Coupling Granular Activated Carbon with Waste Iron Scraps Enhances Anaerobic Digestion of PBAT Wastewater: Performance Improvement and Mechanistic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Sludge and PBAT Wastewater
2.2. Batch Experiment Design
2.3. Sampling and Analytical Methods
3. Results
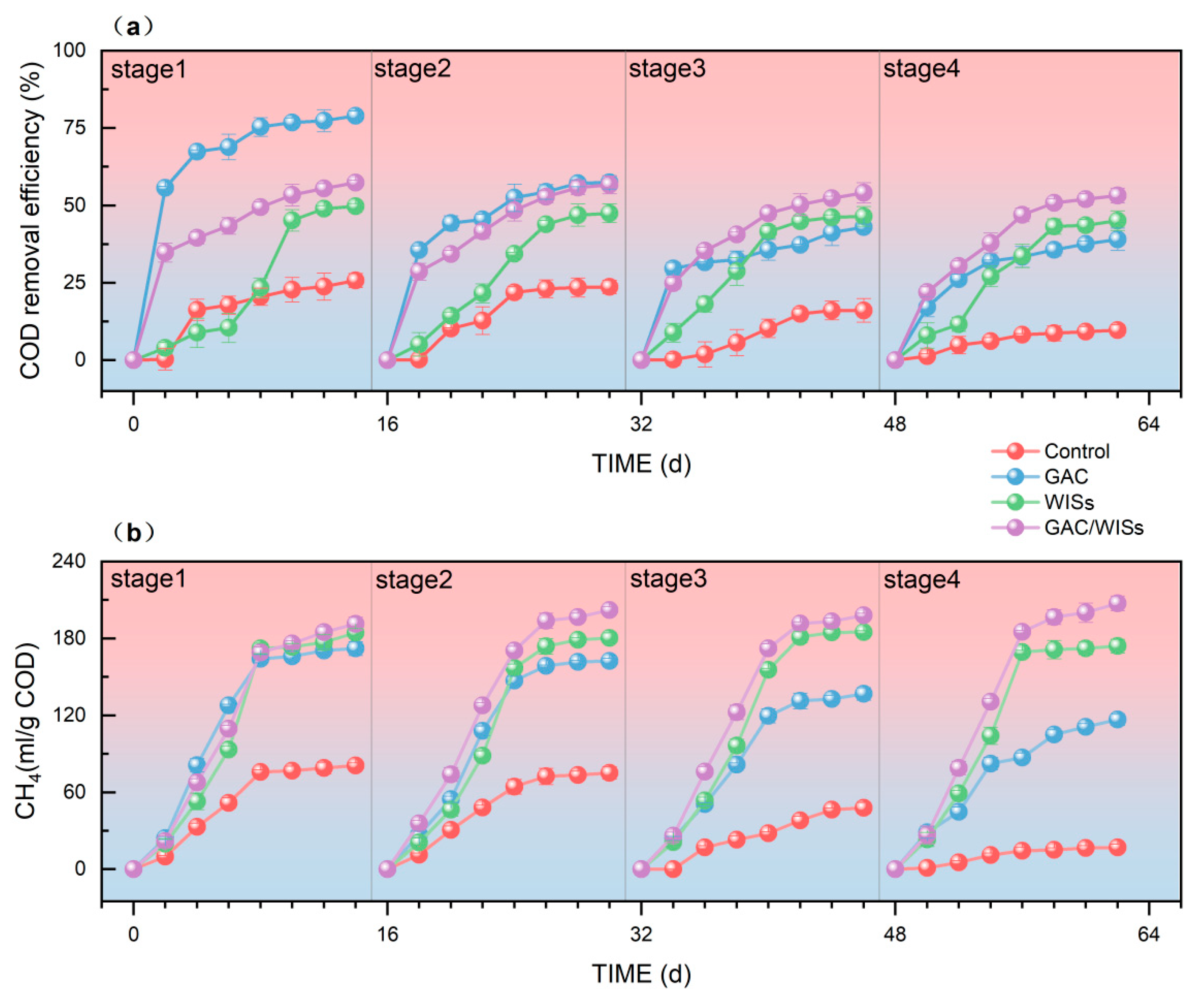
3.1. COD Removal Efficiency and Methane Production Performance
3.2. Removal of Typical Refractory Organic Compounds
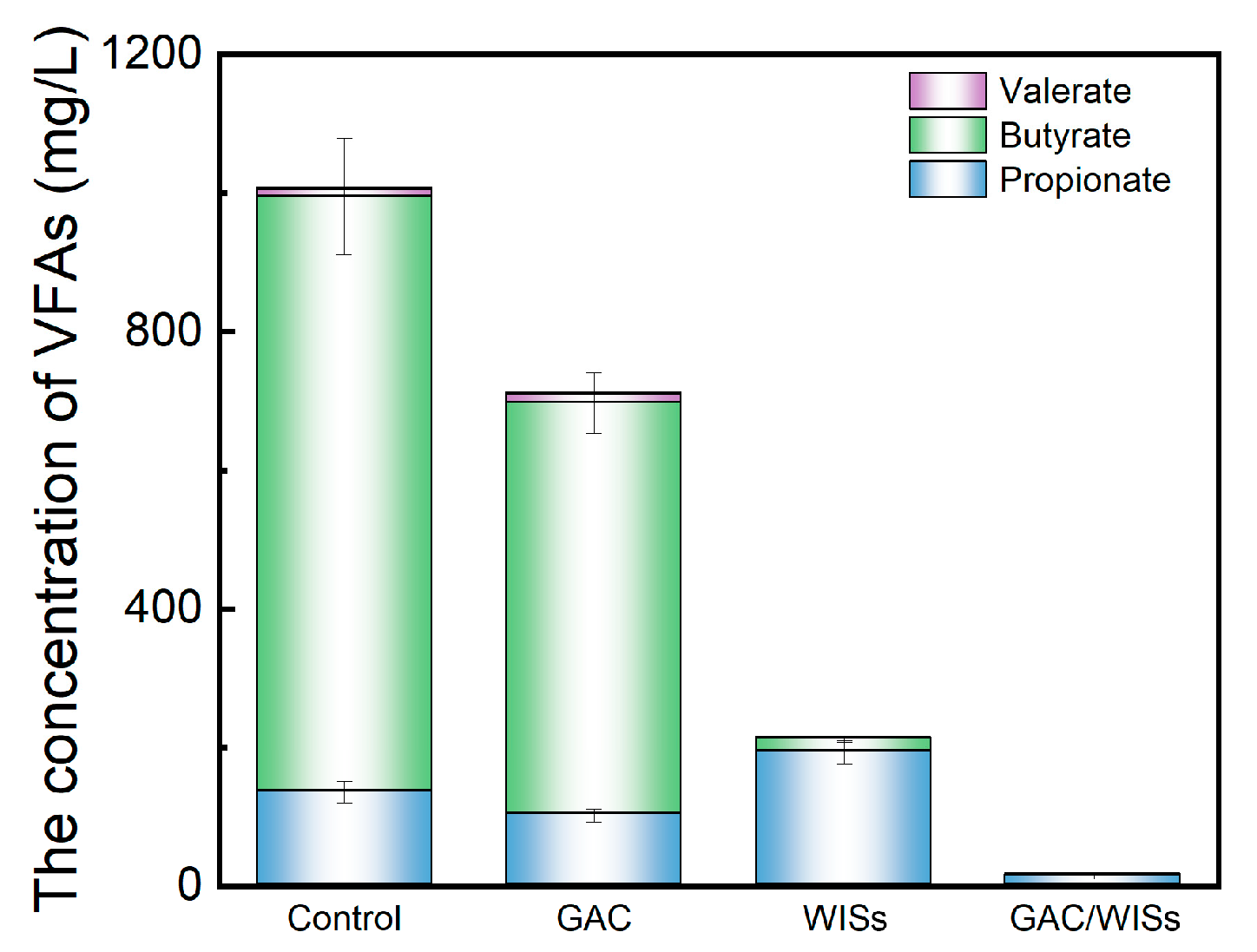
3.3. VFAs Accumulation
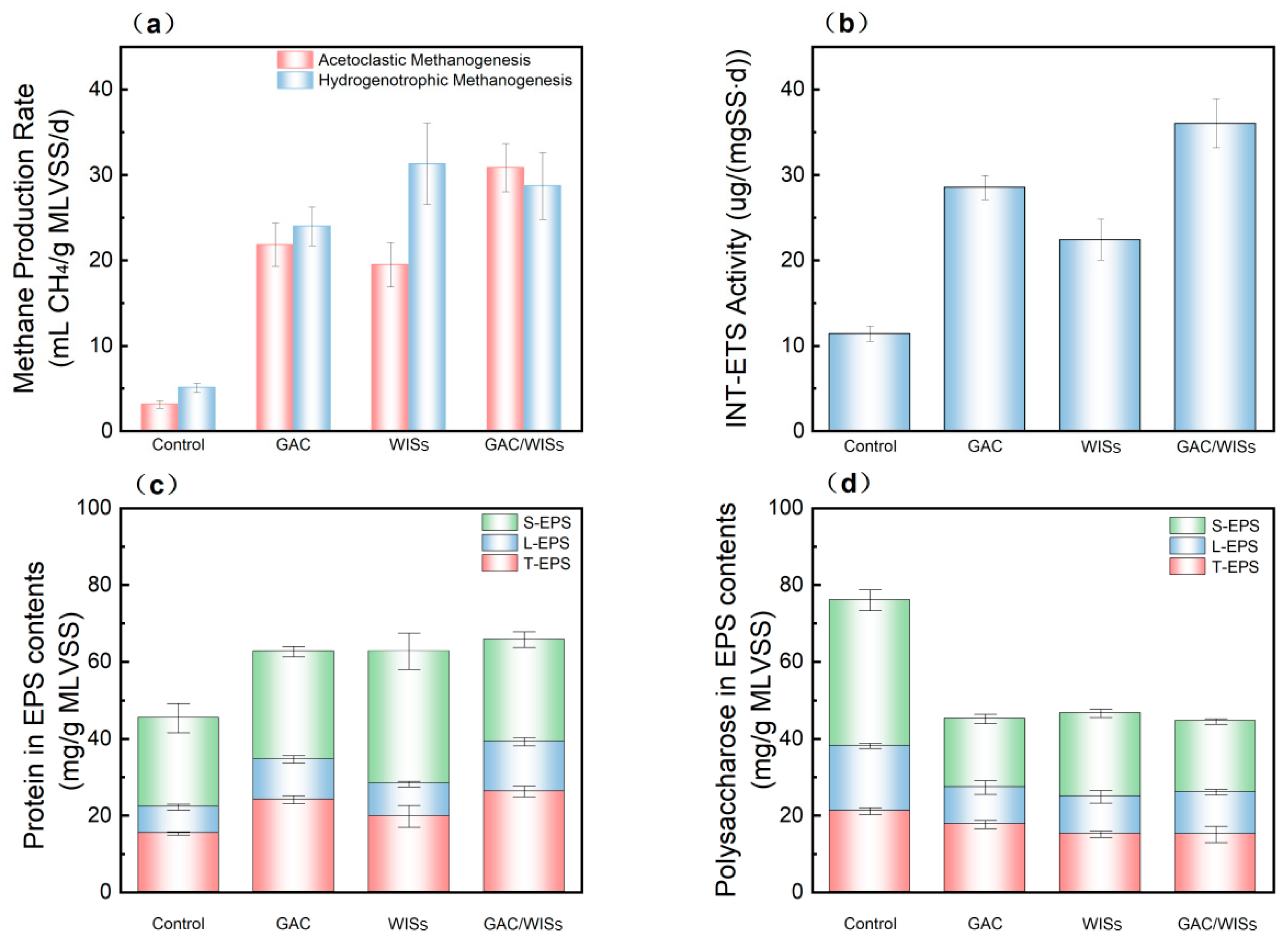
3.4. Methanogenic Activity and Electron Transfer Activity
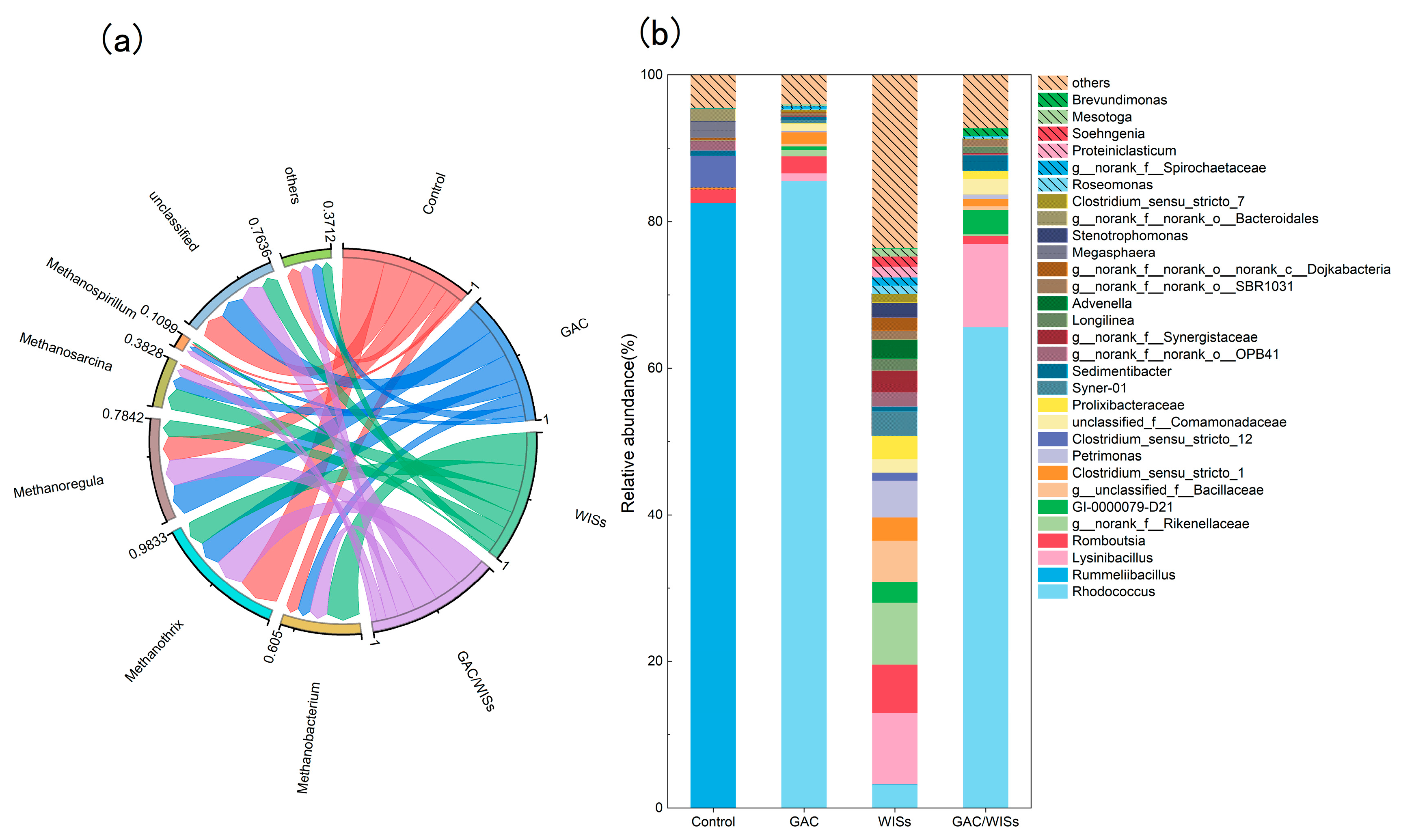
3.5. Microbial Community Structure
- (1)
- Archaeal community
- (2)
- Bacterial community
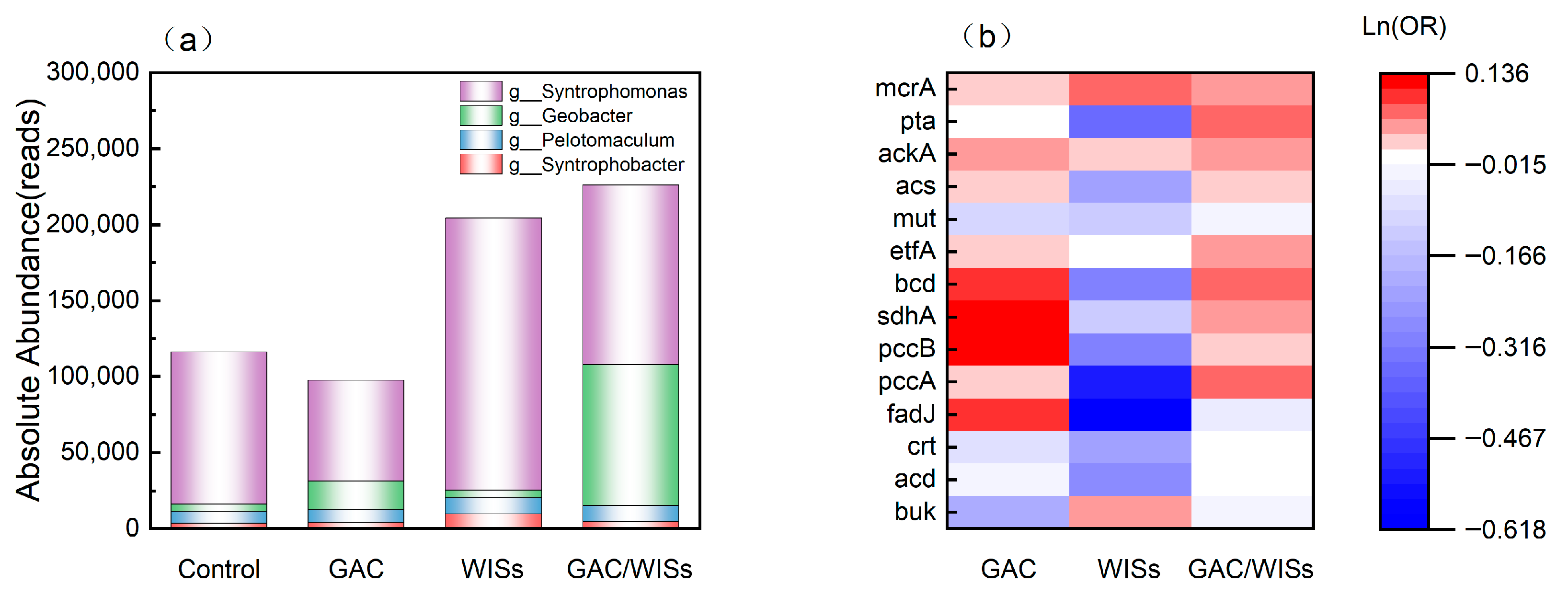
3.6. Metagenomic Analysis of Functional Genes and Pathways
- (1)
- Syntrophic bacteria and DIET-related genera
- (2)
- Metabolic enzyme profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBAT | Poly(butylene adipate-co-terephthalate) |
| COD | Chemical oxygen demand |
| AD | Anaerobic digestion |
| VFAs | Volatile fatty acids |
| GAC | Granular activated carbon |
| WISs | Waste iron scraps |
| DIET | Direct interspecies electron transfer |
| MLSSs | Mixed liquor suspended solids |
| MLVSSs | Mixed liquor volatile suspended solids |
| ETS | Electron transport system |
| EPSs | Extracellular polymeric substances |
| AM | Acetoclastic methanogenic |
| HM | Hydrogenotrophic methanogenic |
References
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, Y.; Chen, Z.; Bian, X.; Chen, N.; Li, J.; Wu, Y.; Yang, Z. Comparative Life Cycle Assessment of PBAT from Fossil-Based and Second-Generation Generation Bio-Based Feedstocks. Sci. Total Environ. 2024, 954, 176421. [Google Scholar] [CrossRef]
