Comparison of In Vitro Fermentation Characteristics Among Five Maize Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting, Harvesting and Sample Preparation
2.2. Inoculum Preparation, Inoculation and Incubation
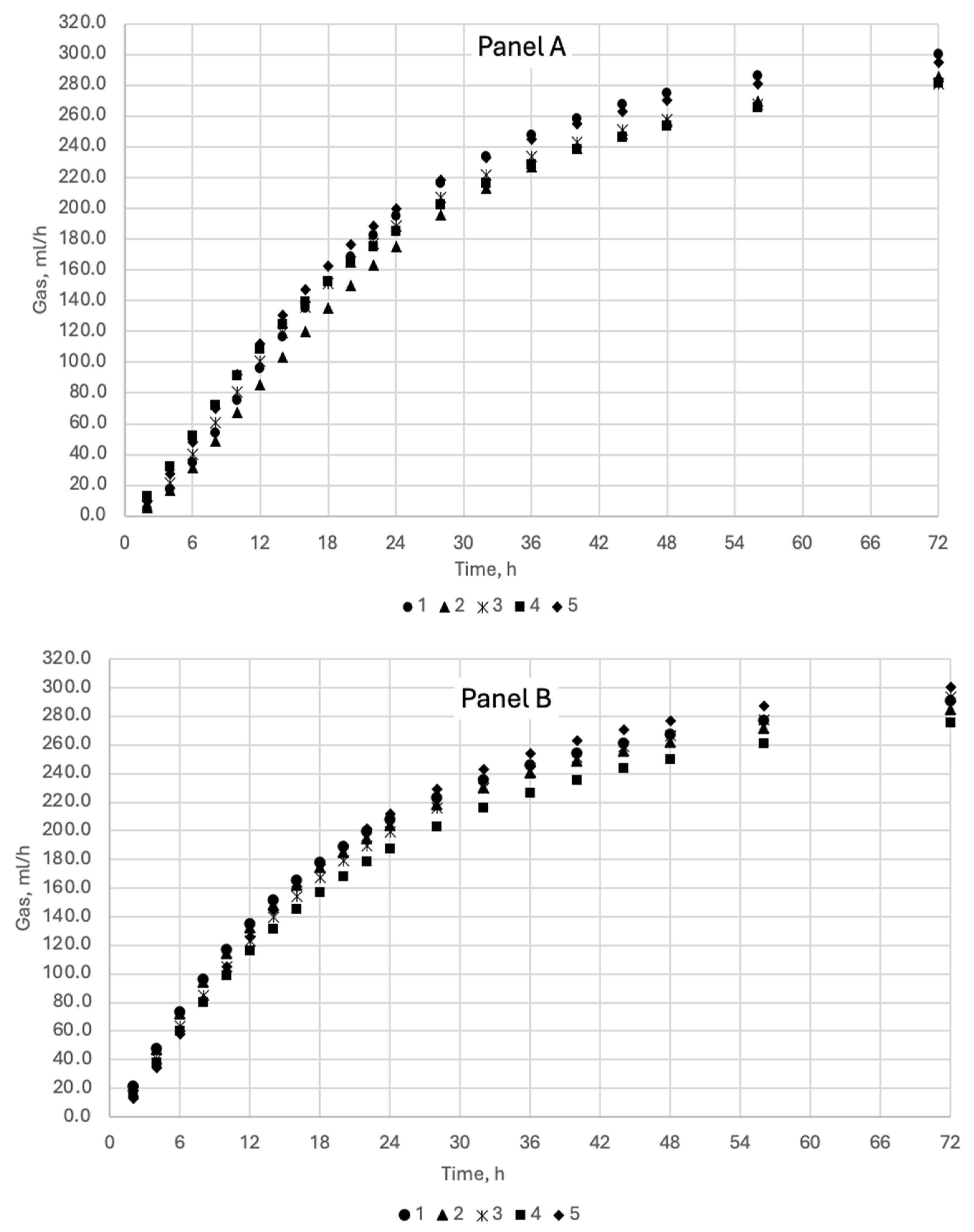
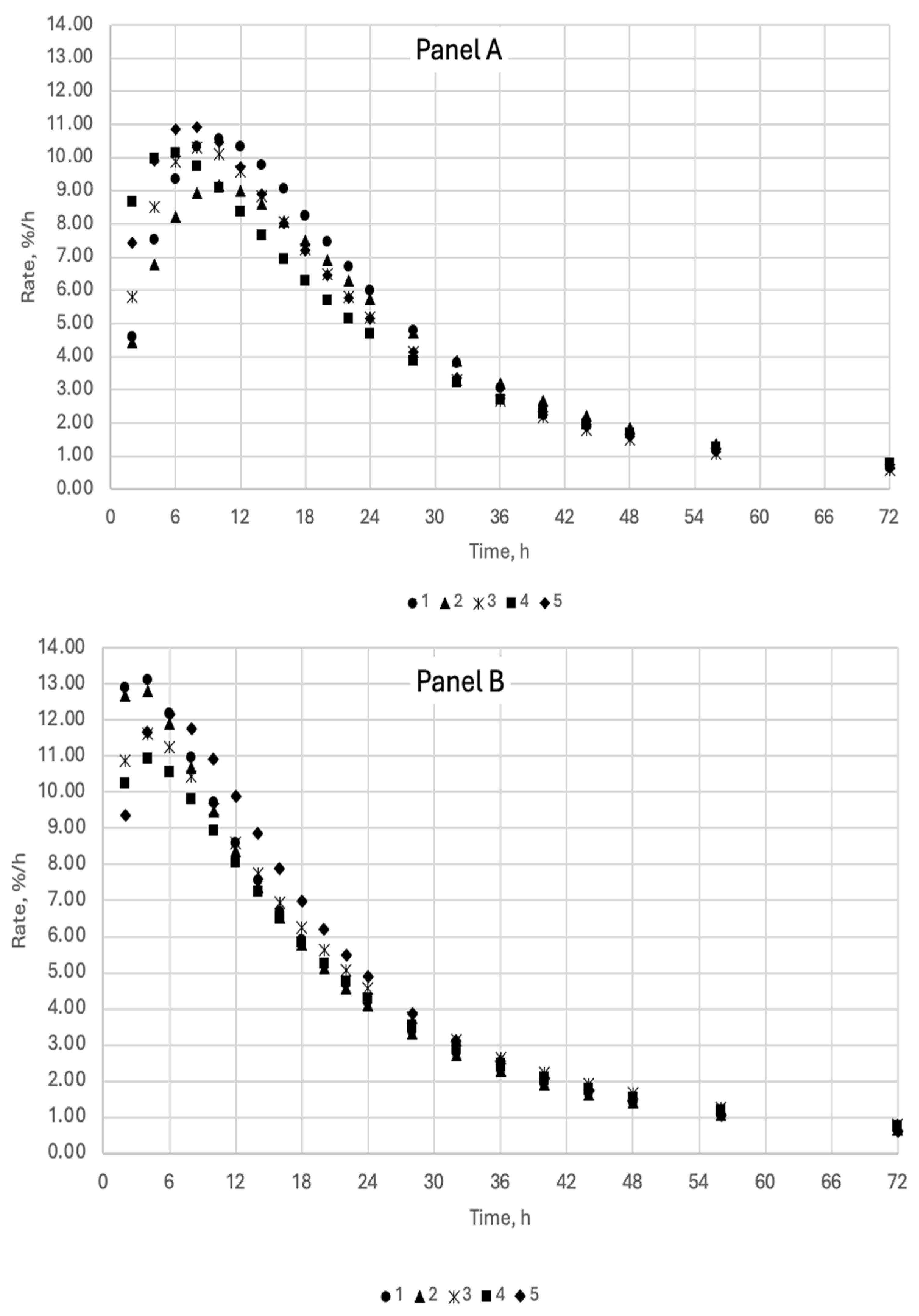
2.3. Measurements of Gas and Residual Substrate
2.4. Fermentation End Products
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deaville, R.; Givens, D.I. Use of the automated gas production technique to determine the fermentation kinetics of cabohydrate fractions in maize silage. Anim. Feed Sci. Technol. 2001, 93, 205–215. [Google Scholar] [CrossRef]
- Neylon, J.M.; Kung, L. Effects of Cutting Height and Maturity on the Nutritive Value of Corn Silage for Lactating Cows. J. Dairy Sci. 2003, 86, 2163–2169. [Google Scholar] [CrossRef]
- Mastellone, V.; Musco, N.; Infascelli, F.; Scandurra, A.; D’Aniello, B.; Pero, M.E.; Iommelli, P.; Tudisco, R.; Lombardi, P. Higher forage: Concentrate ratio and space availability may favor positive behaviors in dairy cows. J. Vet. Behav. 2022, 51, 16–22. [Google Scholar] [CrossRef]
- Huhtanen, P.; Südekum, K.H.; Nousiainen, J.; Shingfield, K.J. Forage conservation, feeding value and milk quality. Grassl. A Chang. World 2010, 15, 379–400. [Google Scholar]
- Spanghero, M.; Zanfi, C.; Signor, M.; Davanzo, D.; Volpe, V.; Venerus, S. Effects of plant vegetative stage and field drying time on chemical composition and in vitro ruminal degradation of forage soybean silage. Anim. Feed Sci. Technol. 2015, 200, 102–106. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Ge, Q.; Yang, B.; Li, S. Effects of harvest period and mixed ratio on the characteristic and quality of mixed silage of alfalfa and maize. Anim. Feed Sci. Technol. 2023, 306, 115796. [Google Scholar] [CrossRef]
- Phipps, R.; Sutton, J.; Beever, D.; Jones, A. The effect of crop maturity on the nutritional value of maize silage for lactating dairy cows. 3. Food intake and milk production. Anim. Sci. 2000, 71, 401–409. [Google Scholar] [CrossRef]
