Species and Harvest Time of Fresh Tropical Grasses Affect Rumen Fermentation as Determined by In Sacco and In Vitro Incubations
Abstract
1. Introduction
2. Materials and Methods
2.1. Grass Sample Collection
2.2. Chemical Analyses
2.3. In Sacco Determination of Rumen Degradability
2.3.1. Sampling
2.3.2. In Sacco Procedure
2.3.3. In Sacco Experiment: Curve Fitting and Calculations
2.4. In Vitro Determination of Gas and Methane (CH4) Production
2.4.1. In Vitro Gas and CH4 Measurement and Donor Animals
2.4.2. Gas and Methane Production: Curve Fitting and Calculations
2.5. Statistical Analyses
3. Results
3.1. Chemical Composition of Tropical Grasses at Different Maturity Stages
3.2. Effects of Tropical Grasses at Different Maturity Stages on In Vitro Gas Production
3.3. In Sacco Degradation of Tropical Grasses at Different Maturity Stages
3.4. Relationships Between Chemical Composition and Ruminal Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ADF | Acid detergent fiber |
| ADL | Acid detergent lignin |
| ANOVA | Analysis of variance |
| CH4 | Methane |
| CP | Crude protein |
| D | Potentially degradable fraction |
| DM | Dry matter |
| ED | Effective degradability |
| EE | Ether extract |
| GP | Gas production |
| IVGP | In vitro gas production |
| IVGPT | In vitro gas production technique |
| Kd | Rate of degradation of D |
| NDF | Neutral detergent fiber |
| NDFD | Neutral detergent fiber degradability |
| OM | Organic matter |
| OMD | Organic matter degradability |
| Rmax | Maximum rate of gas production |
| TRmax | Time to reach Rmax |
| U | Undegradable fraction |
| VFA | Volatile fatty acid |
| W | Washable fraction |
| WSC | Water-soluble carbohydrate |
References
- Williams, S.R.O.; Hannah, M.C.; Jacobs, J.L.; Wales, W.J.; Moate, P.J. Volatile fatty acids in ruminal fluid can be used to predict methane yield of dairy cows. Animals 2019, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Allen, M.S. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: Effects on dry matter intake and milk yield of dairy cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.D.; Dhanoa, M.S.; Morant, S.V.; France, J.; Napper, D.J.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef]
- Elizalde, J.C.; Merchen, N.R.; Faulkner, D.B. In situ dry matter and crude protein degradation of fresh forages during the spring growth. J. Dairy Sci. 1999, 82, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Archimède, H.; Eugène, M.; Marie Magdeleine, C.; Boval, M.; Martin, C.; Morgavi, D.P.; Lecomte, P.; Doreau, M. Comparison of methane production between C3 and C4 grasses and legumes. Anim. Feed Sci. Technol. 2011, 166–167, 59–64. [Google Scholar] [CrossRef]
- Ortega-Gómez, R.; Castillo-Gallegos, E.; Jarillo-Rodríguez, J.; Escobar-Hernández, R.; Ocaña-Zavaleta, E.; de la Mora, B.V. Nutritive quality of ten grasses during the rainy season in a hot-humid climate and ultisol soil. Trop. Subtrop. Agroecosyst. 2011, 13, 481–491. [Google Scholar]
- Wongpom, B.; Koonawootrittriron, S.; Elzo, M.A.; Suwanasopee, T. Milk yield, fat yield and fat percentage associations in a Thai multibreed dairy population. Agric. Nat. Resour. 2017, 51, 218–222. [Google Scholar] [CrossRef]
