Developing a Symbiotic Fermented Milk Product with Microwave-Treated Hawthorn Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Microwave-Treated Hawthorn Extracts
2.3. Preparation of Symbiotic Fermented Milk Products
2.4. Microscopic Examination
2.5. Enumeration of B. bifidum and L. acidophilus in Symbiotic Fermented Milk Products
2.6. Amino Acid Determination
2.7. Mineral Content Determination
2.8. Water-Soluble Antioxidant Content Determination
2.9. Analysis of Experimental Data
3. Results and Discussion
3.1. Development of Symbiotic Fermented Milk Product Technology
3.2. Microbiological Parameters
3.3. Main Characteristics of Symbiotic Fermented Milk Products Over 14 Days
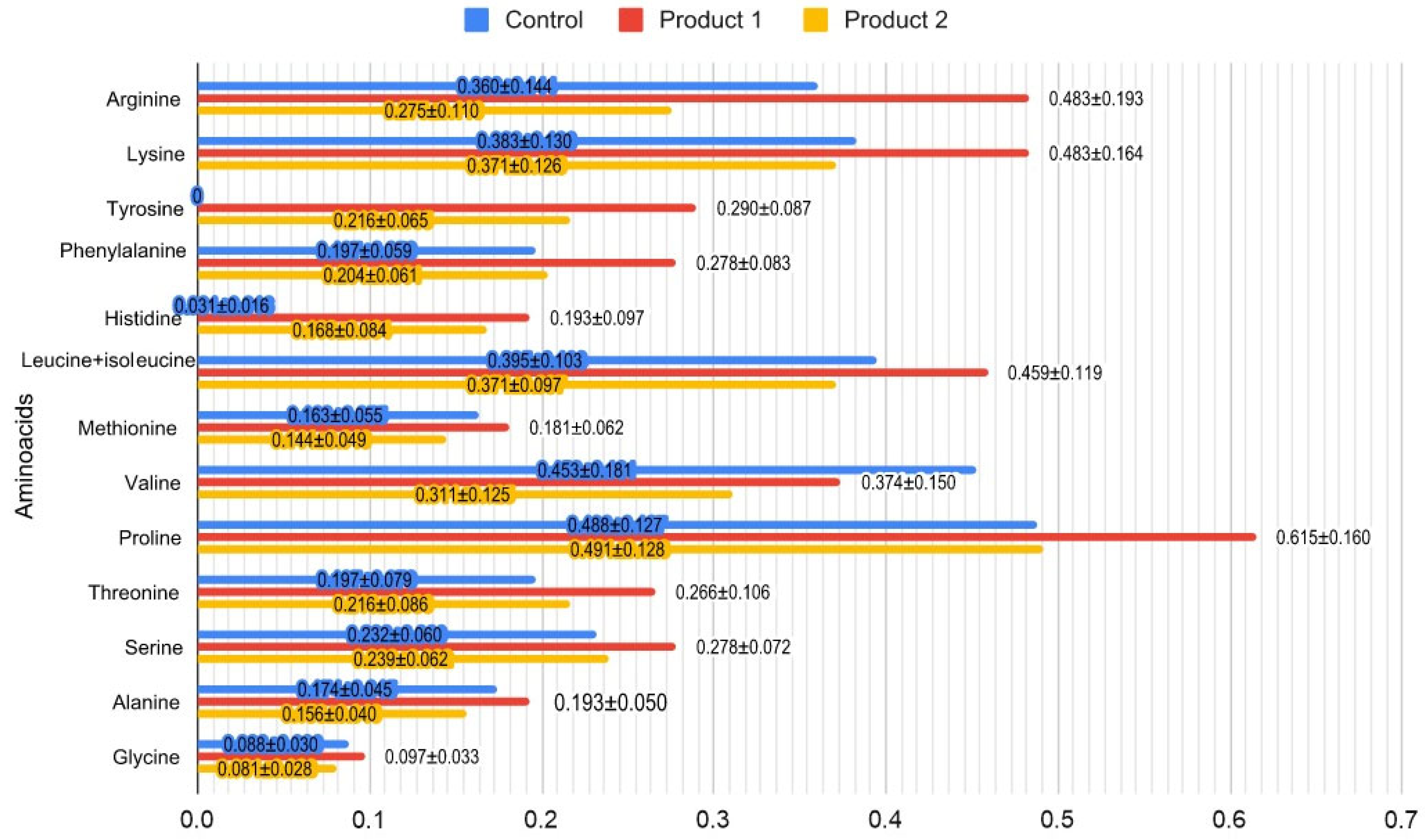
3.4. Amino Acid Composition of Symbiotic Fermented Milk Products
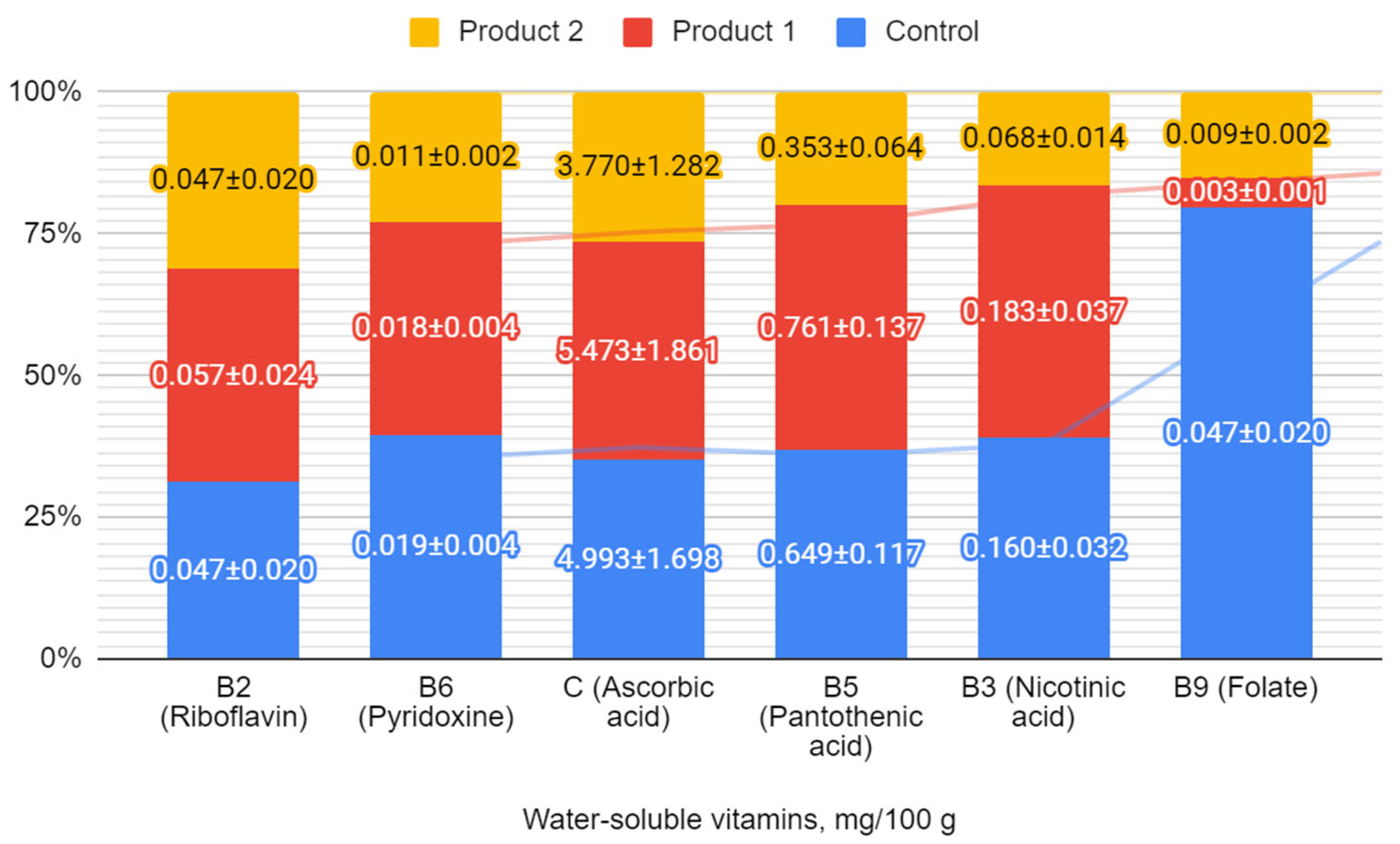
3.5. Vitamin and Mineral Composition of Symbiotic Fermented Milk Products
3.6. Antioxidant Composition of Symbiotic Fermented Milk Products
3.7. Nutritional Values of Symbiotic Fermented Milk Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of Prebiotic Dietary Fibers and Probiotics on Human Health: With Special Focus on Recent Advancement in Their Encapsulated Formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.J.; Rosikiewicz, M.; Pimentel, G.; Bütikofer, U.; von Ah, U.; Voirol, M.-J.; Croxatto, A.; Aeby, S.; Drai, J.; McTernan, P.G.; et al. Probiotic Yogurt and Acidified Milk Similarly Reduce Postprandial Inflammation and Both Alter the Gut Microbiota of Healthy, Young Men. Br. J. Nutr. 2017, 117, 1312–1322. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yeboah, P.J.; Ayivi, R.D.; Eddin, A.S.; Wijemanna, N.D.; Paidari, S.; Bakhshayesh, R.V. A Review and Comparative Perspective on Health Benefits of Probiotic and Fermented Foods. Int. J. Food Sci. Tech. 2023, 58, 4948–4964. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic Potential of Popular Fermented Dairy Products and Its Benefits on Human Health. Front. Nutr. 2024, 11, 1328620. [Google Scholar] [CrossRef]