- ISTAT. Data Warehouse of Statistics Produced by ISTAT-Italian National Institute of Statistics. 2022. Available online: http://dati.istat.it/Index.aspx?QueryId=33702 (accessed on 6 November 2024).
- Bonaiuto, F.; De Dominicis, S.; Ganucci Cancellieri, U.; Crano, W.D.; Ma, J.; Bonaiuto, M. Italian Food? Sounds Good! Made in Italy and Italian Sounding Effects on Food Products’ Assessment by Consumers. Front. Psychol. 2021, 12, 581492. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Brogna, N.; Formigoni, A. The link between feeding dairy cows and Parmigiano-Reggiano cheese production area. Prof. Anim. Sci. 2017, 33, 520–529. [Google Scholar] [CrossRef]
- Colombini, S.; Galassi, G.; Crovetto, G.M.; Rapetti, L. Milk production, nitrogen balance, and fiber digestibility prediction of corn, whole plant grain sorghum, and forage sorghum silages in the dairy cow. J. Dairy Sci. 2012, 95, 4457–4467. [Google Scholar] [CrossRef]
- McGrath, J.; Duval, S.M.; Tamassia, L.F.; Kindermann, M.; Stemmler, R.T.; de Gouvea, V.N.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Vet. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef]
- De la Roza-Delgado, B.; Garrido-Varo, A.; Soldado, A.; Gonz’alezArrojo, A.; Cuevas Vald’es, M.; Maroto, F.; Pérez-Marínb, D. Matching portable NIRS instruments for in situ monitoring indicators of milk composition. Food Control 2017, 76, 74–81. [Google Scholar] [CrossRef]
- Hancock, D.W.; Collins, M. Forage preservation method influences alfalfa nutritive value and feeding characteristics. Crop Sci. 2006, 46, 688–694. [Google Scholar] [CrossRef]
- Kugler, J. Producing high quality orchardgrass and timothy hay. Proc. Natl. Alfalfa Symp. 2004, 13, 15. [Google Scholar]
- Kumar, S.; Choudhury, P.K.; Carro, M.D.; Griffith, G.W.; Dagar, S.S.; Puniya, M.; Calabrò, S.; Ravella, S.R.; Dhewa, T.; Upadhyay, R.C. New aspects and strategies for methane mitigation from ruminants. Appl. Microbiol. Biotechnol. 2014, 98, 31–44. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1838–1844. [Google Scholar] [CrossRef]