- Huyen, N.T.D.; Schonewille, J.T.; Pellikaan, W.F.; Nguyen, T.X.; Hendriks, W.H. Grasses in response to variable regrowth periods in Vietnam. Fermentation 2024, 10, 280. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of inoculum source in a gas production method. Anim. Feed Sci. Technol. 2005, 124–125, 95–105. [Google Scholar] [CrossRef]
- Bezabih, M.; Pellikaan, W.F.; Tolera, A.; Khan, N.A.; Hendriks, W.H. Chemical composition and in vitro total gas and methane production of forage species from the Mid Rift Valley grasslands of Ethiopia. Grass Forage Sci. 2014, 69, 635–643. [Google Scholar] [CrossRef]
- Melesse, A.; Steingass, H.; Schollenberger, M.; Rodehutscord, M. Screening of common tropical grass and legume forages in Ethiopia for their nutrient composition and methane production profile in vitro. Trop. Grassl. 2017, 5, 163–175. [Google Scholar] [CrossRef]
- Macome, F.M.; Pellikaan, W.F.; Hendriks, W.H.; Warner, D.; Schonewille, J.T.; Cone, J.W. In vitro gas and methane production in rumen fluid from dairy cows fed grass silages differing in plant maturity, compared to in vivo data. J. Anim. Physiol. Anim. Nutr. 2017, 102, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Tung, L.X.; Hao, B.V.; Quyen, N.T.; Thao, V.T.; Quang, N.H. Evaluation on adaptability and potentiality of some forage species in Northwestern regions of Vietnam in the year of establishment. J. Anim. Husb. Sci. Tech. 2014, 12, 77–83. [Google Scholar]
- AOAC; Horwitz, W. Official Methods of Analysis of AOAC International, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2005; pp. 69–80. [Google Scholar]
- ISO 5983-2; Animal Feeding Stuffs. Determination of Nitrogen Content and Calculation Of Crude Protein Content. Part 2: Block Digestion/Steam Distillation Method. ISO: Geneva, Switzerland, 2009.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Collaborative study of acid-detergent fiber and lignin. J. AOAC 1973, 56, 781–784. [Google Scholar] [CrossRef]
- Tas, B.M.; Taweel, H.Z.; Smit, H.J.; Elgersma, A.; Dijkstra, J.; Tamminga, S. Rumen degradation characteristics of perennial ryegrass cultivars during the growing season. Anim. Feed Sci. Technol. 2006, 131, 102–119. [Google Scholar] [CrossRef]
- Ali, M.; Cone, J.W.; van Duinkerken, G.; Klop, A.; Kruisdijk, J.; Blok, M.C.; Bruinenberg, M.; Hendriks, W.H. Relationship between chemical composition and in situ rumen degradation characteristics of grass silages in dairy cows. NJAS—Wagening. J. Life Sci. 2014, 70, 9–15. [Google Scholar] [CrossRef]
- De Jonge, L.H.; van Laar, H.; Hendriks, W.H.; Dijkstra, J. A modified rinsing method for the determination of the S, W-S and D + U fraction of protein and starch in feedstuff within the in situ technique. Animal 2013, 7, 1289–1297. [Google Scholar] [CrossRef]
- Ali, M.; Cone, J.W.; van Duinkerken, G.; Klop, A.; Blok, M.C.; Bruinenberg, M.; Khan, N.A.; Hendriks, W.H. Variation between individual cows in in situ rumen degradation characteristics of maize and grass silages. NJAS—Wagening. J. Life Sci. 2016, 78, 167–173. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Fraseer, C.; Corse, E.L. The effect on protein utilization of feeding different protein supplements via the rumen or via the abomasum in young growing sheep. Br. J. Nutr. 1970, 24, 803–809. [Google Scholar] [CrossRef]
- Dhanoa, M.S. On the analysis of dacron bag data for low degradability feeds. Grass Forage Sci. 1988, 43, 441–444. [Google Scholar] [CrossRef]
