1. Introduction
In tropical regions, the beef industry can rely on grazing systems as it is economically more attractive; however, it has some disadvantages regarding pastures’ vulnerability to climatic seasonality. Alternatives have been studied for growing beef cattle in tropical grazing systems as a means to improve performance and mitigate the negative effects of seasonality [
1,
2,
3,
4]. The intensification of grazing systems by pasture management (stockpiling or rotation) and adoption of supplementation are the most used tools to overcome the seasonality of the tropics and ensure ideal beef cattle production [
5]. Non-protein nitrogen (NPN) supplementation is used as a strategy to increase the proportion of protein into the diet and meet the requirement of nitrogen ammonia for microbial protein synthesis in the rumen.
Notably, urea is one of the most well-known NPN sources that can be efficiently used in beef cattle diets. Alternative sources, such as nitrates, have also been used [
6,
7,
8,
9]; however, to our understanding, most of the research has been performed with cattle in feedlot systems, and fewer results are found in the literature with cattle in grazing systems being supplemented with nitrate. NPN supplementation can be nutritionally favorable, especially in tropical regions, where forage quality and availability vary greatly through the year [
10], displaying lower protein content and increased lignification during the dry season, which are enough to directly affect feed intake and the digestion of structural carbohydrates in the rumen [
11].
Nitrate metabolism leads to a higher release of negative Gibbs free energy compared to other NPN sources, potentially supporting greater microbial activity [
12]. Coupled with fomenting bacteria growth, nitrate has the capability to lower methane production, and thus to play an important role in the mitigation of greenhouse gases emissions to the atmosphere [
4,
13] while preserving the energy that can be used by the animal.
Nonetheless, nitrate bitterness can be a limiting factor affecting feed intake [
14,
15], which might elicit lower digestibility of the diet’s nutrients of cattle in tropical grazing systems and thus negatively compromise the synthesis and efficiency of microbial protein in the rumen. This effect can even be potentialized through the seasons of the year if any supplementation is adopted, as forage quality and its availability changes through the year [
10].
Several nitrates can be used as NPN sources for ruminants, such as ammonium nitrate, calcium nitrate, sodium nitrate, and potassium nitrate, including encapsulated formulations with more than one N source [
16,
17,
18,
19]. In the rumen, bacteria can utilize H
2 as an electron donor to reduce nitrate to nitrite and ammonia. Furthermore, nitrite is toxic to methanogenic microorganisms [
19]. When compared to urea, nitrates undergo a slower metabolism in the rumen, which reduces the risk of ammonia toxicity. In the bloodstream, nitrite converts hemoglobin to methemoglobin, causing methemoglobinemia. Therefore, careful dietary adaptation and not exceeding the upper supplementation limit are important [
19,
20].
Therefore, the hypothesis of this study is that the intensification of grazing systems through the adoption of rotational and deferred grazing methods, associated with non-protein nitrogen supplementation, has a positive effect on beef cattle ruminal metabolism. Furthermore, we hypothesize that the use of ammonium nitrate will favor the synchronization of nutrient utilization by rumen microorganisms, presenting an alternative to urea supplementation.
2. Materials and Methods
The study was conducted at the College of Veterinary Medicine and Animal Science (FMVZ/USP) in Pirassununga, Sao Paulo State, Brazil, at coordinates 21°56′47″ S 47°27′05″ W, over a two-year period.
The experiment began in June 2019 and ended in June 2021, with the second year being considered a repetition of the first year. The samples were taken two months after the beginning of each season (spring, summer, autumn, and winter). The study was approved by and followed the guidelines of the Committee for the Use and Care of Institutional Animals of the College of Veterinary Medicine and Animal Science of the University of São Paulo (No. 2347040422). This article was written based on the observations of the lead author in his doctoral research [
21].
2.1. Experimental Design, Pasture System, and Treatments
The experimental area has Köppen climate classification: Cwa (Monsoon-influenced humid subtropical climate), with precipitation of approximately 1200 mm per year and an average annual temperature of 23 °C, at an altitude of approximately 590 m above sea level (more details on precipitation and average temperature can be found in
Supplementary Materials File S1). The area was planted in 2018 with
Urochiloa brizantha cv. Marandu, receiving liming and nitrogen fertilization of 110 kg of N per ha per year throughout the experiment, divided into two applications (January and March).
The experiment used a total area of 14.4 ha, divided into eight grazing units, in four of which a grazing rotated system was installed, again divided into six paddocks of 0.3 ha with a fixed grazing period of 7 days and a rest period of 35 days (
Figure 1). The other four undivided units with 1.8 ha each were used to install the grazing deferred system. An auxiliary area of approximately 7 ha was used to keep the experimental animals of the grazing deferred treatment during the pasture rest period of 84 days, from April to June (
Figure 1).
