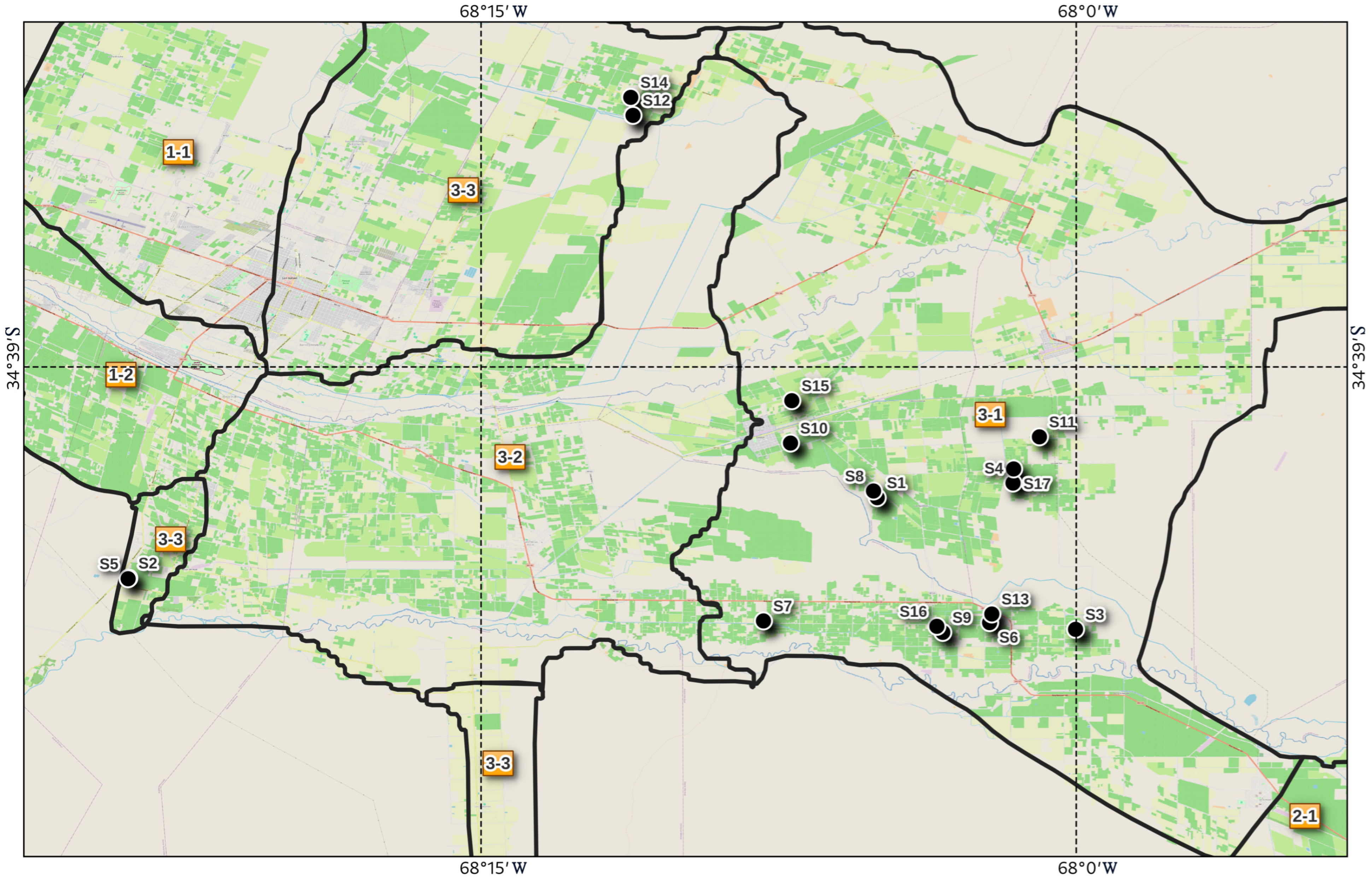
2.1. Study Area and Sampling Procedure
2.2. Enumeration and Isolation of Filamentous Fungi and Yeasts from Rotten Grapes
2.3. Identification of Filamentous Fungi, Yeast and Yeast-like Organisms
2.4. Tenuazonic Acid Extraction, Detection and Quantification
2.5. In Vitro Evaluation of Spoilage Features in Isolated Yeasts
2.5.1. Acidity Production
2.5.2. Hydrogen Sulphide Production
2.5.3. Unpleasant Aroma Production
2.6. Microvinification Trials
2.6.1. Physicochemical Analyses of Wines
2.6.2. Sensory Analysis of Wines