- Xu, P.; Liu, H.; Liu, C.; Zhu, G. Syntrophic Methane Production from Volatile Fatty Acids: Focus on Interspecies Electron Transfer. Sci. Total Environ. 2024, 946, 174410. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Z.; He, C.; Mei, H.; Wang, Y.; Hu, Z.-H.; Wang, W. Waste Iron Shavings to Advance Anaerobic Treatment of Acidic Poly (Butylene Adipate-Co-Terephthalate) Wastewater in Submerged Anaerobic Membrane Reactor. J. Hazard. Mater. 2025, 490, 137813. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; Zhang, Z.; Fu, Y.; Huo, Z.; Wu, Y.; Shi, H. Chemical Features and Biological Effects of Degradation Products of Biodegradable Plastics in Simulated Small Waterbody Environment. Sci. Total Environ. 2023, 904, 166829. [Google Scholar] [CrossRef]
- Wirasembada, Y.C.; Shin, B.; Shin, J.; Kurniawan, A.; Cho, J. Effects of Sudden Shock Load on Simultaneous Biohythane Production in Two-Stage Anerobic Digestion of High-Strength Organic Wastewater. Bioresour. Technol. 2024, 394, 130186. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zheng, Z.; Wang, X. Obstacles Faced by Methanogenic Archaea Originating from Substrate-Driven Toxicants in Anaerobic Digestion. J. Hazard. Mater. 2021, 403, 123938. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Su, Y.; Wu, D.; Xie, B. Plasticizers Inhibit Food Waste Anaerobic Digestion Performance by Affecting Microbial Succession and Metabolism. J. Hazard. Mater. 2024, 473, 134554. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Xing, T.; Xiao, F.; Liu, Y.; Zhen, F.; Sun, Y. An Integrated Evaluation Strategy for Anaerobic Digestion Monitoring Based on Acid-Base Balance and Thermodynamics of Volatile Fatty Acid Degradation. Chem. Eng. J. 2024, 486, 150340. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy Mechanism, Microbial Population, and Process Optimization for Volatile Fatty Acids Metabolism in Anaerobic Digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Nayeri, D.; Mohammadi, P.; Bashardoust, P.; Eshtiaghi, N. A Comprehensive Review on the Recent Development of Anaerobic Sludge Digestions: Performance, Mechanism, Operational Factors, and Future Challenges. Results Eng. 2024, 22, 102292. [Google Scholar] [CrossRef]