A total of eight Nellore female cows, with an average weight of approximately 551 ± 7.01 kg, were used as experimental animals for rumen fermentation data. The experimental animals were randomly assigned to 8 paddocks in a randomized block design, based on terrain location, over a two-year period and evaluated at each station. In total, 64 experimental units were used, a number considered adequate by the scientific community and the ethics committee on the use of animals for research with rumen-cannulated cattle in this type of experimental design.
The treatments involved the combinations of two grazing systems (deferred and rotational) and two types of supplementations of ammonium nitrate (NH
4NO
3) or urea: deferred grazing with NH
4NO
3, deferred grazing with urea, rotational grazing with NH
4NO
3, and rotational grazing with urea. Each block was divided by management corridors. A variable number of ‘non-experimental’ animals (regulators) were used to adjust the stocking rate using the “put and take” technique described by Mott and Lucas [
22], aiming to maintain a specific intermediate pasture height (maximum of 45 cm and minimum of 30 cm) as an indirect assessment of forage mass availability [
23] in each grazing unit.
Two different formulations of energy-protein supplement, with ammonium nitrate or urea, were adopted throughout the year to better meet the animals’ nutritional requirements. The animals received a concentrate (isoproteic) composed of ground corn, salt, mineral supplement, and either urea or ammonium nitrate. Urea was included at 13% and 22% of the total dry matter, while ammonium nitrate was included at 18% and 30% for the dry (autumn and winter) and rainy (spring and summer) seasons, respectively. Animals were gradually adapted to the urea and nitrate supplements over a 14-day period. Further details regarding supplement composition and feeding are provided in
Section 2.3.
2.2. Hand-Plucking Technique to Estimate Forage Nutritional Value
The hand-plucking technique, adapted from Sollenberger and Sherney [
24], was used to estimate diet nutritive value. The second month of each season was taken to perform all sampling. Hand-plucking was performed on the 1st, 3rd, and 7th days of the rotational grazing method, and in the same days, samples from the deferred method were also taken, dried at 65 °C for 72 h, and milled for subsequent analysis. The nutritional composition of forage can be seen in
Table 1.
2.3. Experimental Supplementation
The supplement was provided in measured amounts along the seasons due to toxicity risks. Due to the better nutritional quality of forage during the rainy season, a supplement containing approximately 6.2% N was used. In contrast, pastures during the dry season have higher fiber content and lower protein concentrations, which can limit microbial activity in the rumen and reduce fiber digestion. Therefore, during the dry season, a new formulation with a higher nitrogen inclusion (10.2% N) was used. The formulations and compositions of the supplement can be found in
Table 2.
2.4. Dry Matter Intake of Forage and Supplement
The intake (forage and supplement) and feces excretion were determined using two internal markers (titanium dioxide [TiO
2] and chromium oxide [Cr
2O
3]) to determine feces excretion, and one internal marker (indigestible neutral detergent fiber [iNDF]) for forage intake. Feces were collected for marker analysis, and the dry matter intake of forage and supplement was calculated. Therefore, to assess forage intake, during 10 days of each experimental period, TiO
2 was administered (15 g/cow.day) directly into the rumen through the rumen cannula (
Figure 1) twice a day at 8 a.m. (7.5 g) and at 4 p.m. (7.5 g), the first five days for adaptation, and the last five days for feces collection, twice a day (8 a.m. and 4:00 p.m.) [
25].
Marker concentrations were obtained in ppm and subsequently converted to kilograms (kg) for the determination of feces excretion by means of a known amount of external marker administered (kg/day) and that found on the feces as follows:
In which: TDFE: total daily feces excretion (kg); MDiet: marker in the diet (kg); MFeces: marker in the feces (kg).
Chromium concentration followed the methodology described by Almeida et al., [
26], which used an energy dispersive X-ray fluorescence technique. Thus, for the determination of supplement dry matter intake, we used the following equation:
In which: DMIS: dry matter intake of supplement (kg/day); IndFeces: indigestibility of feces (%); MIndDiet: indigestibility of diet (%).
Forage dry matter intake (DMI) was calculated by internal marker iNDF concentration (%) and determined by means of its incubation for 288 h in the rumen of cannulated animals kept in grazing pastures. The iNDF concentration on dried samples of forage and feces was used to calculate and estimate the forage feed intake as follows:
In which: DMIF: dry matter intake of forage (kg/day); : indigestibility of feces (%); MIndForage: indigestibility of forage (%).