- Warner, D.; Dijkstra, J.; Hendriks, W.H.; Pellikaan, W.F. Passage kinetics of 13C-labeled corn silage components through the gastrointestinal tract of dairy cows. J. Dairy Sci. 2013, 96, 5844–5858. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Marvin, H.J.P. Influence of drying method and ageing on chemical and physical properties and in vitro degradation characteristics of grass and maize samples. J. Agric. Sci. 1996, 126, 7–14. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Visscher, G.J.W.; Oudshoorn, L. Influence of rumen fluid and substrate concentration on fermentation kinetics measured with a fully automated time related gas production apparatus. Anim. Feed Sci. Technol. 1996, 61, 113–128. [Google Scholar] [CrossRef]
- Pellikaan, W.F.; Hendriks, W.H.; Uwimana, G.; Bongers, L.J.G.M.; Becker, P.M.; Cone, J.W. A novel method to determine simultaneously methane production during in vitro gas production using fully automated equipment. Anim. Feed Sci. Technol. 2011, 168, 196–205. [Google Scholar] [CrossRef]
- Pellikaan, W.F.; Stringano, E.; Leenaars, J.; Bongers, D.J.G.M.; van Laar-van Schuppen, S.; Plant, J.; Mueller-Harvey, I. Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Anim. Feed Sci. Technol. 2011, 166–167, 377–390. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Van Gelder, A.H.; Hetta, M.; Rodrigues, M.A.M.; de Boever, J.L.; de Hartigh, H.; Rymer, C.; van Oostrum, M.; van Kaathoven, R.; Cone, J.W. Ranking of in vitro fermentability of 20 feedstuffs with an automated gas production technique: Results of a ring test. Anim. Feed Sci. Technol. 2005, 123–124, 243–253. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Driehuis, F. Description of gas production profiles with a three-phasic model. Anim. Feed Sci. Technol. 1997, 66, 31–45. [Google Scholar] [CrossRef]
- Nutrient Requirements of Dairy Cattle, 7th ed.; National Research Council: Washington, DC, USA, 2001.
- Lounglawan, P.; Lounglawan, W.; Suksombat, W. Effect of Cutting Interval and Cutting Height on Yield and Chemical Composition of King Napier Grass (Pennisetum purpureum x Pennisetum americanum). APCBEE Procedia 2014, 8, 27–31. [Google Scholar] [CrossRef]
- De Oliveira, J.K.S.; da Corrêa, D.C.; Cunha, A.M.Q.; Do Rêgo, A.C.; Faturi, C.; da Silva, W.L.; Domingues, F.N. Effect of nitrogen fertilization on production, chemical composition and morphogenesis of guinea grass in the humid tropics. Agronomy 2020, 10, 1840. [Google Scholar] [CrossRef]
- Rook, J.A.F.; Balch, C.C. The effects of intraruminal infusions of acetic, propionic and butyric acids on the yield and composition of the milk of the cow. Br. J. Nutr. 1961, 15, 361–369. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, B.; Zhao, X. Could propionate formation be used to reduce enteric methane emission in ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef]
- Hoffman, P.C.; Sievert, S.J.; Shaver, R.D.; Welch, D.A.; Combs, D.K. In situ dry matter protein, and fiber degradation of perennial forages. J. Dairy Sci. 1993, 76, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.V.; Waghorn, G.C.; Brookes, I.M.; Woodfield, D.R. Effect of maturation and initial harvest dates on the nutritive characteristics of ryegrass (Lolium perenne L.). Anim. Feed Sci. Technol. 2006, 127, 293–318. [Google Scholar] [CrossRef]
