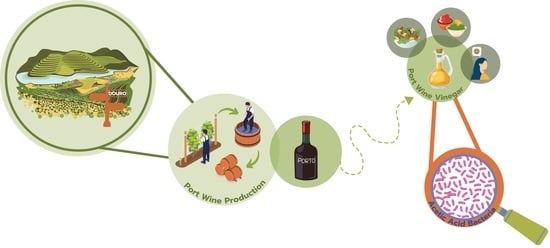
Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria
Abstract
1. Introduction
2. Biochemistry and Physiology of Acetic Acid Bacteria (AAB)
2.1. General Overview, Classification, and Identification
2.2. Metabolic Pathways and Respiratory Chains in AAB
2.2.1. Respiratory Machinery and Energy Yield
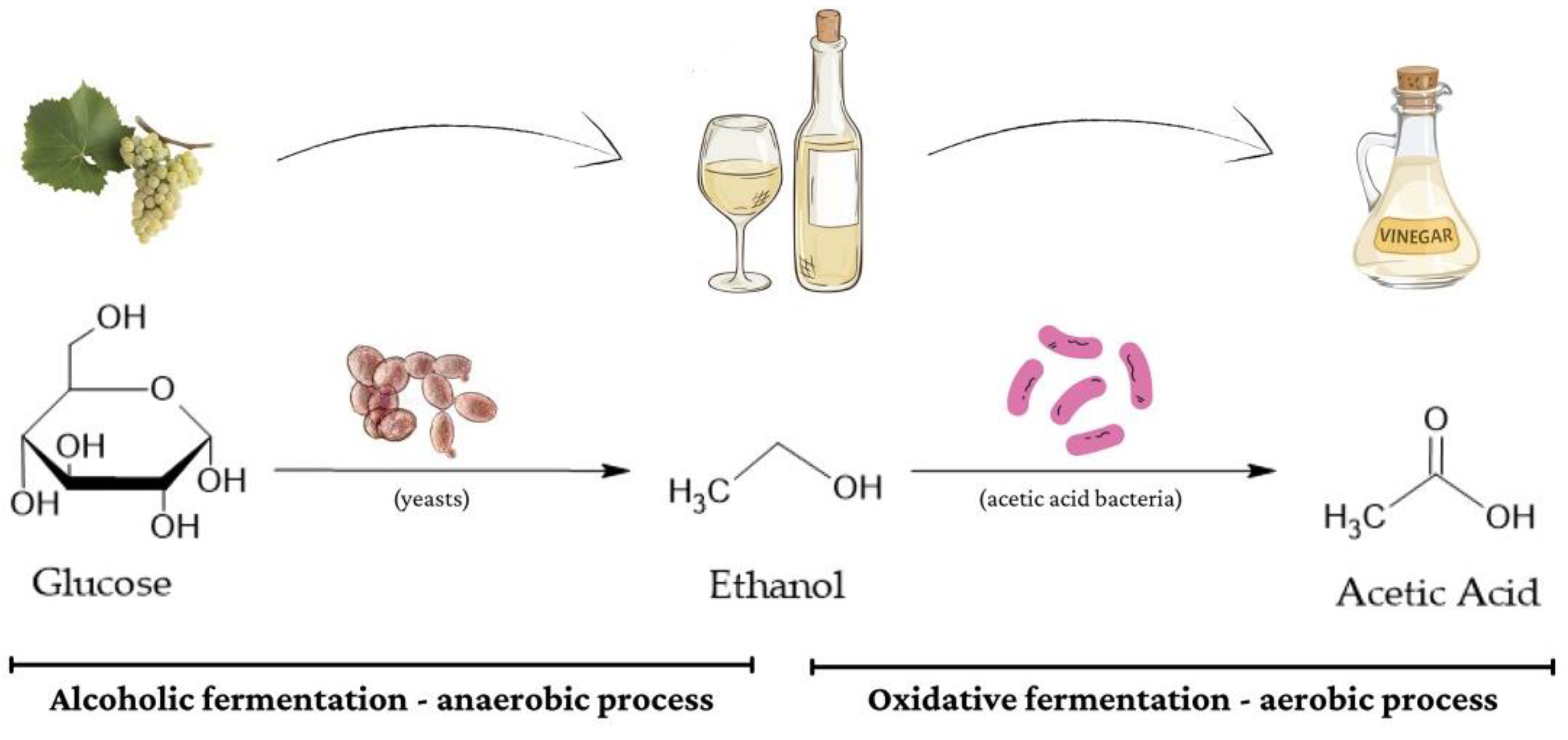
2.2.2. Acetic Acid Production: Oxidative Fermentation
2.2.3. Oxidation of Carbohydrates, Alcohols, and Organic Acids
2.2.4. Resistance to Acidic Environments
2.2.5. Resistance to Alcoholic Environments
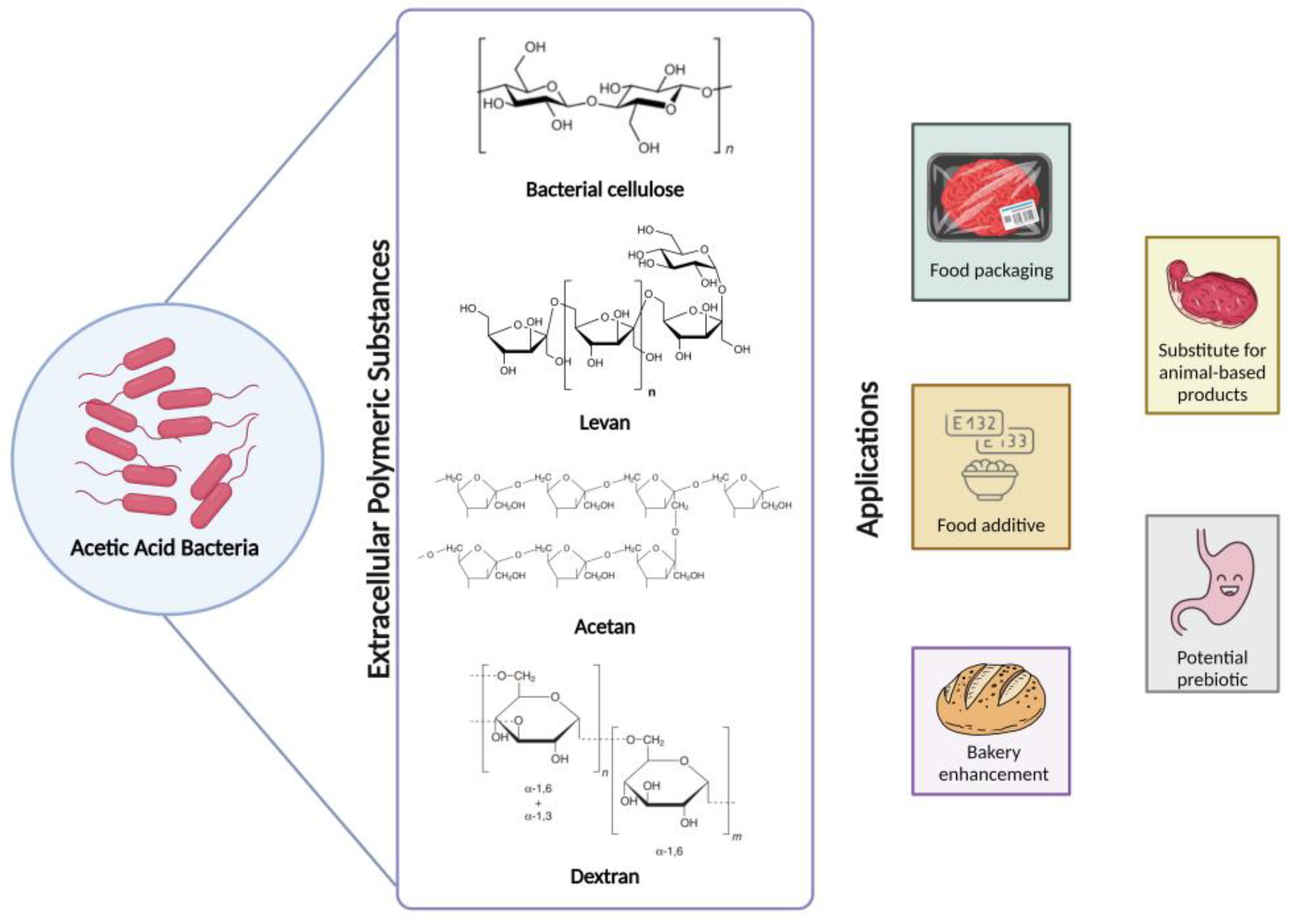
2.2.6. Extracellular Polymeric Substances Produced by AAB
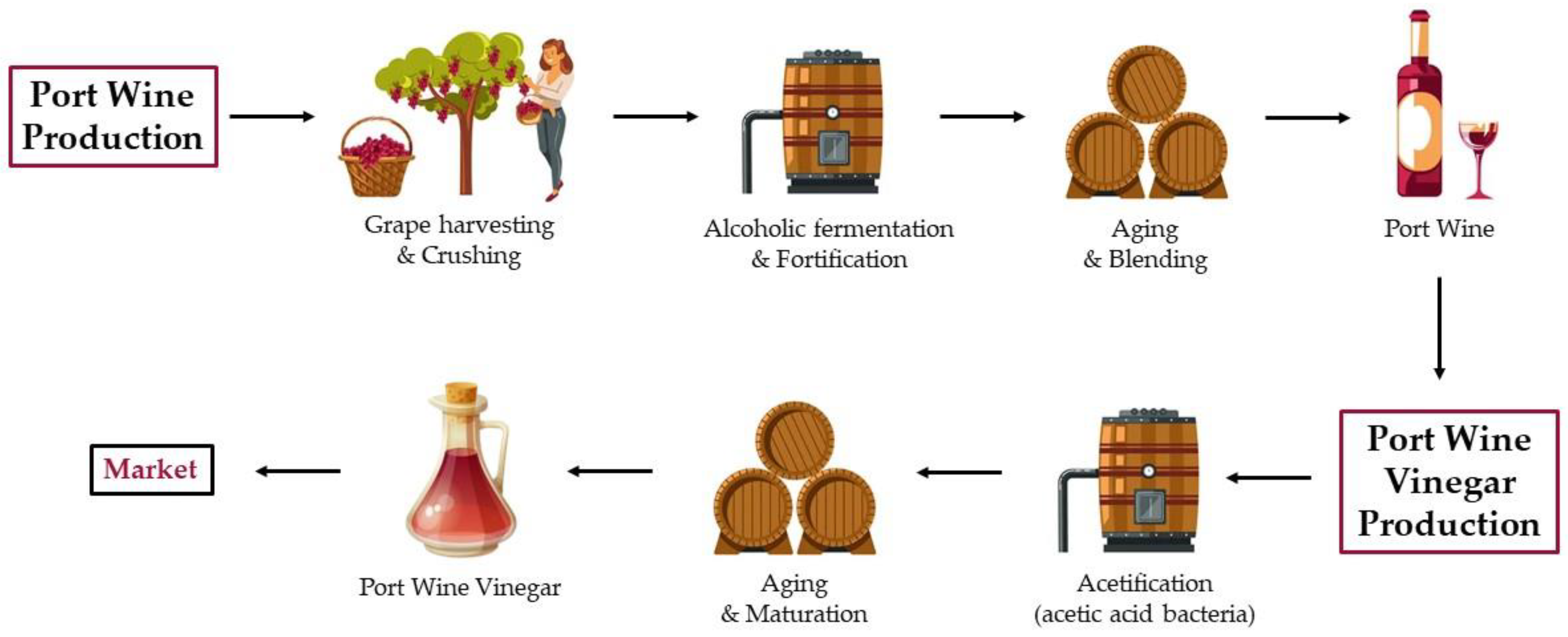
3. Overview of Port Wine Production
4. Port Wine Vinegar
4.1. Vinegar Production: From Fermentation to Quality
4.2. Wine Vinegar Characteristics
4.3. Improving Port Wine Production and Its Effect on Vinegar
5. Exploring Taste: Port Wine and Vinegar Sensory Analysis
6. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Table of contents
| 1. Introduction……………………………………………………………………………………… | 1 |