- El-Menawy, R.K.; Mohamed, D.M.; Ismail, M.M.; Hassan, A.M. Optimal Combination of Cow and Quinoa Milk for Manufacturing of Functional Fermented Milk with High Levels of Antioxidant, Essential Amino Acids and Probiotics. Sci. Rep. 2023, 13, 20638. [Google Scholar] [CrossRef]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, Prebiotic, Synbiotic and Fermented Food Supplementation in Psychiatric Disorders: A Systematic Review of Clinical Trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.; Drunkler, D.A.; Colla, E. Probiotic Functional Yogurt: Challenges and Opportunities. Fermentation 2023, 10, 6. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and Potential Applications of Probiotic Encapsulation in Fermented Milk Products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Hamid Nour, A.; Ruth Oluwaseun, A.; Hamid Nour, A.; Suliman Omer, M.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds (Review). In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects, 1st ed.; Churyumov, I.G., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Suksaeree, J.; Monton, C. Maximizing Curcuminoid Extraction from Curcuma Aromatica Salisb. Rhizomes via Environmentally Friendly Microwave-Assisted Extraction Technique Using Full Factorial Design. Int. J. Food Sci. 2024, 2024, 4566123. [Google Scholar] [CrossRef]
- Ngoc, P.C.; Leclercq, L.; Rossi, J.-C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.-S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules 2019, 24, 4420. [Google Scholar] [CrossRef] [PubMed]