2.5. Total Apparent Digestibility of Dry Matter and Its Fractions
Apparent digestibility coefficients were calculated based on TiO
2 content in the diet and feces. Concentrations of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), lignin (Lig), ether extract (EE), and mineral matter (MM) in forage were determined using a near-infrared spectrophotometer (NIRS, model NIRFlex N-500 Solids, BÜCHI) with calibration developed and validated by Embrapa Southeast Livestock for samples of
Urochloa spp., respectively: R
2 for CP = 0.979; R
2 for NDF = 0.930; R
2 for ADF = 0.970; R
2 for lignin = 0.943; R
2 for EE = 0.920; R
2 for MM = 0.960. Supplement and feces were dried for DM [
27], milled to 1 mm, and analyzed for CP (micro-Kjeldahl [
28]), EE (ANKOM XT15 Extractor
® [
29]), and MM (muffle furnace at 550 °C for 4 h). OM was calculated as 100 minus MM. GE of feces, supplement, and forage was determined by complete oxidation in a calorimetric bomb (C5000 control, IKA
®, Staufen, Germany). The non-fiber carbohydrates (NFC) content of feed and feces was obtained by subtracting CP, EE, MM, and NDF percentages from 100. The total digestible nutrients (TDN) of feed and feces was calculated by summing the products of the apparent digestibility coefficients for CP, NDF, corrected EE, and NFC, divided by 100.
2.6. Ruminal Kinetics and Degradability of DM and Nutrients
The ruminal dynamics were assessed by total rumen emptying. Ruminal digesta was manually removed through the rumen cannula from each cow pressing the ruminal contents over a sieve (4 mm) to separate liquid and solid phases, to determine the disappearance rate (kt) in the rumen. Then, 1 kg of each solid and liquid sample were dried at 65 °C (forced-air oven) for 72 h. Ruminal solid and liquid mass data were used to calculate the solid disappearance rate using the equation suggested by Robinson et al. [
30].
The ruminal degradability of DM, NDF, and CP was assessed following the Mehrez and Ørskov [
31] methods, in which, nylon tissue bags with 50 μm pores in the dimensions of 10 × 20 cm were filled with 5 g of forage from each treatment, identified and then incubated in the rumen for 0, 3, 6, 12, 24, 48, 72, and 96 h. Degradability parameters were obtained and calculated using the NLIN procedure of SAS as suggested by Ørskov and McDonald [
32].
2.7. Determination of Urinary Parameters
Microbial protein production was calculated by determining urinary volume through creatinine concentration, following Valadares et al. [
33].
Urine samples were collected twice daily (8 a.m. and 4 p.m.) over five days via spontaneous urination. Each time, 10 mL of urine was preserved in 40 mL of 0.036 N sulfuric acid and stored at −20 °C for analysis of allantoin, uric acid, urea, and creatinine. Allantoin was measured using the colorimetric method of Chen and Gomes [
34], uric acid by colorimetric enzymatic reaction with uricase and peroxidase (Bioclin
® Kit Ref K139, Belo Horizonte, Brazil), and urea and creatinine by commercial kits (Bioclin
® Ref K047 and K067, respectively). Daily urinary creatinine excretion (CE) was estimated using Chizzotti et al. [
35]:
In which: CE: daily urinary creatinine excretion; EBW: empty body weight.
Total daily urinary volume (L/cow) was calculated by dividing daily urinary creatinine excretion by the observed creatinine concentration in spot samples. This volume estimated daily excretions of urea, allantoin, and uric acid.
Purine derivatives (PuD) excretion was calculated by multiplying daily urine volume by PuD concentration. Absorbed microbial purines (AP, mmol/day) were calculated from urinary PuD excretion using:
In which: PuD: purine derivatives; AP = absorbed microbial purines; BW: body weight.
Intestinal flow of microbial nitrogen compounds (MicN, g N/day) was calculated with:
In which: MicN = microbial nitrogen; AP = absorbed microbial purines.
Microbial nitrogen synthesis efficiency (EMNS) was determined by the ratio of microbial N production to digested organic matter (OM).