| 2. Biochemistry and physiology of Acetic Acid Bacteria (AAB)………………………………. | 2 |
| 2.1. General overview, classification, and identification…………………………………….. | 2 |
| 2.2. Metabolic pathways and respiratory chains in AAB……………………………………. | 6 |
| 2.2.1. Respiratory machinery and energy yield…………………………………………... | 6 |
| 2.2.2. Acetic acid production: Oxidative fermentation…………………………………... | 6 |
| 2.2.3. Oxidation of carbohydrates, alcohols, and organic acids………………………… | 8 |
| 2.2.4. Resistance to acidic environments…………………………………………………... | 9 |
| 2.2.5. Resistance to alcoholic environments………………………………………………. | 10 |
| 2.2.6. Extracellular polymeric substances produced by AAB…………………………… | 11 |
| 3. Overview of Port Wine production……………………………………………………………. | 13 |
| 4. Port Wine Vinegar………………………………………………………………………………. | 13 |
| 4.1. Vinegar production: From fermentation to quality……………………………………… | 14 |
| 4.2. Wine vinegar characteristics……………………………………………………………….. | 18 |
| 4.3. Improving Port Wine production and its effect on vinegar……………………………. | 19 |
| 5. Exploring taste: Port wine and vinegar sensory analysis…………………………………… | 20 |
| 6. Final remarks…………………………………………………………………………………….. | 22 |
| References…………………………………………………………………………………………... | 23 |
References
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Hashimoto, W. Adaptation of Yeast Saccharomyces cerevisiae to Grape-Skin Environment. Sci. Rep. 2023, 13, 9279. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; de Pinho, P.G. Port Wine. Adv. Food Nutr. Res. 2011, 63, 119–146. [Google Scholar]
- Martins, J.P. The Pleasure of Port: The Inside Story of a Unique Fortified Wine; LIVROS D’HOJE: Lisbon, Portugal, 2011; ISBN 9789722046619. [Google Scholar]
- Cristovam, E.; Paterson, A. PORT|Composition and Analysis. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4638–4644. [Google Scholar]
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Wines: Madeira, Port and Sherry Fortified Wines—The Sui Generis and Notable Peculiarities. Major Differences and Chemical Patterns. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 534–555. [Google Scholar]
- Grandes Escolhas Vinagre de Vinho Do Porto Já é Marca Registada. Available online: https://grandesescolhas.com/vinagre-de-vinho-do-porto-ja-e-marca-registada/ (accessed on 31 January 2024).