- Kilic, G.; Sengun, I.Y. Bioactive Properties of Kombucha Beverages Produced with Anatolian Hawthorn (Crataegus Orientalis) and Nettle (Urtica Dioica) Leaves. Food Biosci. 2023, 53, 102631. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Haji Ghafarloo, M.; Jouki, M.; Tabari, M. Production and Characterization of Synbiotic Doogh, a Yogurt-Based Iranian Drink by Gum Arabic, Ginger Extract and B. Bifidum. J. Food Sci. Technol. 2020, 57, 1158–1166. [Google Scholar] [CrossRef]
- Rahmani, F.; Gandomi, H.; Noori, N.; Faraki, A.; Farzaneh, M. Microbial, Physiochemical and Functional Properties of Probiotic Yogurt Containing Lactobacillus Acidophilus and Bifidobacterium Bifidum Enriched by Green Tea Aqueous Extract. Food Sci. Nutr. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Rodríguez-Mínguez, E.; Ríos, M.G.; Sánchez, C.; Picon, A. Mangosteen Extracts: Effects on Intestinal Bacteria, and Application to Functional Fermented Milk Products. Food Res. Int. 2024, 191, 114720. [Google Scholar] [CrossRef]
- Szołtysik, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Dąbrowska, A.; Bobak, Ł.; Chrzanowska, J. The Effect of Rosa Spinosissima Fruits Extract on Lactic Acid Bacteria Growth and Other Yoghurt Parameters. Foods 2020, 9, 1167. [Google Scholar] [CrossRef] [PubMed]
- Al-Hindi, R.R.; Abd El Ghani, S. Production of Functional Fermented Milk Beverages Supplemented with Pomegranate Peel Extract and Probiotic Lactic Acid Bacteria. J. Food Qual. 2020, 2020, 4710273. [Google Scholar] [CrossRef]
- Vanaki, E.; Kamkar, A.; Noori, N.; Azizian, A.; Mohammadkhan, F. The Effect of Aqueous Extract of Arctium Lappa Root on the Survival of Lactobacillus Acidophilus La-5 and Bifidobacterium Bifidum Bb-12 and Sensorial and Physicochemical Properties of Synbiotic Yogurt. Food Sci. Nutr. 2024, 12, 2182–2191. [Google Scholar] [CrossRef]
- Burmasova, M.A.; Utebaeva, A.A.; Sysoeva, E.V.; Sysoeva, M.A. Melanins of Inonotus Obliquus: Bifidogenic and Antioxidant Properties. Biomolecules 2019, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Utebaeva, A.; Gabrilyants, E.; Abish, Z.; Yevlash, V. Evaluation of Quality Characteristics of Fermented Acidophilic Product with B. Bifidum and Prunus padus extract. East.-Eur. J. Enterp. Technol. 2024, 2, 13–25. [Google Scholar] [CrossRef]