- Sompong, O.; Suksong, W.; Promnuan, K.; Thipmunee, M.; Mamimin, C.; Prasertsan, P. Two-Stage Thermophilic Fermentation and Mesophilic Methanogenic Process for Biohythane Production from Palm Oil Mill Effluent with Methanogenic Effluent Recirculation for pH Control. Int. J. Hydrogen Energy 2016, 41, 21702–21712. [Google Scholar] [CrossRef]
- Sarker, A.; Nikhil, G.N. Bioprospecting Syntrophic Microbial-Material Interactions during Anaerobic Digestion: A Review on Applications of Biocatalysis and Challenges. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced Anaerobic Digestion of Primary Sludge with Additives: Performance and Mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Shen, Y.; Chen, H.; Li, Z.; Cai, L.; Yang, H.; Zhu, C.; Shen, J.; Kang, J.; et al. Simultaneous Regeneration of Activated Carbon and Removal of Adsorbed Atrazine by Ozonation Process: From Laboratory Scale to Pilot Studies. Water Res. 2024, 251, 121113. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, H.; Zhang, Y.; Cui, M.; Fu, B.; Liu, H. Insight into Sludge Anaerobic Digestion with Granular Activated Carbon Addition: Methanogenic Acceleration and Methane Reduction Relief. Bioresour. Technol. 2021, 319, 124131. [Google Scholar] [CrossRef]
- Xia, A.; Feng, D.; Huang, Y.; Zhu, X.; Wang, Z.; Zhu, X.; Liao, Q. Activated Carbon Facilitates Anaerobic Digestion of Furfural Wastewater: Effect of Direct Interspecies Electron Transfer. ACS Sustain. Chem. Eng. 2022, 10, 8206–8215. [Google Scholar] [CrossRef]
- Yu, N.; Guo, B.; Liu, Y. Shaping Biofilm Microbiomes by Changing GAC Location during Wastewater Anaerobic Digestion. Sci. Total Environ. 2021, 780, 146488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Yu, N.; Guo, B.; Liu, Y. Enhancing the Resistance to H2S Toxicity during Anaerobic Digestion of Low-Strength Wastewater through Granular Activated Carbon (GAC) Addition. J. Hazard. Mater. 2022, 430, 128473. [Google Scholar] [CrossRef]