2.8. Statistical Analysis
Data were statistically analyzed using the online version of the software Statistical Analysis Systems—OnDemand for Academics SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Prior to statistical analysis, the data were assessed for the presence of disparate information (“outliers”) and the normality assumption of the residuals was assessed by the Shapiro–Wilk test. When the normality assumption was not accepted, the logarithmic or the square root transformation was applied. Data were analyzed according to the mixed procedure (PROC MIXED), in which season was considered as a repeated variable (split-plot in time). A total of 15 different covariance structures were tested, and the chosen one was based on the lower value of the Corrected Akaike Information Criterion (AICC) [
36]. The Variance Components (VC), Compound Symmetry (CS), and Heterogeneous Compound Symmetry (CSH) are the covariance structures most commonly used. The model includes the effect of grazing method, nitrogen source, period of the year (winter, spring, summer and autumn), and the interaction between grazing method, nitrogen source and season of the year. The effects of blocks were considered as random factors.
where Y
ijkl: experimental answer; u: constant; b
i: effect of the block; g
j: effect of grazing; n
k: effect of nitrogen source; (gn)
jk: interaction effect of grazing and nitrogen source; e(1)
ijk: random error; s
l: effect of season; (sg)
lj: interaction effect of season and grazing; (sn)
lk: interaction effect of season and nitrogen source; (sgn)
ljk: interaction effect of season, grazing and nitrogen source; e(2)
ljk: random error.
In the presence of interaction, the effects of one factor inside the other were evaluated using the SLICE command of the Mixed Procedure. All means were presented as least squares means and statistical differences by treatment effects were obtained by pairwise difference test (PDIFF) using the Fisher test, considering a significance of p ≤ 0.05.
3. Results
Feed intake variables showed mainly significant effects for season. There was an effect of grazing method on NPN intake and microbial nitrogen synthesis (MicN: microbial nitrogen compounds synthesis; EMNS: efficiency of microbial protein synthesis), where animals in deferred grazing had the highest MicN and EMNS values compared to animals under rotational grazing (
Table 3).
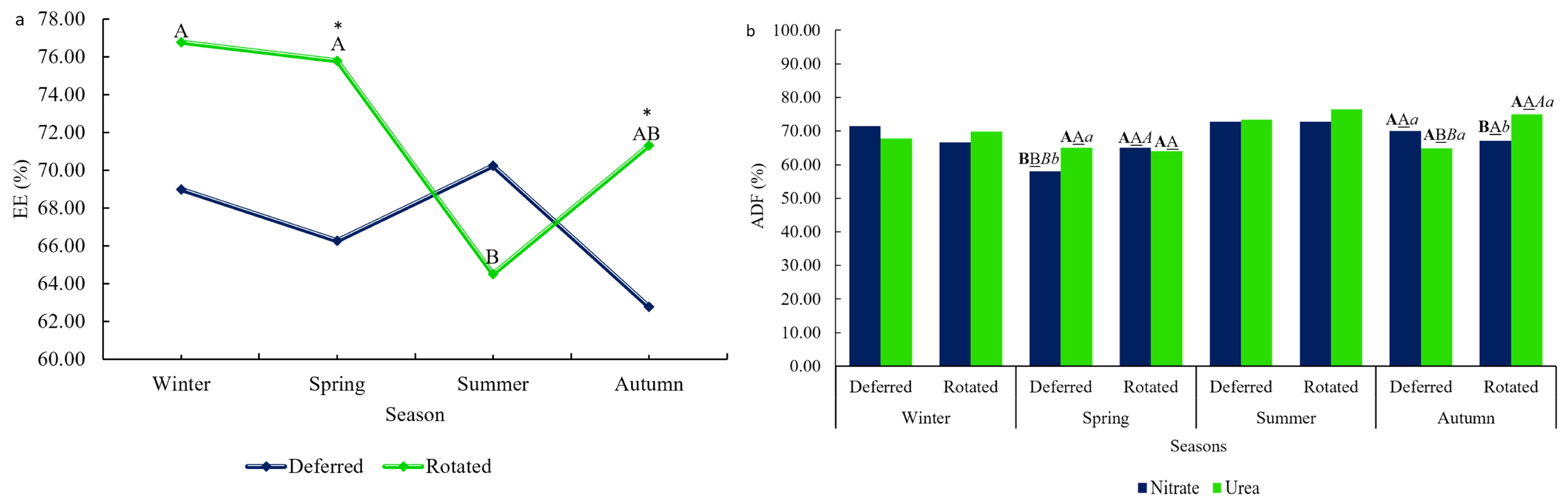
In
Table 4, we show that nitrate was the treatment with the highest digestibility coefficient for the NFC variable. With the exception of EE, all variables showed seasonal effects. EE showed a Grazing × Season interaction, and ADF showed an interaction between Grazing × N Source × Season.
A higher digestibility coefficient of EE was detected in spring and autumn for the rotational grazing method (
Figure 2a).
ADF (%) digestibility was higher when animals were fed urea within deferred grazing during the spring season. The same pattern was detected in autumn but within rotational grazing, with animals fed urea having higher ADF digestibility. Yet, in
Figure 2b, it is possible to notice that nitrate supplementation increased ADF (%) digestibility in the rotational grazing method, when compared to deferred. Meanwhile, in autumn, the same pattern of ADF (%) digestibility was observed, with greater digestibility in rotated grazing methods within urea supplementation.