2.7. Statistical Analysis
3. Results and Discussion
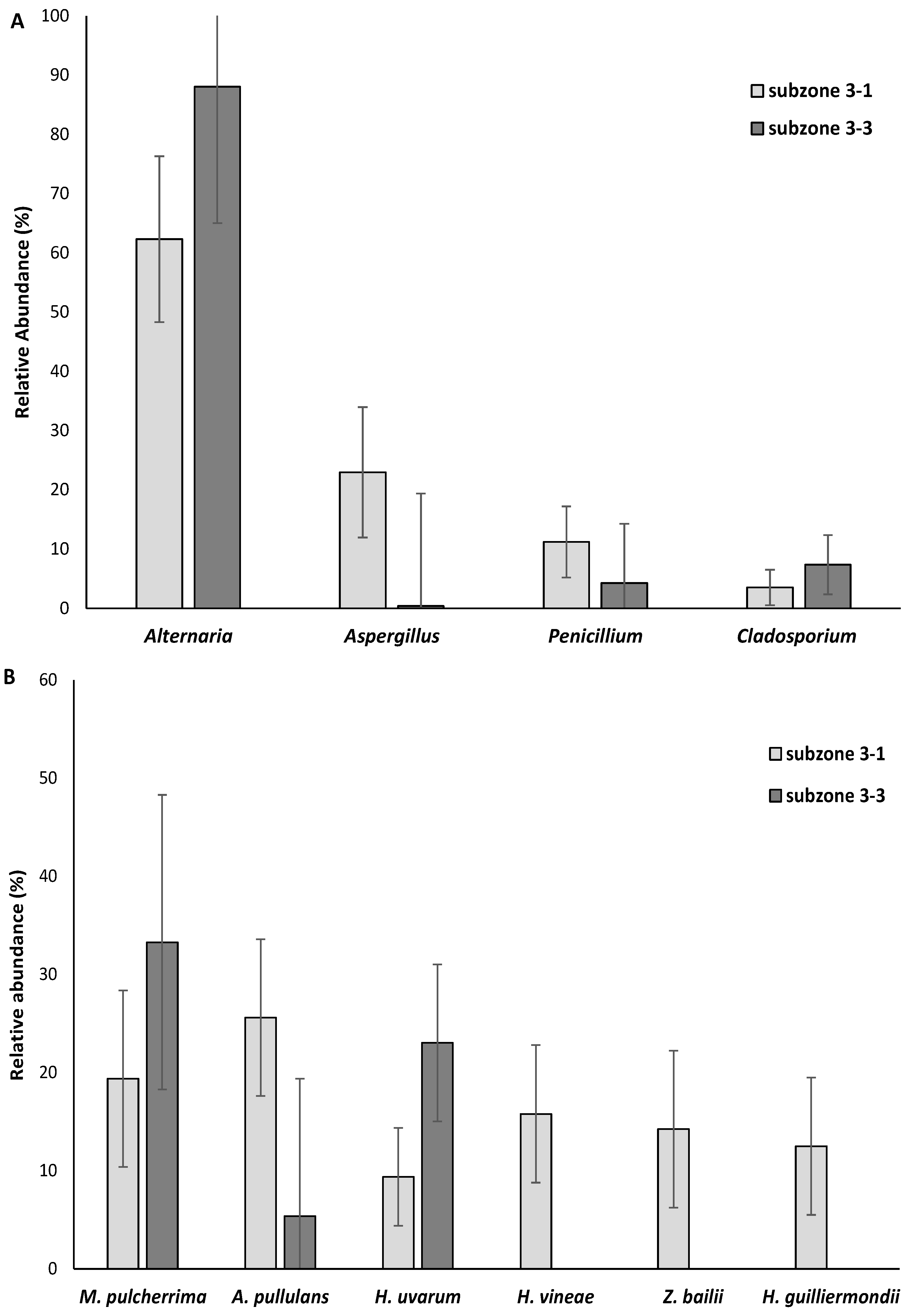
3.1. Filamentous Fungi and Yeasts Present in Wine Grape Bunch Rots
3.2. TA Natural Occurrence
3.3. Evaluation of the Wine Spoilage Potential of the Isolated Yeasts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV International Organisation of Vine and Wine. Statistical Report on World Vitiviniculture. 2024. Available online: https://www.oiv.int/sites/default/files/2025-04/OIV-State_of_the_World_Vine-and-Wine-Sector-in-2024.pdf (accessed on 23 April 2025).