- He, Z.-W.; Zou, Z.-S.; Ren, Y.-X.; Tang, C.-C.; Zhou, A.-J.; Liu, W.; Wang, L.; Li, Z.; Wang, A. Roles of Zero-Valent Iron in Anaerobic Digestion: Mechanisms, Advances and Perspectives. Sci. Total Environ. 2022, 852, 158420. [Google Scholar] [CrossRef]
- Hao, X.; Wei, J.; van Loosdrecht, M.C.; Cao, D. Analysing the Mechanisms of Sludge Digestion Enhanced by Iron. Water Res. 2017, 117, 58–67. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Huang, Y.; Cai, B.; Dong, H.; Li, H.; Yuan, J.; Xu, H.; Wu, H.; Xu, Z.; Sun, D.; Dang, Y.; et al. Enhancing Anaerobic Digestion of Food Waste with Granular Activated Carbon Immobilized with Riboflavin. Sci. Total Environ. 2022, 851, 158172. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Zhang, J.; Hu, Z.; Zhang, X.; Muñoz Sierra, J. Degradation Kinetics of Pentachlorophenol and Changes in Anaerobic Microbial Community with Different Dosing Modes of Co-Substrate and Zero-Valent Iron. Int. Biodeterior. Biodegrad. 2016, 113, 126–133. [Google Scholar] [CrossRef]
- Wu, B.; Wang, H.; Dai, X.; Chai, X. Influential Mechanism of Water Occurrence States of Waste-Activated Sludge: Specifically Focusing on the Roles of EPS Micro-Spatial Distribution and Cation-Dominated Interfacial Properties. Water Res. 2021, 202, 117461. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Q.; Yoza, B.A.; Li, Q.X.; Ke, M.; Chen, C. Degradation of Guar in an Up-Flow Anaerobic Sludge Blanket Reactor: Impacts of Salinity on Performance Robustness, Granulation and Microbial Community. Chemosphere 2019, 232, 327–336. [Google Scholar] [CrossRef]
- Cai, G.; Zhao, L.; Wang, T.; Lv, N.; Li, J.; Ning, J.; Pan, X.; Zhu, G. Variation of Volatile Fatty Acid Oxidation and Methane Production during the Bioaugmentation of Anaerobic Digestion System: Microbial Community Analysis Revealing the Influence of Microbial Interactions on Metabolic Pathways. Sci. Total Environ. 