- GOST 33491-2015; Product Fermented-Milk, Enriched Bifidobacteriae Bifidum. Specifications. Interstate Council for Standardization, Metrology and Certification, Standardinform: Moscow, Russia, 2015.
- Zaitsev, S.Y.; Voronina, O.A.; Savina, A.A.; Ignatieva, L.P.; Bogolyubova, N.V. Correlations between the Total Antioxidant Activity and Biochemical Parameters of Cow Milk Depending on the Number of Somatic Cells. Int. J. Food Sci. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- GOST 32901-2014; Milk and milk products. Methods of microbiological analysis. Methods of microbiological analysis. Interstate Council for Standardization, Metrology and Certification, Standardinform: Moscow, Russia, 2014.
- Kariyawasam, K.M.G.M.M.; Lee, N.-K.; Paik, H.-D. Synbiotic Yoghurt Supplemented with Novel Probiotic Lactobacillus Brevis KU200019 and Fructooligosaccharides. Food Biosci. 2021, 39, 100835. [Google Scholar] [CrossRef]
- Shahein, M.R.; Elkot, W.F.; Albezrah, N.K.A.; Abdel-Hafez, L.J.M.; Alharbi, M.A.; Massoud, D.; Elmahallawy, E.K. Insights into the Microbiological and Physicochemical Properties of Bio-Frozen Yoghurt Made with Probiotic Strains in Combination with Jerusalem Artichoke Tubers Powder. Fermentation 2022, 8, 390. [Google Scholar] [CrossRef]
- Saatloo, N.V.; Mehdizadeh, T.; Aliakbarlu, J.; Tahmasebi, R. Physicochemical, Sensory and Microbiological Characteristics of Coriander Seed Powder Yogurt. AMB Expr. 2023, 13, 66. [Google Scholar] [CrossRef]
- GOST 54340-2011Fermented dairy products and dairy compound products. General specifications, Interstate Council for Standardization, Metrology and Certification, Standardinform: Moscow, Russia, 2011.