- Straffelini, E.; Carrillo, N.; Schilardi, C.; Aguilera, R.; Estrella Orrego, M.J.; Tarolli, P. Viticulture in Argentina under Extreme Weather Scenarios: Actual Challenges, Future Perspectives. Geogr. Sustain. 2023, 4, 161–169. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Sgubin, G.; Bois, B.; Ollat, N.; Swingedouw, D.; Zito, S.; Gambetta, G.A. Climate Change Impacts and Adaptations of Wine Production. Nat. Rev. Earth Environ. 2024, 5, 258–275. [Google Scholar] [CrossRef]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Martín, M.; Prendes, L.; Morata, V.; Merín, M. Biocontrol and Enzymatic Activity of Non-Saccharomyces Wine Yeasts: Improvements in Winemaking. Fermentation 2024, 10, 218. [Google Scholar] [CrossRef]
- Kepner, C.; Swett, C.L. Previously Unrecognized Diversity within Fungal Fruit Rot Pathosystems on Vitis Vinifera and Hybrid White Wine Grapes in Mid-Atlantic Vineyards. Australas. Plant Pathol. 2018, 47, 181–188. [Google Scholar] [CrossRef]
- Prendes, L.P.; Merín, M.G.; Andreoni, M.A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Mycobiota and Toxicogenic Alternaria Spp. Strains in Malbec Wine Grapes from DOC San Rafael, Mendoza, Argentina. Food Control 2015, 57, 122–128. [Google Scholar] [CrossRef]
- Prendes, L.P.; Zachetti, V.G.L.; Pereyra, A.; Morata de Ambrosini, V.I.; Ramirez, M.L. Water Activity and Temperature Effects on Growth and Mycotoxin Production by Alternaria Alternata Strains Isolated from Malbec Wine Grapes. J. Appl. Microbiol. 2017, 122, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Prendes, L.P.; Morata, V.I.; Bottini, R. High-Throughput Modified QuEChERS Method for the Determination of the Mycotoxin Tenuazonic Acid in Wine Grapes. RSC Adv. 2016, 6, 95670–95679. [Google Scholar] [CrossRef]
- Prendes, L.P.; Fontana, A.R.; Merín, M.G.; D’Amario Fernández, A.; Bottini, R.; Ramirez, M.L.; Morata de Ambrosini, V.I. Natural Occurrence and Production of Tenuazonic Acid in Wine Grapes in Argentina. Food Sci. Nutr. 2018, 6, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M.; Lawrence, G.A.; Lau, B.P.Y. Analysis of Wines, Grape Juices and Cranberry Juices ForAlternaria Toxins. Mycotoxin Res. 2006, 22, 142–147. [Google Scholar] [CrossRef]
- Broggi, L.; Reynoso, C.; Resnik, S.; Martinez, F.; Drunday, V.; Bernal, Á.R. Occurrence of Alternariol and Alternariol Monomethyl Ether in Beverages from the Entre Rios Province Market, Argentina. Mycotoxin Res. 2013, 29, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.R.; de Kok, A.; Scholten, J.; Righi, L.W.; Cardoso, C.D.; Necchi Rohers, G.; da Silva, R.C. Development, Optimization and Validation of a Multimethod for the Determination of 36 Mycotoxins in Wines by Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2014, 129, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Cao, X.; Liu, M.; Wang, W. Determination of Alternaria Mycotoxins in Wine and Juice Using Ionic Liquid Modified Countercurrent Chromatography as a Pretreatment Method Followed by High-Performance Liquid Chromatography. J. Chromatogr. A 2016, 1436, 133–140. [Google Scholar] [CrossRef]
- López, P.; Venema, D.; de Rijk, T.; de Kok, A.; Scholten, J.M.; Mol, H.G.J.; de Nijs, M. Occurrence of Alternaria Toxins in Food Products in The Netherlands. Food Control 2016, 60, 196–204. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.; Farmer, P. Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary Exposure Assessment to Alternaria Toxins in the European Population. EFSA J. 2016, 14, e04654. [Google Scholar] [CrossRef]
- EC-European Commission. Commission Recommendation (EU) 2022/553 of 5 April 2022 on Monitoring the Presence of Alternaria Toxins in Food. Off. J. Eur. Communities 2022, 107, 90. Available online: https://eur-lex.europa.eu/eli/reco/2022/553/oj/eng (accessed on 23 April 2025).