- Vilela, A. Microbial Dynamics in Sour–Sweet Wine Vinegar: Impacts on Chemical and Sensory Composition. Appl. Sci. 2023, 13, 7366. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Bhat, S.V.; Akhtar, R.; Amin, T. An Overview on the Biological Production of Vinegar. Int. J. Fermented Foods 2014, 3, 139. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. Acetic Acid Bacteria Spoilage of Bottled Red Wine—A Review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef] [PubMed]
- De Vero, L.; Giudici, P. Genus-Specific Profile of Acetic Acid Bacteria by 16S RDNA PCR-DGGE. Int. J. Food. Microbiol. 2008, 125, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of Acetic Acid Bacteria in Food Industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Yassunaka Hata, N.N.; Surek, M.; Sartori, D.; Serrato, R.V.; Spinosa, W.A. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef]
- De Filippis, F.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different Temperatures Select Distinctive Acetic Acid Bacteria Species and Promotes Organic Acids Production during Kombucha Tea Fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Raspor, P.; Goranovič, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, J.M.; Mas, A. Chapter 9-Acetic Acid Bacteria. Mol. Wine Microbiol. 2011, 227–255. [Google Scholar] [CrossRef]
- Mizzi, J.; Gaggìa, F.; Bozzi Cionci, N.; Di Gioia, D.; Attard, E. Selection of Acetic Acid Bacterial Strains and Vinegar Production From Local Maltese Food Sources. Front. Microbiol. 2022, 13, 897825. [Google Scholar] [CrossRef] [PubMed]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Kilic, G.; Charoenyingcharoen, P.; Yukphan, P.; Yamada, Y. Investigation of the Microbiota Associated with Traditionally Produced Fruit Vinegars with Focus on Acetic Acid Bacteria and Lactic Acid Bacteria. Food Biosci. 2022, 47, 101636. [Google Scholar] [CrossRef]
- Plioni, I.; Bekatorou, A.; Terpou, A.; Mallouchos, A.; Plessas, S.; Koutinas, A.A.; Katechaki, E. Vinegar Production from Corinthian Currants Finishing Side-Stream: Development and Comparison of Methods Based on Immobilized Acetic Acid Bacteria. Foods 2021, 10, 3133. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyama, H.; Tonouchi, N.; Okamoto-Kainuma, A. Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; ISBN 978-4-431-55931-3. [Google Scholar]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.-M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of Acetic Acid Bacteria and Their Acid Resistant Mechanism. AMB Express 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Azuma, Y.; Kosaka, T.; Yakushi, T.; Hoshida, H.; Akada, R.; Yamada, M. Genomic Analyses of Thermotolerant Microorganisms Used for High-Temperature Fermentations. Biosci. Biotechnol. Biochem. 2016, 80, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Torija, M.-J.; del Carmen García-Parrilla, M.C.; Troncoso, A.M. Acetic Acid Bacteria and the Production and Quality of Wine Vinegar. Sci. World J. 2014, 2014, 394671. [Google Scholar] [CrossRef] [PubMed]
- Plessi, M. Vinegar. Encycl. Food Sci. Nutr. 2003, 5996–6004. [Google Scholar] [CrossRef]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and Microbiological Aspects of Traditional Balsamic Vinegar and Their Influence on Quality and Sensorial Properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of Acetic Acid. Bacteria in “Traditional Balsamic Vinegar.”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in Bacterial Cellulose Matrices for Biotechnological Applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- González, Á.; Guillamón, J.M.; Mas, A.; Poblet, M. Application of Molecular Methods for Routine Identification of Acetic Acid Bacteria. Int. J. Food Microbiol. 