- Vahdat, F.; Mehdizadeh, T.; Kazemeini, H.; Reale, A.; Kaboudari, A. Physicochemical, Microbial, and Sensory Characteristics of Yogurt with Persian Shallot (Allium Hirtifolium Boiss) and Probiotic Bacteria. Food Sci. Nutr. 2024, 12, 3653–3662. [Google Scholar] [CrossRef]
- Eris, F.R.; Pamela, V.Y.; Kusumasari, S.; Meindrawan, B. Extraction of Inulin from Beneng Tuber (Xanthosoma Undipes) and Its Application to Yogurt. Future Foods 2024, 9, 100339. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Larasati, B.A.; Panunggal, B.; Afifah, D.N.; Anjani, G.; Rustanti, N. Total Lactic Acid Bacteria, Antioxidant Activity, and Acceptance of Synbiotic Yoghurt with Red Ginger Extract (Zingiberofficinale Var. Rubrum). IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012037. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine Metabolism and Nutrition in Growth, Health and Disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Hancock, C.N.; Fischer, J.W. Proline Metabolism and Cancer: Emerging Links to Glutamine and Collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Beitāne, I.; Ciproviča, I. Nutritional Benefits of Bifidobacterium Lactis in Dairy Products. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2013, 67, 378–382. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shadan, A.; Ma, Y. Biotechnological Applications of Probiotics: A Multifarious Weapon to Disease and Metabolic Abnormality. Probiotics Antimicro. Prot. 2022, 14, 1184–1210. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Li, P. Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health; Rao, V., Rao, L.G., Eds.; InTech: Houston, TX, USA, 2016. [Google Scholar] [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J.B. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera—A promising approach. Croat. J. Food Sci. Technol. 2013, 5, 85–91. [Google Scholar]