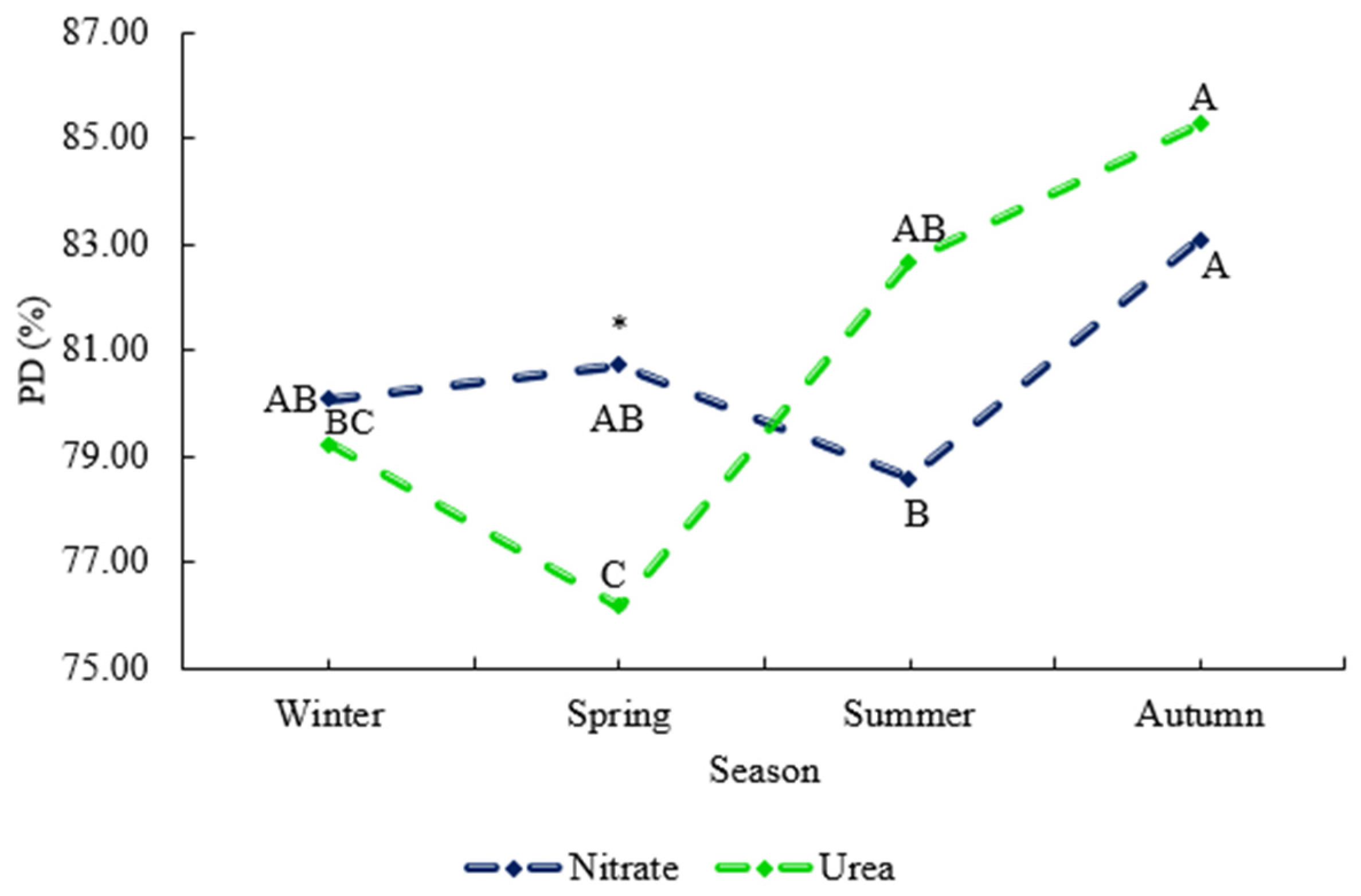
Only the PD of DM (potential degradability of dry matter; a + b) had a significant effect, with an interaction for N source × Season (
Figure 3), in which we can see greater potential digestibility of DM in spring when animals were fed nitrate as opposed to urea. The variables De5 and De8 (DM degradability at 5 and 8%) did not have a significant effect (
Table 5), while the variables a, b, c, PD, and De2 differed between seasons (
Table 5).