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Spoilage Yeasts in Red Wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–235. [Google Scholar]
- Kesmen, Z.; Özbekar, E.; Büyükkiraz, M.E. Multifragment Melting Analysis of Yeast Species Isolated from Spoiled Fruits. J. Appl. Microbiol. 2018, 124, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Regiones Vitivinícolas Argentinas. Available online: https://caracterizacion-fisico-ambiental-coviar.hub.arcgis.com/ (accessed on 23 April 2025).
- Serra, R.; Lourenço, A.; Alípio, P.; Venâncio, A. Influence of the Region of Origin on the Mycobiota of Grapes with Emphasis on Aspergillus and Penicillium Species. Mycol. Res. 2006, 110, 971–978. [Google Scholar] [CrossRef]
- Castrillo Cachón, D.; Rabuñal Crego, E.; Neira González, N.; Blanco Camba, P. Yeast Diversity on Grapes from Galicia, NW Spain: Biogeographical Patterns and the Influence of the Farming System. Oeno One 2019, 53, 573–587. [Google Scholar] [CrossRef]
- Cordoba, M.; Balzarini, M.; Paccioretti, P.; Vallone, R.; Corvalán, F. Zonificación Estadística de Regiones Vitivinícolas de Mendoza, San Juan, Salta y Tucumán Basada En Datos de Suelo y Clima; COVIAR: Miyazaki, Japan, 2023. [Google Scholar]
- Abrunhosa, L.; Paterson, R.R.M.; Kozakiewicz, Z.; Lima, N.; Venancio, A. Mycotoxin Production from Fungi Isolated from Grapes. Lett. Appl. Microbiol. 2001, 32, 240–242. [Google Scholar] [CrossRef]
- Combina, M.; Mercado, L.; Borgo, P.; Elia, A.; Jofre, V.; Ganga, A.; Martinez, C.; Catania, C. Yeasts Associated to Malbec Grape Berries from Mendoza, Argentina. J. Appl. Microbiol. 2005, 98, 1055–1061. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Querol, A.; Barrio, E.; Ramón, D. A Comparative Study of Different Methods of Yeast Strain Characterization. Syst. Appl. Microbiol. 1992, 15, 439–446. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of Yeasts by RFLP Analysis of the 5.8S RRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Evol. Microbiol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Espinar, M.T.F.; Martorell, P.; de Llanos, R.; Querol, A. Molecular Methods to Identify and Characterize Yeasts in Foods and Beverages. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 55–82. [Google Scholar]
- Siegel, D.; Merkel, S.; Koch, M.; Nehls, I. Quantification of the Alternaria Mycotoxin Tenuazonic Acid in Beer. Food Chem. 2010, 120, 902–906. [Google Scholar] [CrossRef]
- Merín, M.G.; Luciana, P.; Mario, A. Morata Aislamiento e Identificación de Levaduras de Uva Para Vinificar Con Síntomas de Podredumbre de La Región Vitivinícola de San Rafael. In Proceedings of the CLICAP Congreso Latinoamericano de Ingeniería y Ciencias Aplicadas; Facultad de Ciencias Aplicadas a la Industria: San Rafael, Argentina, 2022; p. 866. [Google Scholar]
- Sangorrín, M.P.; Lopes, C.A.; Jofré, V.; Querol, A.; Caballero, A.C. Spoilage Yeasts from Patagonian Cellars: Characterization and Potential Biocontrol Based on Killer Interactions. World J. Microbiol. Biotechnol. 2008, 24, 945–953. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Vargas Perucca, M.F.; Petrignani, D.B.; Vergara, S.C.; Leiva-Alaniz, M.J.; Maturano, Y.P.; Vazquez, F.; Dellacassa, E. Enhancing Flavor Complexity in Craft Beer: Sequential Inoculation with Indigenous Non-Saccharomyces and Commercial Saccharomyces Yeasts. Fermentation 2024, 10, 657. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces Cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Portugal, C.; Pinto, L.; Ribeiro, M.; Tenorio, C.; Igrejas, G.; Ruiz-Larrea, F. Potential Spoilage Yeasts in Winery Environments: Characterization and Proteomic Analysis of Trigonopsis Cantarellii. Int. J. Food Microbiol. 2015, 210, 113–120. [Google Scholar] [CrossRef] [PubMed]
- 3591:1977; Sensory Analysis — Apparatus — Wine-Tasting Glass. Organización Internacional de Normalización (ISO): Ginebra, Switzerland, 1977.