- Cunningham, M.; Vinderola, G.; Charalampopoulos, D.; Lebeer, S.; Sanders, M.E.; Grimaldi, R. Applying Probiotics and Prebiotics in New Delivery Formats—Is the Clinical Evidence Transferable? Trends Food Sci. Technol. 2021, 112, 495–506. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Ariff, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of Probiotics, Prebiotics, Synbiotics and Bioactive Phytochemicals on the Formulation of Functional Yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Alhamdan, A.M.; Al Juhaimi, F.Y.; Hassan, B.H.; Ehmed, K.A.; Mohamed Ahmed, I.A. Physicochemical, Microbiological, and Sensorial Quality Attributes of a Fermented Milk Drink (Laban) Fortified with Date Syrup (Dibs) during Cold Storage. Foods 2021, 10, 3157. [Google Scholar] [CrossRef] [PubMed]
- Varvara, R.-A.; Vodnar, D.C. Probiotic-Driven Advancement: Exploring the Intricacies of Mineral Absorption in the Human Body. Food Chem. X 2024, 21, 101067. [Google Scholar] [CrossRef]
- Yangilar, F.; Cakmakci, S. Probiotic Shelf-life, Mineral Contents and Others Properties of Probiotic Yogurts Supplemented with Corn Flour. J. Agric. Sci. 2017, 23, 472–481. [Google Scholar]
- Jena, R.; Choudhury, P.K. Bifidobacteria in Fermented Dairy Foods: A Health Beneficial Outlook. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Nikitina, E. Investigation on Fermented Milk Quality after the Addition of Flaxseed Mucilage and the Use of Lactobacillus Delbrueckii Subsp. Bulgaricus and Lactiplantibacillus Plantarum AG9. Front. Biosci. (Elite Ed) 2024, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S.; et al. Biological Properties and Potential Application of Hawthorn and Its Major Functional Components: A Review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus Monogyna Subsp. Monogyna Jacq.). J. Intercult. Ethnopharmacol. 2014, 3, 51. [Google Scholar] [CrossRef]