- Zicarelli, F.; Sarubbi, F.; Iommelli, P.; Grossi, M.; Lotito, D.; Tudisco, R.; Infascelli, F.; Musco, N.; Lombardi, P. Nutritional Characteristics of Corn Silage Produced in Campania Region Estimated by Near Infrared Spectroscopy (NIRS). Agronomy 2023, 13, 634. [Google Scholar] [CrossRef]
- Kiatti, D.; Bossima, I.K.; Vastolo, A.; Chiacchio, M.F.; Vitaglione, P.; Dossa, L.H.; Cutrignelli, M.I.; Calabrò, S. Sustainable ruminant nutrition in West Africa by in vitro characterization of cashew apple by-products. Heliyon 2024, 10, e37737. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Searle, P.L. Determination of ammonium in seawater based on the indophenol reaction with ophenylphenol (OPP): A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Crovetto, G.M.; Rapetti, L.; Galassi, G. Gas production and nutritive value of the whole plant and its components in four hybrids of maize. Ital. J. Anim. Sci. 2003, 2 (Suppl. 1), 198–200. [Google Scholar]
- Udén, P. Fresh and ensiled forage plants—Total composition, silage losses and the prediction of silage composition from the crop. Grass Forage Sci. 2018, 73, 420–431. [Google Scholar] [CrossRef]
- De Boever, J.L.; Aerts, J.M.; Vanacker, J.M.; De Brabander, D.L. Evaluation of the nutritive value of maize silages using a gas production technique. Anim. Feed Sci. Technol. 2005, 123–124, 255–265. [Google Scholar] [CrossRef]
- Lovet, D.K.; Deaville, E.R.; Mould, F.; Givens, D.I.; Owen, E. Using near infrared reflectance spectroscopy (NIRS) to predict the biologial parameters of maize silage. Anim. Feed Sci. Technol. 2004, 115, 179–187. [Google Scholar] [CrossRef]
- Doane, P.H.; Pell, A.N.; Schofield, P. The effect of preservation method on the neutral detergent soluble fraction of forages. J. Anim. Sci. 1997, 75, 1140–1148. [Google Scholar] [CrossRef]