2021, 754, 142425. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Sun, F.; Liu, J.; Zhou, Y. Enhanced Anaerobic Phenol Degradation by Conductive Materials via EPS and Microbial Community Alteration. Chem. Eng. J. 2018, 352, 1–9. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Dong, H.; Cui, P.; Lu, W.; Xu, H.; Qiu, B.; Sun, D.; Ma, J.; Dang, Y. Enrichment of Methanothrix Species via Riboflavin-Loaded Granular Activated Carbon in Anaerobic Digestion of High-Concentration Brewery Wastewater amidst Continuous Inoculation of Methanosarcina Barkeri. Water Res. 2025, 268, 122739. [Google Scholar] [CrossRef]
- Hellal, M.S.; Gamon, F.; Cema, G.; Kadimpati, K.K.; Ziembińska-Buczyńska, A.; Surmacz-Górska, J. Improving In-Situ Biomethanation of Sewage Sludge under Mesophilic Conditions: Performance and Microbial Community Analysis. Biomass Bioenergy 2024, 191, 107487. [Google Scholar] [CrossRef]
- Zheng, S.; Li, M.; Liu, Y.; Liu, F. Desulfovibrio Feeding Methanobacterium with Electrons in Conductive Methanogenic Aggregates from Coastal Zones. Water Res. 2021, 202, 117490. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Yang, Y.; Luo, Y.; Liu, F.; Song, D.; Sun, G.; Li, D.; Guo, J.; Dong, M.; Xu, M. Cysteine-Mediated Extracellular Electron Transfer of Lysinibacillus Varians GY32. Microbiol. Spectr. 2022, 10, e02798-22. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, H.; Chen, Y.; Guo, M.; Xia, Z.; Hu, B.; Nie, Y.; Tang, Y. Identification of Novel Butyrate- and Acetate-Oxidizing Bacteria in Butyrate-Fed Mesophilic Anaerobic Chemostats by DNA-Based Stable Isotope Probing. Microb. Ecol. 2020, 79, 285–298. [Google Scholar] [CrossRef]
- Nguyen, D.; Vigil, W.; Niks, D.; Hille, R. The Rapid-Reaction Kinetics of an Electron-Bifurcating Flavoprotein, the Crotonyl-CoA-Dependent NADH:Ferredoxin Oxidoreductase EtfAB:Bcd. J. Biol. Chem. 2024, 300, 107745. [Google Scholar] [CrossRef]