- Heeren, J.A.H.; Podesta, S.C.; Hatew, B.; Klop, G.; van Laar, H.; Bannink, A.; Warner, D.; de Jonge, L.H.; Dijkstra, J. Rumen degradation characteristics of ryegrass herbage and ryegrass silage are affected by interactions between stage of maturity and nitrogen fertilisation rate. Anim. Prod. Sci. 2014, 54, 1263–1267. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Marita, J.M.; Frost, K.; Grabber, J.; Ralph, J.; Lu, F.; Kim, H. Grass lignin acylation: P-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta 2009, 229, 1253–1267. [Google Scholar] [CrossRef]
- Jung, H.G.; Vogel, K.P. Influence of lignin on digestibility of forage cell wall material. J. Anim. Sci. 1986, 62, 1703–1712. [Google Scholar] [CrossRef]
- Weimer, P.J. Why Don’t Ruminal Bacteria Digest Cellulose Faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Tamminga, S.; Zemmelink, G. Degradation of tropical roughages and concentrate feeds in the rumen. Anim. Feed Sci. Technol. 1995, 54, 81–92. [Google Scholar] [CrossRef]
- Aung, M.; Kyawt, Y.Y.; Htun, M.T.; Mu, K.S.; Aung, A. Comparison of in vitro fermentation with in situ degradation to estimate dry matter degradability and energy protein synchronization of roughage based diets. Iran. J. Appl. Anim. Sci. 2019, 9, 17–24. [Google Scholar]
- Rahmat, S.F.I.; Permana, I.G.; Despal, D. Rumen Degradation properties of tropical legumes feed under in sacco studies. IOP Conf. Ser. Earth Environ. Sci. 2021, 888, 012071. [Google Scholar] [CrossRef]
- Huyen, N.T.D.; Schonewille, J.T.; Pellikaan, W.F.; Trach, N.X.; Hendriks, W.H. In vitro gas and methane production of some common feedstuffs used for dairy rations in Vietnam and Thailand. Anim. Biosci. 2024, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.W.; van Gelder, A.H.; Soliman, I.A.; de Visser, H.; van Vuuren, A.M. Different techniques to study rumen fermentation characteristics of maturing grass and grass silage. J. Dairy Sci. 1999, 82, 957–966. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Chen, Z.; Karowe, D.N.; Spickard, A. C3 grasses have higher nutritional quality than C3 grasses under ambient and elevated atmospheric CO2. Glob. Change Biol. 2004, 10, 1565–1575. [Google Scholar] [CrossRef]
- Van Vuuren, A.M.; Tamminga, S.; Ketelaar, R.S. In sacco degradation of organic matter and crude protein of fresh grass (Lolium perenne) in the rumen of grazing dairy cows. J. Agric. Sci. 1991, 116, 429–436. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Piccolo, G.; Bovera, F.; Zicarelli, F.; Gazaneo, M.P.; Infascelli, F. In vitro fermentation kinetics of fresh and dried silage. Anim. Feed Sci. Technol. 2005, 123–124, 129–137. [Google Scholar] [CrossRef]
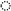 and ···), normal (× and - -), and late (- and —) from rumen of cattle. Error bars indicate SEM; lines in each panel indicate values for 336 h incubation.
and ···), normal (× and - -), and late (- and —) from rumen of cattle. Error bars indicate SEM; lines in each panel indicate values for 336 h incubation.
 and ···), normal (× and - -), and late (- and —) from rumen of cattle. Error bars indicate SEM; lines in each panel indicate values for 336 h incubation.
and ···), normal (× and - -), and late (- and —) from rumen of cattle. Error bars indicate SEM; lines in each panel indicate values for 336 h incubation.