- Solopova, A.; Van Sinderen, D. Determination of Bifidobacterial Carbohydrate Utilization Abilities and Associated Metabolic End Products. In Bifidobacteria; Van Sinderen, D., Ventura, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2278, pp. 117–129. [Google Scholar] [CrossRef]
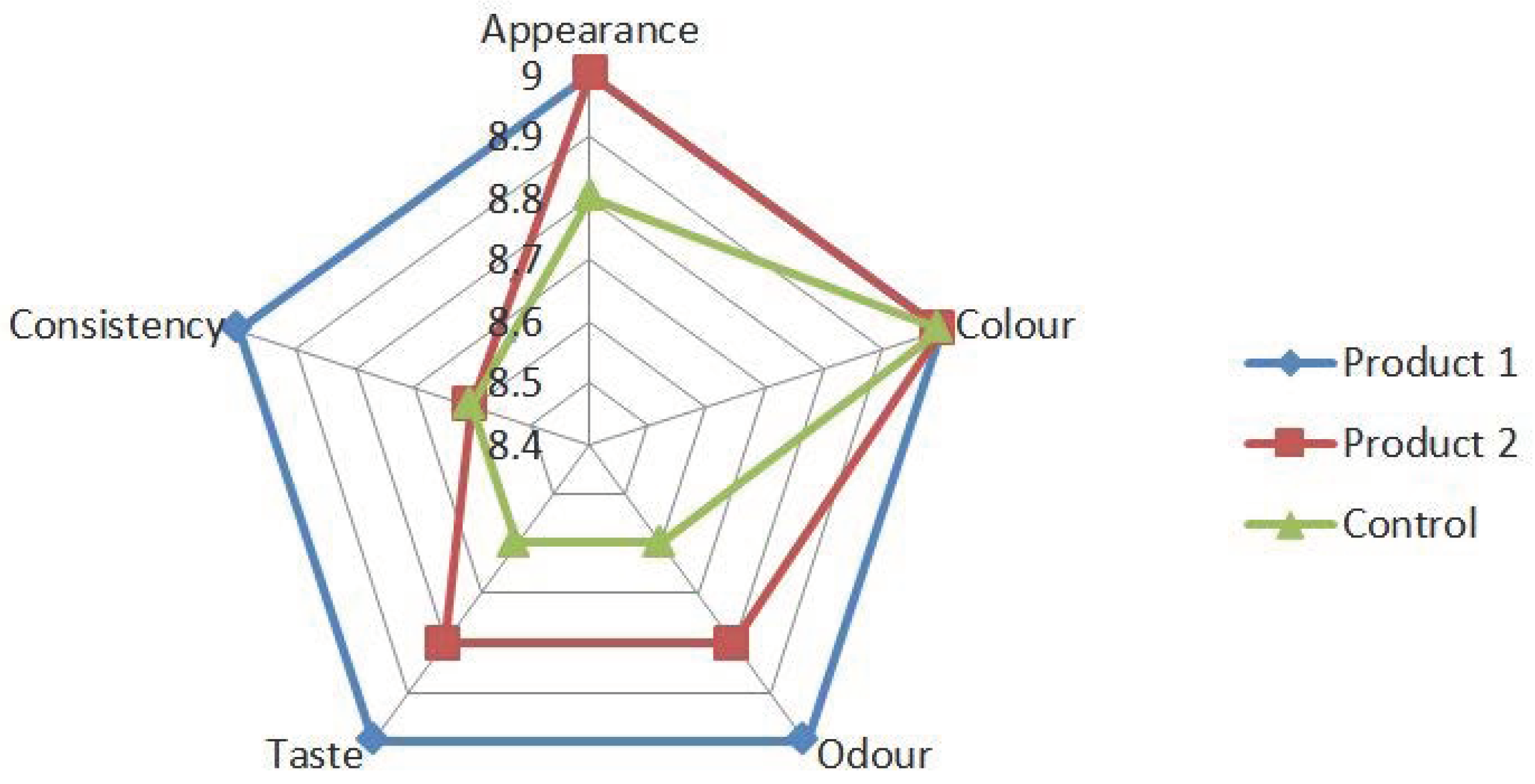
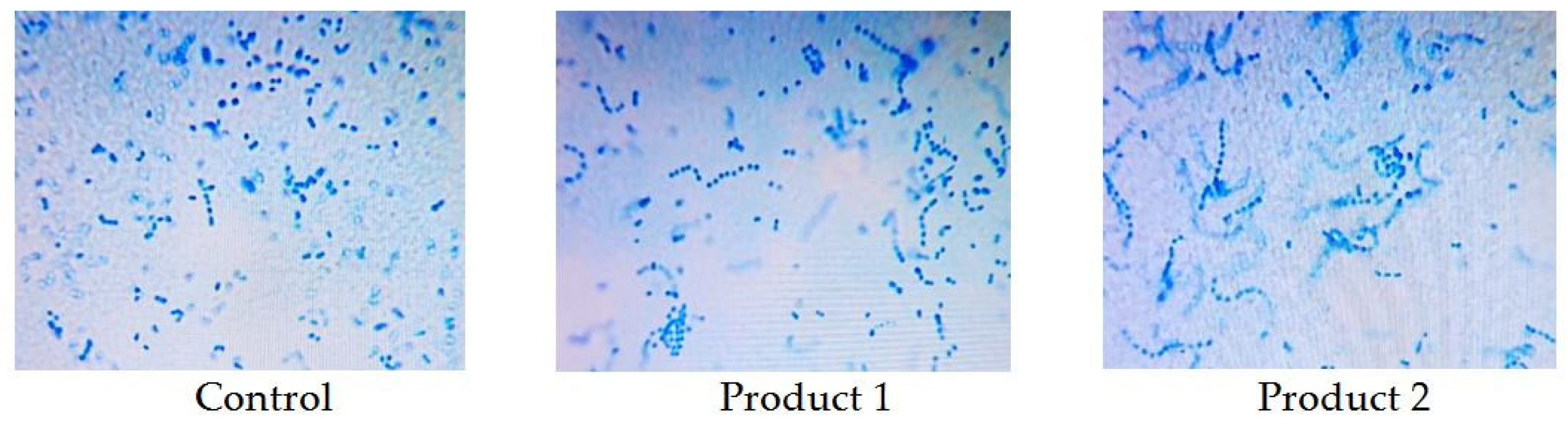

| Indicator Name | Standardized Indicator | Control | Product 1 | Product 2 |
|---|---|---|---|---|
| Lactic acid microorganisms | not less than 1 × 107 [CFU/g]. | more than 1 × 107 CFU/g | more than 1 × 108 CFU/g | more than 1 × 108 CFU/g |
| E. coli (coliforms) | not present in 0.1 [g]. | not detected | not detected | not detected |
| Staphylococcus aureus | not present in 0.1 [g]. | not detected | not detected | not detected |
| Pathogenic microorganisms (including Salmonella) | not present in 25 [g]. | not detected | not detected | not detected |
| Yeast | not more than 50 [CFU/g]. | less than 1 × 101 CFU/g | less than 1 × 101 CFU/g | less than 1 × 101 CFU/g |
| Molds | not more than 50 [CFU/g]. | less than 1 × 101 CFU/g | less than 1 × 101 CFU/g | less than 1 × 101 CFU/g |
| Days | Products | Titratable Acidity [°T] | Number of L. acidophilus [lg CFU/cm3] | Number of B. bifidum [lg CFU/cm3] | Viscosity [s] | Water-Holding Capacity [%] |
|---|---|---|---|---|---|---|
| 1 | Control | 108.5 ± 0.16 a | 8.92 ± 0.02 a | 9.17 ± 0.12 a | 9.8 a | 30 a |
| Product 1 | 106.2 ± 0.22 b | 9.12 ± 0.04 b | 10.52 ± 0.05 b | 10.2 b | 15 b | |
| Product 2 | 105.8 ± 0.44 b | 9.24 ± 0.03 c | 9.31 ± 0.03 a | 10.4 c | 15 b | |
| 7 | Control | 125.4 ± 0.74 a | 10.26 ± 0.03 a | 11.53 ± 0.05 a | 10.2 a | 25 a |
| Product 1 | 120.2 ± 0.28 b | 11.32 ± 0.02 b | 12.07 ± 0.07 b | 11.3 b | 20 a | |
| Product 2 | 120.6 ± 0.16 b | 11.28 ± 0.04 b | 11.61 ± 0.02 a | 11.2 b | 20 b | |