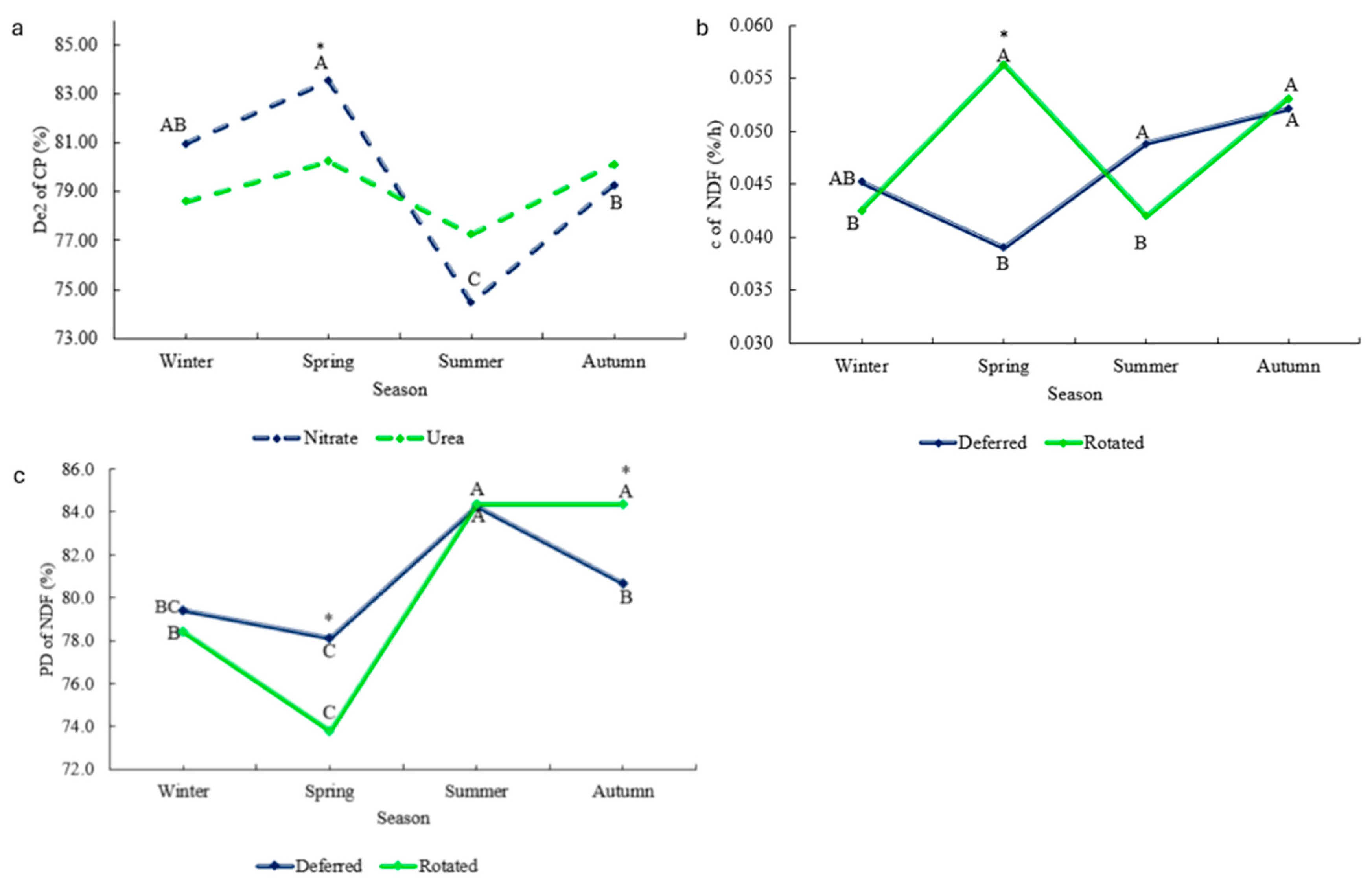
For in situ degradability of CP and NDF, we did not find isolated effects of Grazing and N source (
Table 6). However, the season effect was detected for all CP variables and all NDF variables except De5 (
Table 6).
Significant N source × Season interactions were observed in De2 of CP, with greater degradability within spring when animals were fed nitrate (
Figure 4a). Significant Grazing × Season interactions were observed in c and PD of FDN (
Table 6;
Figure 4b,c).
Animals subject to the rotated grazing method had a greater rate of degradation of the potentially degradable fraction (c %/h) within spring; however, PD of NDF in situ degradability showed lower mean values for animals in rotated grazing within spring but greater values within autumn when compared to deferred grazing.