| Grass | Harvest Time * | DM, % | OM | CP | EE | NDF | ADF | ADL |
|---|---|---|---|---|---|---|---|---|
| Guinea | Early (−2) | 16.4 | 890 | 218 | 28.7 | 573 | 315 | 20.2 |
| Normal | 15.7 | 883 | 169 | 26.3 | 663 | 410 | 31.6 | |
| Late (+2) | 15.5 | 877 | 143 | 28.5 | 661 | 397 | 30.6 | |
| King | Early (−2) | 13.8 | 881 | 139 | 33.4 | 557 | 297 | 19.4 |
| Normal | 13.3 | 909 | 88.7 | 27.9 | 628 | 360 | 23.6 | |
| Late (+2) | 18.5 | 931 | 89.9 | 23.7 | 642 | 399 | 47.3 | |
| Mulato II | Early (−2) | 19.9 | 864 | 145 | 25.7 | 441 | 223 | 19.3 |
| Normal | 17.5 | 875 | 107 | 19.2 | 586 | 320 | 24.5 | |
| Late (+2) | 17.4 | 879 | 136 | 18.1 | 600 | 326 | 30.9 |
| Parameter | Guinea | King | Mulato II | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early (−2) | Normal | Late (+2) | Early (−2) | Normal | Late (+2) | Early (−2) | Normal | Late (+2) | G | H | G × H | ||
| GP-72 h, mL/g OM | 312 b | 260 cde | 295 bc | 298 b | 350 a | 264 de | 323 ab | 291 bcd | 259 e | 7.5 | 0.049 | <0.001 | <0.001 |
| A1, mL/g OM | 41 c | 27 e | 37 cde | 54 b | 71 a | 54 b | 54 b | 40 cd | 29 de | 2.5 | <0.001 | 0.001 | <0.001 |
| A2, mL/g OM | 178 a | 130 de | 156 bc | 150 c | 171 ab | 120 e | 172 ab | 144 cd | 119 e | 4.2 | 0.022 | <0.001 | <0.001 |
| A1 + A2, mL/g OM | 219 abc | 156 fg | 193 cde | 204 bcd | 241 a | 174 efg | 226 ab | 184 def | 147 g | 6.2 | <0.001 | <0.001 | <0.001 |
| C1, h | 1.0 | 1.1 | 1.1 | 1.1 | 1.0 | 1.0 | 1.1 | 1.1 | 1.1 | 0.04 | 0.023 | 0.938 | 0.089 |
| C2, h | 10.5 ab | 10.9 a | 9.9 c | 9.5 d | 9.1 de | 9.0 e | 10.3 bc | 10.2 bc | 10.2 bc | 0.09 | <0.001 | <0.001 | <0.001 |
| Rmax1, mL/g OM/h | 24.6 cd | 15.5 d | 21.2 cd | 31.6 bc | 45.2 a | 38.0 ab | 31.1 bc | 23.0 cd | 15.8 d | 2.56 | <0.001 | 0.208 | <0.001 |
| Rmax2, mL/g OM/h | 12.8 a | 9.6 def | 11.7 ab | 11.0 bcd | 12.7 a | 9.0 ef | 11.6 abc | 10.2 cde | 8.4 f | 0.34 | <0.001 | <0.001 | <0.001 |
| CH4-72 h | |||||||||||||
| mL/g OM | 41 b | 34 b | 41 b | 41 b | 47 a | 35 b | 41 b | 35 c | 31 c | 1.2 | <0.001 | <0.001 | <0.001 |
| % of GP-72 | 13.0 | 12.9 | 13.9 | 13.8 | 13.5 | 13.7 | 12.8 | 11.9 | 12.2 | 0.22 | <0.001 | 0.226 | 0.226 |
| Parameter | Grass Species (G) | Harvest Time (H) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Guinea | King | Mulato II | Early | Normal | Late | G | H | Linear | ||
| Organic matter | ||||||||||
| W | 6.9 | 19.4 | 10.4 | 15.9 | 7.4 | 13.4 | 2.77 | 0.073 | 0.197 | 0.562 |
| U | 37.5 | 33.3 | 35.1 | 27.3 b | 38.5 a | 40.1 a | 1.30 | 0.179 | 0.004 | 0.002 |
| D | 55.6 | 47.4 | 54.5 | 56.8 | 54.2 | 46.5 | 2.92 | 0.213 | 0.138 | 0.067 |
| W + D | 62.5 | 66.8 | 64.9 | 72.7 a | 61.5 b | 59.9 b | 1.30 | 0.179 | 0.004 | 0.002 |
| Kd (%/h) | 2.54 | 2.54 | 2.79 | 3.09 | 2.40 | 2.38 | 0.159 | 0.508 | 0.057 | 0.034 |