- Merín, M.G.; de Ambrosini, V.I.M. Kinetic and Metabolic Behaviour of the Pectinolytic Strain Aureobasidium Pullulans GM-R-22 during Pre-Fermentative Cold Maceration and Its Effect on Red Wine Quality. Int. J. Food Microbiol. 2018, 285, 18–26. [Google Scholar] [CrossRef]
- Organización Internacional de la Viña y el Vino (OIV). Compendium of International Methods of Wine and Must Analysis; International Organization of Vine and Wine: Paris, France, 2009. [Google Scholar]
- Herbert, S.; Rebecca, N.; Bleibaum, H.A.T. Sensory Evaluation Practices, 5th ed.; Academic Press: Oxford, UK, 2020; ISBN 9780128153345. [Google Scholar]
- Ghuffar, S.; Irshad, G.; Shahid, M.; Naz, F.; Riaz, A.; Khan, M.A.; Mehmood, N.; Sattar, A.; Asadullah, H.M.; Gleason, M.L. First Report of Alternaria Alternata Causing Fruit Rot of Grapes in Pakistan. Plant Dis. 2018, 102, 1659. [Google Scholar] [CrossRef]
- Kakalíková, Ľ.; Jankura, E.; Šrobárová, A. First Report of Alternaria Bunch Rot of Grapevines in Slovakia. Australas. Plant Dis. Notes 2009, 4, 68–69. [Google Scholar] [CrossRef]
- Nair, N. Fungi Associated with Bunch Rot of Grapes in the Hunter Valley. Aust. J. Agric. Res. 1985, 36, 435. [Google Scholar] [CrossRef]
- Swart, A.E.; Holz, G. Colonization of Table Grape Bunches by Alternaria Alternata and Rot of Cold-Stored Grapes. South Afr. J. Enol. Vitic. 2017, 15, 19–25. [Google Scholar] [CrossRef][Green Version]
- Tournas, V.H.; Katsoudas, E. Mould and Yeast Flora in Fresh Berries, Grapes and Citrus Fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, M. Bunch Rot of Rhine Riesling Grapes in the Lower South-West of Western Australia. Aust. J. Exp. Agric. 1980, 20, 247. [Google Scholar] [CrossRef]
- Cosseboom, S.D.; Hu, M. Diversity, Pathogenicity, and Fungicide Sensitivity of Fungal Species Associated with Late-Season Rots of Wine Grape in the Mid-Atlantic United States. Plant Dis. 2021, 105, 3101–3110. [Google Scholar] [CrossRef]
- Lorenzini, M.; Azzolini, M.; Tosi, E.; Zapparoli, G. Postharvest Grape Infection of Botrytis Cinerea and Its Interactions with Other Moulds under Withering Conditions to Produce Noble-Rotten Grapes. J. Appl. Microbiol. 2013, 114, 762–770. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Characterization and Pathogenicity of Alternaria Spp. Strains Associated with Grape Bunch Rot during Post-Harvest Withering. Int. J. Food Microbiol. 2014, 186, 1–5. [Google Scholar] [CrossRef]
- Serra, R.; Braga, A.; Venâncio, A. Mycotoxin-Producing and Other Fungi Isolated from Grapes for Wine Production, with Particular Emphasis on Ochratoxin A. Res. Microbiol. 2005, 156, 515–521. [Google Scholar] [CrossRef]
- Sage, L.; Garon, D.; Seigle-Murandi, F. Fungal Microflora and Ochratoxin A Risk in French Vineyards. J. Agric. Food Chem. 2004, 52, 5764–5768. [Google Scholar] [CrossRef]
- Chiotta, M.L.; Ponsone, M.L.; Combina, M.; Torres, A.M.; Chulze, S.N. Aspergillus Section Nigri Species Isolated from Different Wine-Grape Growing Regions in Argentina. Int. J. Food Microbiol. 2009, 136, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bau, M.; Bragulat, M.R.; Abarca, M.L.; Minguez, S.; Cabañes, F.J. Ochratoxigenic Species from Spanish Wine Grapes. Int. J. Food Microbiol. 2005, 98, 125–130. [Google Scholar] [CrossRef]
- Medina, A.; Mateo, R.; López-Ocaña, L.; Valle-Algarra, F.M.; Jiménez, M. Study of Spanish Grape Mycobiota and Ochratoxin A Production by Isolates of Aspergillus Tubingensis and Other Members of Aspergillus Section Nigri. Appl. Environ. Microbiol. 2005, 71, 4696–4702. [Google Scholar] [CrossRef]