4. Discussion
This study used females of the Nelore breed. However, nitrate supplementation can be applied to any cattle on a low-protein diet (especially grazing cattle). The only limitations include the need for animal adaptation and the potential for very high dietary N levels to cause toxicity and increase nitrous oxide emissions on the farm [
19]. It is important to emphasize that during the entire study period, not a single animal became ill or showed signs of intoxication. This indicates that the doses of N consumed by the animals were safe.
Nitrate resulted in lower NPN intake (kg/day), yet no significant effect on microbial protein synthesis or the efficiency of microbial N synthesis was detected. This lower intake of NPN may be associated with the bitterness of nitrate [
14,
15]; however, based on our findings, we understand that ammonium nitrate is a potential and suitable NPN source for beef cattle in the grazing system. The reduction of nitrate to ammonia represents a metabolic path that is thermodynamically favorable and incorporates more energy into the rumen by increasing the overall flow of microbial protein in the rumen coupled with reduction of methane emission [
11]. On the other hand, grazing methods had an influence on MicN and EMNS with 19.72% lower values detected for animals under rotated grazing when compared to deferred grazing (
Table 3). A possible reason for the lower EMNS (g/kg.OM) in rotated grazing is that animals under rotated grazing have access to pasture with a profile of forage with higher content of nutrients, and a higher concentration ratio of cell soluble compounds to structural compounds, which makes the forage highly concentrated in nutrients and, thus, more digestible, as observed in our findings (
Table 4;
Figure 2a,b).
No significant effect was detected on supplement intake. However, we expected that the animals would prefer harvesting new green leaf, a great source of PDR, to supplementation. Possibly, this led to a lower rate of conversion of protein to ammonia and thus resulted in a lower MicN when animals were in rotated grazing. The most likely and probable second reason that accounts for the lower MicN and EMNS is the greater rumen solids content expected from animals in rotated pasture, especially during the summer season [
37]. Thereby, the pressure of fiber content on a full rumen into the rumen wall influences ruminal motility, which increases the disappearance rate by %.h and by kg/h. Theoretically, a greater amount of ruminal content leaving the rumen means lower retention of microbial protein, degradability, and consequently, lower EMNS as well [
37].
Although nitrogen sources did not significantly affect feed intake or diet digestibility, N supplementation in beef cattle is intended to enhance these parameters by accelerating rumen passage and aiding forage cell wall breakdown, a result potentially influenced by the isoproteic nature of the diets. Martinez-Fernandez et al. [
38] observed that supplementation in cattle grazing on tropical pastures increased the alpha diversity and Chao richness of the rumen microbial community. This increase is crucial for degrading forage cell components and improving overall rumen kinetics.
When studying the microbial community of pasture-fed steers and supplemented with encapsulated nitrate, Granja-Salcedo et al. [
7] reported a reduction in
Methanobrevibacter populations and a negative correlation between methane emissions and populations of
Proteobacteria,
Prevotellaceae,
Selemonas, and
Succinivibrio spp. However, further studies are necessary to elucidate the microbiome and the modulation exerted by nitrate on the microbial community.
The higher disappearance rate by %/h and by kg/h over summer and autumn might be accounted for by the fact that in both seasons, pastures displayed overall higher forage availability and nutrients digestibility as well (
Table 4). A greater content of dry matter in the rumen is expected in rainy seasons, which can lead to constant interaction and pressure into the rumen wall and pillars, where there are chemoreceptors that recognize the ruminal volume size and its chemical composition. This effect causes changes in the rumen motility, which ultimately causes the more dense and fine rumen content made up of concentrated and smaller degraded pieces of forage to leave the rumen, thus possibly increasing the disappearance rate by %/h and by kg/h.
It was observed that nitrate does not have a negative effect on forage, supplement, or total dry matter intake (kg/day). However, the pasture system impacted NPN intake (kg/day), which was 32.5% higher in animals grazing on rotational pastures compared to those on deferred pastures. This may be due to the synergistic effect of supplementation and the superior quality of rotational pastures, which led to higher NDF digestibility (
Table 4). Rotational grazing systems typically have higher overall digestibility and nutrient concentration in leaves compared to deferred pastures, likely due to management practices that prioritize forage use at ideal plant height and maturity [
39]. Our findings indicate that animals in rotational grazing systems had higher NDF (%) digestibility compared to those in deferred systems.
The ADF (%) digestibility was improved when animals were under rotated grazing and/or being supplemented with urea as the main source of non-protein nitrogen, as seen in
Figure 2b. The effect might be related to the fact that in pastures of rotated grazing, animals can be selective and thus defoliation occurs primarily to high palatable leaves, the newly emerged ones, which have a lower concentration of lignified structural carbohydrates [
40], therefore increasing fermentation activity and improving digestibility of the ADF (%).