- Iommelli, P.; Zicarelli, F.; Musco, N.; Sarubbi, F.; Grossi, M.; Lotito, D.; Lombardi, P.; Infascelli, F.; Tudisco, R. Effect of Cereals and Legumes Processing on In Situ Rumen Protein Degradability: A Review. Fermentation 2022, 8, 363. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell Unvesity Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Charmley, E. Towards improved silage quality–A review. Can. J. Anim. Sci. 2001, 81, 157–168. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
| Variety | DM | CP | Ash | Starch | NDF | ADF | TS |
|---|---|---|---|---|---|---|---|
| Fresh | |||||||
| Tiesto | 290 ± 21 | 73 ± 3 | 45 ± 4 | 325 ± 22 | 428 ± 38 | 243 ± 21 | 105 ± 12 |
| R700 1 | 300 ± 22 | 74 ± 2 | 40 ± 7 | 311 ± 24 | 395 ± 36 | 228 ± 26 | 79 ± 8 |
| MAS 78.T | 285 ± 19 | 72 ± 3 | 42 ± 5 | 318 ± 23 | 398 ± 41 | 228 ± 28 | 70 ± 9 |
| DKC 7074 | 298 ± 20 | 78 ± 4 | 44 ± 4 | 294 ± 19 | 401 ± 40 | 238 ± 28 | 76 ± 11 |
| KWS Kantico | 301 ± 23 | 71 ± 3 | 41 ± 4 | 314 ± 19 | 402 ± 40 | 227 ± 30 | 70 ± 10 |
| Ensiled | |||||||
| Tiesto | 303 ± 21 | 79 ± 2 | 44 ± 5 | 324 ± 24 | 428 ± 42 | 231 ± 21 | <12 |
| R700 1 | 303 ± 22 | 81 ± 3 | 46 ± 5 | 309 ± 27 | 443 ± 43 | 242 ± 22 | <12 |
| MAS 78.T | 332 ± 23 | 83 ± 3 | 44 ± 7 | 328 ± 28 | 423 ± 38 | 232 ± 22 | <12 |
| DKC 7074 | 322 ± 25 | 86 ± 4 | 47 ± 5 | 303 ± 28 | 434 ± 37 | 242 ± 25 | <12 |
| KWS Kantico | 332 ± 21 | 76 ± 3 | 45 ± 4 | 335 ± 22 | 421 ± 37 | 230 ± 19 | <12 |
| Variety | OMCV | B | tRM | RM | OM Loss | pH |
|---|---|---|---|---|---|---|
| mL·g−1 | h | h | %/h | % | ||
| Fresh | ||||||
| Tiesto | 302 | 20.7 | 12.5 | 10.24 | 76.4 | 6.51 |
| R700 1 | 292 | 20.6 | 12.7 | 9.96 | 76.9 | 6.57 |
| MAS 78.T | 282 | 20.9 | 13.4 | 9.61 | 77.4 | 6.56 |
| DKC 7074 | 281 | 22.1 | 13.0 | 8.85 | 74.9 | 6.54 |
| KWS Kantico | 296 | 21.3 | 13.9 | 9.98 | 77.0 | 6.56 |
| MSE | 75 | 0.60 | 0.33 | 0.35 | 6.76 | 0.004 |
| Ensiled | ||||||
| Tiesto | 286 | 21.3 | 15.1 | 10.12 | 78.1 AB | 6.52 |
| R700 1 | 281 | 21.1 | 14.6 | 9.84 | 74.6 B | 6.51 |
| MAS 78.T | 291 | 21.5 | 15.1 | 10.09 | 76.0 AB | 6.54 |
| DKC 7074 | 272 | 22.3 | 14.3 | 8.95 | 77.5 AB | 6.49 |
| KWS Kantico | 292 | 20.4 | 14.4 | 10.7 | 79.7 A | 6.48 |
| MSE | 195 | 1.42 | 0.49 | 0.73 | 2.66 | 0.002 |
| Ensiling effect | NS | NS | NS | NS | NS | NS |