- Díaz, G.A.; Torres, R.; Vega, M.; Latorre, B.A. Ochratoxigenic Aspergillus Species on Grapes from Chilean Vineyards and Aspergillus Threshold Levels on Grapes. Int. J. Food Microbiol. 2009, 133, 195–199. [Google Scholar] [CrossRef]
- Melki Ben Fredj, S.; Chebil, S.; Lebrihi, A.; Lasram, S.; Ghorbel, A.; Mliki, A. Occurrence of Pathogenic Fungal Species in Tunisian Vineyards. Int. J. Food Microbiol. 2007, 113, 245–250. [Google Scholar] [CrossRef]
- Prakitchaiwattana, C.; Fleet, G.; Heard, G. Application and Evaluation of Denaturing Gradient Gel Electrophoresis to Analyse the Yeast Ecology of Wine Grapes. FEMS Yeast Res. 2004, 4, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Gadoury, D.M.; Seem, R.C.; Wilcox, W.F.; Henick-Kling, T.; Conterno, L.; Day, A.; Ficke, A. Effects of Diffuse Colonization of Grape Berries by Uncinula Necator on Bunch Rots, Berry Microflora, and Juice and Wine Quality. Phytopathology 2007, 97, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A.A.; Nychas, G.-J.E. Yeast Populations Residing on Healthy or Botrytis -Infected Grapes from a Vineyard in Attica, Greece. Appl. Environ. Microbiol. 2007, 73, 2765–2768. [Google Scholar] [CrossRef]
- Prendes, L.P.; Merín, M.G.; Fontana, A.R.; Bottini, R.A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Isolation, Identification and Selection of Antagonistic Yeast against Alternaria Alternata Infection and Tenuazonic Acid Production in Wine Grapes from Argentina. Int. J. Food Microbiol. 2018, 266, 14–20. [Google Scholar] [CrossRef]
- Barata, A.; González, S.; Malfeito-Ferreira, M.; Querol, A.; Loureiro, V. Sour Rot-Damaged Grapes Are Sources of Wine Spoilage Yeasts. FEMS Yeast Res. 2008, 8, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Seborro, F.; Belloch, C.; Malfeito-Ferreira, M.; Loureiro, V. Ascomycetous Yeast Species Recovered from Grapes Damaged by Honeydew and Sour Rot. J. Appl. Microbiol. 2008, 104, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Occurrence of Non-Saccharomyces Cerevisiae Yeast Species Over Three Vintages in Four Vineyards and Grape Musts From Four Production Regions of the Western Cape, South Africa. S. Afr. J. Enol. Vitic. 2003, 24, 35–42. [Google Scholar] [CrossRef]
- Chavan, P.; Mane, S.; Kulkarni, G.; Shaikh, S.; Ghormade, V.; Nerkar, D.P.; Shouche, Y.; Deshpande, M.V. Natural Yeast Flora of Different Varieties of Grapes Used for Wine Making in India. Food Microbiol. 2009, 26, 801–808. [Google Scholar] [CrossRef]
- Raspor, P.; Milek, D.M.; Polanc, J.; Smole Možina, S.; Čadež, N. Yeasts Isolated from Three Varieties of Grapes Cultivated in Different Locations of the Dolenjska Vine-Growing Region, Slovenia. Int. J. Food Microbiol. 2006, 109, 97–102. [Google Scholar] [CrossRef]
- Mattos Rocha, R.K.; Andrioli, J.; Scariot, F.J.; Schwarz, L.V.; Longaray Delamare, A.P.; Echeverrigaray, S. Yeast Diversity in Cabernet-Sauvignon and Merlot Grapes Grown in the Highlands of Southern Brazil. OENO One 2022, 56, 101–110. [Google Scholar] [CrossRef]
- Battilani, P.; Logrieco, A.; Giorni, P.; Cozzi, G.; Bertuzzi, T.; Pietri, A. Ochratoxin A Production by Aspergillus Carbonarius on Some Grape Varieties Grown in Italy. J. Sci. Food Agric. 2004, 84, 1736–1740. [Google Scholar] [CrossRef]
- Bellí, N.; Bau, M.; Marín, S.; Abarca, M.L.; Ramos, A.J.; Bragulat, M.R. Mycobiota and Ochratoxin A Producing Fungi from Spanish Wine Grapes. Int. J. Food Microbiol. 2006, 111, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Mendonça, C.; Venâncio, A. Ochratoxin A Occurrence and Formation in Portuguese Wine Grapes at Various Stages of Maturation. Int. J. Food Microbiol. 2006, 111, S35–S39. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria Redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Nichea, M.J.; Cendoya, E.; Romero, C.J.; Humaran, J.F.; Zachetti, V.G.L.; Palacios, S.A.; Ramirez, M.L. Phylogenetic Analysis and Toxigenic Profile of Alternaria Species Isolated from Chickpeas (Cicer Arietinum) in Argentina. Diversity 2022, 14, 924. [Google Scholar] [CrossRef]