- Su, K.; Li, L.; Wang, Q.; Cao, R. A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System. Fermentation 2023, 9, 467. [Google Scholar] [CrossRef]
- Lu, J.; Hou, R.; Peng, W.; Guan, F.; Yuan, Y. Responses of Methane Production and Methanogenic Pathways to Polystyrene Nanoplastics Exposure in Paddy Soil. J. Hazard. Mater. 2024, 465, 133197. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.; Liu, K.; Si, B.; Yang, G.; Tian, C.; Zhang, Y. Multi-Cycle Anaerobic Digestion of Hydrothermal Liquefaction Aqueous Phase: Role of Carbon and Iron Based Conductive Materials in Inhibitory Compounds Degradation, Microbial Structure Shaping, and Interspecies Electron Transfer Regulation. Chem. Eng. J. 2023, 454, 140019. [Google Scholar] [CrossRef]
- Li, Q.; Gao, X.; Liu, Y.; Wang, G.; Li, Y.-Y.; Sano, D.; Wang, X.; Chen, R. Biochar and GAC Intensify Anaerobic Phenol Degradation via Distinctive Adsorption and Conductive Properties. J. Hazard. Mater. 2021, 405, 124183. [Google Scholar] [CrossRef]
- Kim, J.R.; Huling, S.G.; Kan, E. Effects of Temperature on Adsorption and Oxidative Degradation of Bisphenol A in an Acid-Treated Iron-Amended Granular Activated Carbon. Chem. Eng. J. 2015, 262, 1260–1267. [Google Scholar] [CrossRef]
- Kutlar, F.E.; Tunca, B.; Yilmazel, Y.D. Carbon-Based Conductive Materials Enhance Biomethane Recovery from Organic Wastes: A Review of the Impacts on Anaerobic Treatment. Chemosphere 2022, 290, 133247. [Google Scholar] [CrossRef] [PubMed]
- Noguer, M.C.; Magdalena, J.A.; Bernet, N.; Escudié, R.; Trably, E. Enhanced Fermentative Hydrogen Production from Food Waste in Continuous Reactor after Butyric Acid Treatment. Energies 2022, 15, 4048. [Google Scholar] [CrossRef]
- Fu, X.; Jin, X.; Pan, C.; Ye, R.; Wang, Q.; Wang, H.; Lu, W. Enhanced Butyrate Production by Transition Metal Particles during the Food Waste Fermentation. Bioresour. Technol. 2019, 291, 121848. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Wu, S.; Yang, W.; Xiong, L.; Zhang, W.; Hua, M.; Pan, B. Long-Term and Multiscale Assessment of Methanogenesis Enhancement Mechanisms in Magnetite Nanoparticle-Mediated Anaerobic Digestion Reactor. Environ. Res. 2024, 262, 119958. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.F.; Vicente, A.A. Biodegradation of PHB/PBAT Films and Isolation of Novel PBAT Biodegraders from Soil Microbiomes. Chemosphere 2024, 362, 142696. [Google Scholar] [CrossRef]
- Soulenthone, P.; Tachibana, Y.; Suzuki, M.; Mizuno, T.; Ohta, Y.; Kasuya, K. Characterization of a Poly(Butylene Adipate-Co-Terephthalate) Hydrolase from the Mesophilic Actinobacteria Rhodococcus Fascians. Polym. Degrad. Stab. 2021, 184, 109481. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Yang, Y.; Zhang, Y. Dual Roles of Zero-Valent Iron in Dry Anaerobic Digestion: Enhancing Interspecies Hydrogen Transfer and Direct Interspecies Electron Transfer. Waste Manag. 2020, 118, 481–490. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zheng, S.; Zang, H.; Liu, F.; Liu, F.; Liu, J. Stimulatory Effect of Magnetite on the Syntrophic Metabolism of Geobacter Co-Cultures: Influences of Surface Coating. Geochim. Cosmochim. Acta 2019, 256, 82–96. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Cavalcante, W.A.; Gehring, T.A.; Zaiat, M. Stimulation and Inhibition of Direct Interspecies Electron Transfer Mechanisms within Methanogenic Reactors by Adding Magnetite and Granular Actived Carbon. Chem. Eng. J. 2021, 415, 128882. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, C.; Zhu, J.; Jing, X.; Kong, F.; Zhang, C. Effects of Waste Rusted Iron Shavings on Enhancing Anaerobic Digestion of Food Wastes and Municipal Sludge. J. Clean. Prod. 2020, 242, 118195. [Google Scholar] [CrossRef]

| Parameters | pH | COD | BOD5 | NH3-N | TP |
|---|---|---|---|---|---|
| Concentration (mg/L) | 3.86 ± 0.13 | 9456.10 ± 219.96 | 3850.00 ± 50.00 | 135.11 ± 5.22 | 1.25 ± 0.15 |
| No. | Chemical Name | Molecular Formula | Concentration (mg/L) |
|---|---|---|---|