| Variety | Acetic | Propionic | Butyric | VFA | BCR | NGR | NH3-N |
|---|---|---|---|---|---|---|---|
| mM·g−1 | |||||||
| Fresh | |||||||
| Tiesto | 4.68 | 2.38 | 0.836 | 8.31 | 0.097 AB | 6.76 | 1.73 |
| R700 1 | 4.52 | 2.63 | 0.827 | 8.36 | 0.088 B | 6.58 | 1.71 |
| MAS 78.T | 4.63 | 2.79 | 0.822 | 8.63 | 0.09 AB | 6.70 | 1.71 |
| DKC 7074 | 4.44 | 2.74 | 0.826 | 8.45 | 0.105 A | 6.58 | 1.70 |
| KWS Kantico | 4.54 | 2.85 | 0.847 | 8.61 | 0.087 B | 6.63 | 1.68 |
| MSE | 0.034 | 0.036 | 0.003 | 0.074 | 0.00003 | 0.083 | 0.005 |
| Ensiled | |||||||
| Tiesto | 4.29 | 2.46 | 0.999 | 8.15 | 0.095 B | 6.71 | 1.91 |
| R700 1 | 4.25 | 2.56 | 0.941 | 8.19 | 0.105 B | 6.58 | 1.90 |
| MAS 78.T | 4.32 | 2.36 | 0.952 | 8.05 | 0.103 B | 6.64 | 1.92 |
| DKC 7074 | 4.49 | 2.54 | 0.935 | 8.38 | 0.098 B | 6.76 | 1.90 |
| KWS Kantico | 4.35 | 2.48 | 1.074 | 8.46 | 0.126 A | 7.07 | 1.84 |
| MSE | 0.031 | 0.065 | 0.01 | 0.11 | 0.00006 | 0.17 | 0.003 |
| Ensiling effect | NS | NS | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zicarelli, F.; Calabrò, S.; Iommelli, P.; Grossi, M.; Infascelli, F.; Tudisco, R. Comparison of In Vitro Fermentation Characteristics Among Five Maize Varieties. Fermentation 2025, 11, 285. https://doi.org/10.3390/fermentation11050285
Zicarelli F, Calabrò S, Iommelli P, Grossi M, Infascelli F, Tudisco R. Comparison of In Vitro Fermentation Characteristics Among Five Maize Varieties. Fermentation. 2025; 11(5):285. https://doi.org/10.3390/fermentation11050285
Chicago/Turabian StyleZicarelli, Fabio, Serena Calabrò, Piera Iommelli, Micaela Grossi, Federico Infascelli, and Raffaella Tudisco. 2025. "Comparison of In Vitro Fermentation Characteristics Among Five Maize Varieties" Fermentation 11, no. 5: 285. https://doi.org/10.3390/fermentation11050285
APA StyleZicarelli, F., Calabrò, S., Iommelli, P., Grossi, M., Infascelli, F., & Tudisco, R. (2025). Comparison of In Vitro Fermentation Characteristics Among Five Maize Varieties. Fermentation, 11(5), 285. https://doi.org/10.3390/fermentation11050285