| 14 | Control | 146.2 ± 0.08 a | 8.99 ± 0.13 a | 8.33 ± 0.11 a | 10.0 a | 45 a |
| Product 1 | 136.0 ± 0.09 b | 9.98 ± 0.02 b | 10.45 ± 0.12 b | 11.0 b | 37 b | |
| Product 2 | 135.6 ± 0.05 b | 9.59 ± 0.09 b | 10.05 ± 0.11 b | 10.7 c | 40 b |
| Products | Flavonoids, mg/g | Content of Water-Soluble Antioxidants, mg/g |
|---|---|---|
| Control | 0.15 ± 0.001 | 0.70 ± 0.0024 |
| Product 1 | 0.19 ± 0.001 | 0.74 ± 0.0024 |
| Product 2 | 0.17 ± 0.001 | 0.63 ± 0.0020 |
| Indicators | Control | Product 1 | Product 2 |
|---|---|---|---|
| Protein, % | 4.44 ± 0.27 | 4.40 ± 0.26 | 4.44 ± 0.27 |
| Fats, % | 2.41 ± 0.19 | 2.43 ± 0.19 | 2.41 ± 0.19 |
| Carbohydrates, % | 3.21 ± 0.16 | 2.80 ± 0.14 | 2.60 ± 0.13 |
| Moisture, % | 89.47 ± 4.47 | 90.0 ± 4.5 | 90.09 ± 4.5 |
| Ash, % | 0.47 ± 0.02 | 0.37 ± 0.02 | 0.46 ± 0.02 |
| Energy value, kcal/kJ/100g | 52.29/218.78 | 50.83/212.67 | 49.85/208.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utebaeva, A.; Gabrilyants, E.; Abish, Z. Developing a Symbiotic Fermented Milk Product with Microwave-Treated Hawthorn Extract. Fermentation 2024, 10, 377. https://doi.org/10.3390/fermentation10080377
Utebaeva A, Gabrilyants E, Abish Z. Developing a Symbiotic Fermented Milk Product with Microwave-Treated Hawthorn Extract. Fermentation. 2024; 10(8):377. https://doi.org/10.3390/fermentation10080377
Chicago/Turabian StyleUtebaeva, Aidana, Eleonora Gabrilyants, and Zhansaya Abish. 2024. "Developing a Symbiotic Fermented Milk Product with Microwave-Treated Hawthorn Extract" Fermentation 10, no. 8: 377. https://doi.org/10.3390/fermentation10080377
APA StyleUtebaeva, A., Gabrilyants, E., & Abish, Z. (2024). Developing a Symbiotic Fermented Milk Product with Microwave-Treated Hawthorn Extract. Fermentation, 10(8), 377. https://doi.org/10.3390/fermentation10080377