Rotational grazing increased EE (%) digestibility, both independently and in interaction with the season (
Figure 2a). Our findings indicate higher EE (%) digestibility in rotational grazing compared to deferred grazing during spring and autumn. This is likely due to the slightly higher nutrient concentration in rotational pastures and the selective grazing behavior, where animals consume the most digestible parts of the plants. Properly managed rotational pastures offer forage with higher nutrient concentration, digestible energy, and water-soluble carbohydrates [
40], as defoliation occurs at the optimal stage of height and maturity. Over the two-year trial, rotational grazing also led to higher crude energy digestibility compared to deferred grazing.
Degradability parameters for forage-based diets naturally vary with the season, reflecting changes in the diet’s composition and availability. In our study, nitrogen source interacted with the season and had an effect on the potential degradability (PD %) of DM, which was higher in animals fed nitrate during spring compared to those fed urea (
Figure 3). This higher PD (%) with nitrate in spring may be due to improved overall digestibility and nutrient degradability as animals had higher supplement feed intake during spring than in summer (
Table 3). The CP degradability rate at 2% (
Figure 4a) showed the same effect of supplementation. Nitrate supplementation reduces it to ammonia, directly contributing to microbial protein synthesis. Additionally, the metabolic reduction of nitrate to ammonia generates a higher flow of negative Gibbs energy, which supports microbial growth, substrate transport, mobility in the rumen and improved degradation of nutrients fractions [
41].
There was a notable difference for the variable b, the potential degradable fraction, which had lower values in winter and spring and higher values during summer and autumn. Surprisingly, summer had the lowest rate of degradation (3.5%/h) of the fraction b (potential degradable fraction), while no major effect was noticed among the other seasons. The decrease of 40.67% in the supplement dry matter intake over the summer and increase of 38.55% for forage DMI (
Table 3) could be a reason for the lowest rate of degradation (3.5%/h) of the fraction b, since a greater proportion of the CP during that season came from forage.
In rotated grazing systems, the ruminal degradation rate of fraction b for NDF was 30.35% higher than that of deferred systems within the spring season, which might be a direct effect of the greater intake of NPN (kg/day) from animals kept in the rotated method, and the higher digestibility of the NDF (%) content from those experimental units as well. The intake behavior of animals in rotated grazing might play a role in this too. When animals are rotated to a “deferred” grazing area, there is great availability of new leaves and that is the main target of the cattle, as they harvest the foliage’s tips, which are more digestible and nutritious [
15,
22,
23]. In the same experiment that our study was carried out, Lelis [
41] evaluated forage production and quality on deferred and rotated grazing methods and during the spring season; the authors observed that rotated grazing pastures had 40% more leaves and 27.7% more CP when compared to deferred. It is possible that the higher apparent digestibility of the NDF for the rotated grazing method (
Table 4) and the higher availability of CP (27.3%) could have led to higher fermentation activity by the rumen bacterial community, which consequently can affect the rate of degradation of the potential degradable fraction of NDF, culminating in the results obtained. This trend does not happen in the deferred grazing method since it entails continuous grazing, where most of the forage leaves were already under harvesting.
The behavior of cattle in continuous grazing is different, and since they have full time availability of forage for grazing, the more digestible and palatable leaves are constantly taken. This, associated with extrinsic factors that do not contribute to forage growth along the seasons, can lead to the pasture having a lower content of new and nutritious leaves, and a higher content of more lignified and recalcitrant forage compounds that tend to take a greater time within the rumen. Therefore, we suggest further studies to elucidate methane emissions, the ruminal microbial community, and the productive performance of cattle raised in intensified grazing systems and on different sources of N in supplements.
5. Conclusions
The study demonstrates that the rotational grazing method improved NPN intake and the digestibility of NDF, ADF, and gross energy. The absence of negative effects of ammonium nitrate on parameters such as the digestibility of DM, nutrient intake, and ruminal kinetics demonstrate that it is a reliable source of N, like urea. The deferred system as a food reserve strategy in critical seasons (autumn and winter), when combined with NNP supplementation, promoted an improvement in microbial efficiency, which may contribute to animal performance. In addition, the use of ammonium nitrate was shown to be a safe strategy, demonstrating that it is an interesting option as a source of N for beef cattle in a grazing system. Although the initial results are promising, further studies investigating the ruminal microbial community, metabolism, gas production, and animal performance are essential to confirm the real benefit of ammonium nitrate in different grazing systems. Moreover, for a comprehensive understanding of its applicability, research on economic viability, nitrogen use efficiency, and the capacity to mitigate methane emissions is necessary. In this context, the judicious application of nitrate emerges as a strategy within climate-smart agriculture, aiming for sustainability in livestock production.