- Pavón, M.Á.; González, I.; Pegels, N.; Martín, R.; García, T. PCR Detection and Identification of Alternaria Species-Groups in Processed Foods Based on the Genetic Marker Alt a 1. Food Control 2010, 21, 1745–1756. [Google Scholar] [CrossRef]
- Geisen, R.; Touhami, N.; Schmidt-Heydt, M. Mycotoxins as Adaptation Factors to Food Related Environments. Curr. Opin. Food Sci. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Merín, M.G.; Raya, R.R.; Morata de Ambrosini, V.I.; Mendoza, L.M. Killer Yeasts Used as Starter Cultures to Modulate the Behavior of Potential Spoilage Non-Saccharomyces Yeasts during Malbec Wine Fermentation. Food Biosci. 2024, 57, 103424. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia Pulcherrima as Biocontrol Agent and Wine Aroma Enhancer in Combination with a Native Saccharomyces Cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Merín, M.G.; Morata, V.I.; Farías, M.E. Characterization of Wines Produced by Mixed Culture of Autochthonous Yeasts and Oenococcus Oeni from the Northwest Region of Argentina. J. Ind. Microbiol. Biotechnol. 2011, 38, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Gao, H.; Bai, X.; Li, L.; Wei, R.; Dong, Z. Metabolomics and Flavor Diversity in Cabernet Sauvignon Wines Fermented by Various Origins of Hanseniaspora Uvarum in the Presence and Absence of Saccharomyces Cerevisiae. LWT 2024, 203, 116396. [Google Scholar] [CrossRef]
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of Native Non-Saccharomyces Yeasts with Biocontrol Activity against Spoilage Yeasts in Order to Produce Healthy Regional Wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a Future for Non-Saccharomyces Yeasts: Selection of Putative Spoilage Wine Strains to Be Used in Association with Saccharomyces Cerevisiae for Grape Juice Fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Vega-Lopez, G.A.; Fernández de Ullivarri, M.; Raya, R.R. Population and Oenological Characteristics of Non-Saccharomyces Yeasts Associated with Grapes of Northwestern Argentina. Arch. Microbiol. 2019, 201, 235–244. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, N.; Badura, J.; von Wallbrunn, C.; Pretorius, I.S. Exploring Future Applications of the Apiculate Yeast Hanseniaspora. Crit. Rev. Biotechnol. 2024, 44, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda, A. Volatilomics of Fruit Wines. Molecules 2024, 29, 2457. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Wine Spoilage Yeasts: Control Strategy. In Yeast—Industrial Applications; InTech: Nappanee, IN, USA, 2017. [Google Scholar]
- Mestre, M.V.; Maturano, Y.P.; Gallardo, C.; Combina, M.; Mercado, L.; Toro, M.E.; Carrau, F.; Vazquez, F.; Dellacassa, E. Impact on Sensory and Aromatic Profile of Low Ethanol Malbec Wines Fermented by Sequential Culture of Hanseniaspora Uvarum and Saccharomyces Cerevisiae Native Yeasts. Fermentation 2019, 5, 65. [Google Scholar] [CrossRef]
- Granchi, L.; Patrignani, F.; Bianco, A.; Braschi, G.; Budroni, M.; Canonico, L.; Capece, A.; Cauzzi, A.; Ciani, M.; Chinnici, F.; et al. Comparison between Metschnikowia Pulcherrima and Torulaspora Delbrueckii Used in Sequential Wine Fermentations with Saccharomyces Cerevisiae. Front. Microbiol. 2025, 16, 1590561. [Google Scholar] [CrossRef]