| ED | 34.9 b | 43.4 a | 38.9 ab | 47.2 a | 33.9 b | 36.1 b | 1.09 | 0.014 | 0.002 | 0.002 |
| Neutral detergent fiber | ||||||||||
| W | 0.15 | 6.27 | 0.52 | 1.80 | 0.52 | 4.62 | 1.911 | 0.146 | 0.389 | 0.356 |
| U | 34.8 | 35.3 | 33.9 | 26.6 | 37.5 | 40.0 | 3.23 | 0.953 | 0.083 | 0.042 |
| D | 65.0 | 58.4 | 65.6 | 71.7 | 62.0 | 55.3 | 4.20 | 0.475 | 0.118 | 0.052 |
| W + D | 65.2 | 64.7 | 66.1 | 73.5 | 62.5 | 60.0 | 3.23 | 0.953 | 0.083 | 0.042 |
| LT (h) | 0.88 | 4.54 | 2.77 | 1.51 | 4.44 | 2.24 | 2.320 | 0.581 | 0.676 | 0.835 |
| Kd (%/h) | 2.44 | 2.69 | 2.45 | 2.86 | 2.50 | 2.21 | 0.292 | 0.797 | 0.379 | 0.190 |
| ED | 32.3 | 36.6 | 32.7 | 40.0 a | 31.0 b | 30.7 b | 1.59 | 0.221 | 0.023 | 0.014 |
| Equation | Pearson’s r | R2adj | Pconstant | Pslope |
|---|---|---|---|---|
| OM degradation | ||||
| Kd = 5.93 (±0.546) − 0.006 (±0.0001) × NDF | −0.917 | 0.819 | <0.001 | <0.001 |
| ED = 82.4 (±17.24) − 0.073 (±0.0288) × NDF | −0.691 | 0.403 | 0.002 | 0.039 |
| NDF degradation | ||||
| U = −89.8 (±64.72) + 0.88 (±0.189) × ADF | 0.871 | 0.723 | 0.208 | 0.002 |
| D (%) = −89.8 (±4.32) − 0.98 (±0.150) × ADL | −0.926 | 0.837 | <0.001 | <0.001 |
| D (g/kg DM) = −155.8 (±73.65) + 14.9 (±2.07) × hemicellulose | 0.938 | 0.864 | 0.072 | <0.001 |
| Kd = 3.39 (±0.458) − 0.032 (±0.0159) × ADL | −0.601 | 0.269 | <0.001 | 0.087 |
| ED = 52.3 (±9.31) − 0.054 (±0.0271) × ADF | −0.604 | 0.274 | 0.001 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.D.; Derix, J.; Hendriks, W.H.; Schonewille, J.T.; Nguyen, T.X.; Pellikaan, W.F. Species and Harvest Time of Fresh Tropical Grasses Affect Rumen Fermentation as Determined by In Sacco and In Vitro Incubations. Fermentation 2025, 11, 276. https://doi.org/10.3390/fermentation11050276
Nguyen HTD, Derix J, Hendriks WH, Schonewille JT, Nguyen TX, Pellikaan WF. Species and Harvest Time of Fresh Tropical Grasses Affect Rumen Fermentation as Determined by In Sacco and In Vitro Incubations. Fermentation. 2025; 11(5):276. https://doi.org/10.3390/fermentation11050276
Chicago/Turabian StyleNguyen, Huyen Thi Duong, Jill Derix, Wouter Hendrikus Hendriks, Jan Thomas Schonewille, Trach Xuan Nguyen, and Wilbert Frans Pellikaan. 2025. "Species and Harvest Time of Fresh Tropical Grasses Affect Rumen Fermentation as Determined by In Sacco and In Vitro Incubations" Fermentation 11, no. 5: 276. https://doi.org/10.3390/fermentation11050276
APA StyleNguyen, H. T. D., Derix, J., Hendriks, W. H., Schonewille, J. T., Nguyen, T. X., & Pellikaan, W. F. (2025). Species and Harvest Time of Fresh Tropical Grasses Affect Rumen Fermentation as Determined by In Sacco and In Vitro Incubations. Fermentation, 11(5), 276. https://doi.org/10.3390/fermentation11050276