| 1 | Cyclopentanone | C5H8O | 2491 |
| 2 | 1,4-Butanediol | C4H10O2 | 1605 |
| 3 | n-Butanol | C4H10O | 996 |
| 4 | Tetrahydrofuran | C4H8O | 249 |
| 5 | Acetic Acid | C2H4O2 | 125 |
| 6 | Propionic Acid | C3H6O2 | 249 |
| 7 | Butyric Acid | C4H8O2 | 125 |
| 8 | Ammonium Chloride | NH4Cl | 135 |
| 9 | Sodium Dihydrogen Phosphate | NaH2PO4 | 1.25 |
| Group | GAC (g/L) | WISs (g/L) | Wastewater Volume (mL) | Sludge Volume (mL) |
|---|---|---|---|---|
| Control | 0 | 0 | 100 | 50 |
| GAC | 20 | 0 | 100 | 50 |
| WISs | 0 | 20 | 100 | 50 |
| GAC/WISs | 10 | 10 | 100 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Wen, J.; Huang, Z.; Jin, Q.; Li, Z.; Zhang, H.; Yang, H.; Huang, J.; Wang, W.; Hu, H. Coupling Granular Activated Carbon with Waste Iron Scraps Enhances Anaerobic Digestion of PBAT Wastewater: Performance Improvement and Mechanistic Insights. Fermentation 2025, 11, 614. https://doi.org/10.3390/fermentation11110614
He C, Wen J, Huang Z, Jin Q, Li Z, Zhang H, Yang H, Huang J, Wang W, Hu H. Coupling Granular Activated Carbon with Waste Iron Scraps Enhances Anaerobic Digestion of PBAT Wastewater: Performance Improvement and Mechanistic Insights. Fermentation. 2025; 11(11):614. https://doi.org/10.3390/fermentation11110614
Chicago/Turabian StyleHe, Chunhua, Jingjing Wen, Zhiqiang Huang, Qilong Jin, Ziyao Li, Hua Zhang, Houyun Yang, Jian Huang, Wei Wang, and Hao Hu. 2025. "Coupling Granular Activated Carbon with Waste Iron Scraps Enhances Anaerobic Digestion of PBAT Wastewater: Performance Improvement and Mechanistic Insights" Fermentation 11, no. 11: 614. https://doi.org/10.3390/fermentation11110614
APA StyleHe, C., Wen, J., Huang, Z., Jin, Q., Li, Z., Zhang, H., Yang, H., Huang, J., Wang, W., & Hu, H. (2025). Coupling Granular Activated Carbon with Waste Iron Scraps Enhances Anaerobic Digestion of PBAT Wastewater: Performance Improvement and Mechanistic Insights. Fermentation, 11(11), 614. https://doi.org/10.3390/fermentation11110614