| Species | AP a (bp) | Restriction Lengths (bp) | |||
|---|---|---|---|---|---|
| Cfo I b | Dde I c | Hae III d | Hinf I e | ||
| Aureobasidium pullulans | 600 | 100 + 180 + 190 | ND | 150 + 450 | 130 + 180 + 290 |
| Hanseniaspora guilliermondii | 775 | 105 + 320 + 340 | 90 + 120 + 160 + 360 | 775 | 160 + 200 + 360 |
| Hanseniaspora uvarum | 775 | 100 + 320 + 340 | 80 + 100 + 170 + 300 | 775 | 160 + 200 + 370 |
| Hanseniaspora vineae | 775 | 90 + 150 + 180 + 270 | 80 + 230 + 460 | 110 + 660 | 370 + 390 |
| Metschnikowia pulcherrima | 400 | 95 + 100 + 205 | ND | 100 + 280 | 190 + 200 |
| Zygosaccharomyces bailii | 790 | 95 + 95 + 270 + 320 | ND | 90 + 690 | 55 + 160 + 225 + 340 |
| Subzone | Wine Grape Variety | TA in Wine Grapes | |
|---|---|---|---|
| Number of Positive Samples 1 | Range (µg/kg) 2 | ||
| 3-1 | Bonarda b | 5/5 | <LOQ 3; 249.29 ± 0.00 |
| Cabernet Sauvignon a | 1/1 | 1395.68 ± 32.95 | |
| Cereza b | 2/2 | <LOQ 3; 75.61 ± 17.68 | |
| Criolla b | 2/2 | <LOQ 3; 103.47 ± 25.00 | |
| Malbec b | 1/1 | <LOQ 3 | |
| Syrah b | 1/1 | 76.90 ± 0.00 | |
| Torrontés b | 1/1 | 140.14 ± 14.10 | |
| 3-3 | Bonarda | 3/4 | <LOQ; 67.27 ± 19.36 |
| Species | Acetic Acid Production | N° of Positive H2S-Producing Strains 2/Total | Defect in Grape Must | |
|---|---|---|---|---|
| N° of Positive Strains/Total | Width 1 (mm) of the Clarification Halo | |||
| M. pulcherrima | 0/17 | nd | 17/17 | M. pulcherrima L1: solvent and acetate odour; cloudy |
| A. pullulans | 0/9 | nd | 5/9 | nd |
| H. guilliermondii | 7/8 | (0.986 ± 0.305) b | 8/8 | nd |
| H. uvarum | 9/10 | (1.694 ± 0.919) ab | 10/10 | nd |
| H. vineae | 6/10 | (1.104 ± 0.279) ab | 10/10 | nd |
| Z. bailii | 0/5 | nd | 5/5 | nd |
| D. bruxellensis * | 1/1 | (5.375) a | 0/1 | “Brett” defect: smell of horse sweat, rubber; rotten; cloudy |
| S. cerevisiae * | 0/1 | nd | 0/1 | nd |
| Physicochemical Parameters | Wines | ||
|---|---|---|---|
| Mp + Sc | Hu + Sc | Sc (Control) | |
| Ethanol (%, v/v) | 14.40 ± 0.14 | 14.10 ± 0.71 | 14.10 ± 0.62 |
| Residual sugars (g/L) | 1.05 ± 0.21 | 0.80 ± 0.57 | 1.23 ± 0.38 |
| Total acidity (g/L tartaric acid) | 4.55 ± 0.21 | 4.65 ± 0.07 | 4.90 ± 0.20 |
| Volatile acidity (g/L acetic acid) | 0.60 ± 0.00 a | 0.69 ± 0.02 b | 0.66 ± 0.01 ab |
| pH | 3.51 ± 0.02 | 3.49 ± 0.02 | 3.48 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prendes, L.P.; Merín, M.G.; Zamora, F.A.; Courtel, C.; Vega, G.A.; Ferreyra, S.G.; Fontana, A.R.; Ramirez, M.L.; Morata, V.I. Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region. Fermentation 2025, 11, 536. https://doi.org/10.3390/fermentation11090536
Prendes LP, Merín MG, Zamora FA, Courtel C, Vega GA, Ferreyra SG, Fontana AR, Ramirez ML, Morata VI. Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region. Fermentation. 2025; 11(9):536. https://doi.org/10.3390/fermentation11090536
Chicago/Turabian StylePrendes, Luciana Paola, María Gabriela Merín, Fabio Alberto Zamora, Claire Courtel, Gustavo Alberto Vega, Susana Gisela Ferreyra, Ariel Ramón Fontana, María Laura Ramirez, and Vilma Inés Morata. 2025. "Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region" Fermentation 11, no. 9: 536. https://doi.org/10.3390/fermentation11090536
APA StylePrendes, L. P., Merín, M. G., Zamora, F. A., Courtel, C., Vega, G. A., Ferreyra, S. G., Fontana, A. R., Ramirez, M. L., & Morata, V. I. (2025). Alternaria, Tenuazonic Acid and Spoilage Yeasts Associated with Bunch Rots of the Southern Oasis of Mendoza (Argentina) Winegrowing Region. Fermentation, 11(9), 536. https://doi.org/10.3390/fermentation11090536