2006, 108, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.F.; de Souza, A.C.; Ramos, C.L.; Pereira, A.A.; Schwan, R.F.; Dias, D.R. Sensorial, Antioxidant and Antimicrobial Evaluation of Vinegars from Surpluses of Physalis (Physalis pubescens L.) and Red Pitahaya (Hylocereus monacanthus). J. Sci. Food Agric. 2019, 99, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Soumahoro, S.; Ouattara, H.G.; Droux, M.; Nasser, W.; Niamke, S.L.; Reverchon, S. Acetic Acid Bacteria (AAB) Involved in Cocoa Fermentation from Ivory Coast: Species Diversity and Performance in Acetic Acid Production. J. Food Sci. Technol. 2019, 57, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Sombolestani, A.S.; Cleenwerck, I.; Cnockaert, M.; Borremans, W.; Wieme, A.D.; De Vuyst, L.; Vandamme, P. Novel acetic acid bacteria from cider fermentations: Acetobacter conturbans sp. nov. and Acetobacter fallax sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6163–6171. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, K.H.; Moon, J.Y.; Yeo, S.-H.; Jeon, C.O. Acetobacter Oryzoeni Sp. Nov., Isolated from Korean Rice Wine Vinegar. Int. J. Syst. Evol. Microbiol. 2020, 70, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.E.; Al-Barha, N.S.; Sharaf, A.-A.M.; Al-Maqtari, Q.A.; Mohedein, A.; Mohammed, H.H.H.; Chen, F. A Novel Strain of Acetic Acid Bacteria Gluconobacter oxydans FBFS97 Involved in Riboflavin Production. Sci. Rep. 2020, 10, 13527. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Angela, C.; Young, J.; Kordayanti, S.; Virgina Partha Devanthi, P.K. Isolation and Screening of Microbial Isolates from Kombucha Culture for Bacterial Cellulose Production in Sugarcane Molasses Medium. KnE Life Sci. 2020. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Hu, S.; Li, Z.; Qu, J.; Wu, Z.; Chen, S. Comparison of the Effects of Acetic Acid Bacteria and Lactic Acid Bacteria on the Microbial Diversity of and the Functional Pathways in Dough as Revealed by High-Throughput Metagenomics Sequencing. Int. J. Food Microbiol. 2021, 346, 109168. [Google Scholar] [CrossRef]
- Anguluri, K.; La China, S.; Brugnoli, M.; De Vero, L.; Pulvirenti, A.; Cassanelli, S.; Gullo, M. Candidate Acetic Acid Bacteria Strains for Levan Production. Polymers 2022, 14, 2000. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of Acetic Acid Bacteria and Lactic Acid Bacteria in Multispecies Solid-State Fermentation of Traditional Chinese Cereal Vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef] [PubMed]
- El-Askri, T.; Yatim, M.; Sehli, Y.; Rahou, A.; Belhaj, A.; Castro, R.; Durán-Guerrero, E.; Hafidi, M.; Zouhair, R. Screening and Characterization of New Acetobacter fabarum and Acetobacter pasteurianus Strains with High Ethanol–Thermo Tolerance and the Optimization of Acetic Acid Production. Microorganisms 2022, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, M.; La China, S.; Lasagni, F.; Romeo, F.V.; Pulvirenti, A.; Gullo, M. Acetic Acid Bacteria in Agro-Wastes: From Cheese Whey and Olive Mill Wastewater to Cellulose. Appl. Microbiol. Biotechnol. 2023, 107, 3729–3744. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, S.-H.; Lee, C.-Y.; Jo, H.-W.; Lee, W.-H.; Kim, E.-H.; Choi, B.-K.; Huh, C.-K. Screening of Acetic Acid Bacteria Isolated from Various Sources for Use in Kombucha Production. Fermentation 2023, 10, 18. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation 2023, 9, 447. [Google Scholar] [CrossRef]
- Zgardan, D.; Mitina, I.; Sturza, R.; Mitin, V.; Rubtov, S.; Grajdieru, C.; Behta, E.; Inci, F.; Haciosmanoglu, N. A Survey on Acetic Acid Bacteria Levels and Volatile Acidity in Several Wines of the Republic of Moldova. Biol. Life Sci. Forum 2023, 26, 79. [Google Scholar] [CrossRef]
- Parra, A.; Ovejas, A.; González-Arenzana, L.; Gutiérrez, A.R.; López-Alfaro, I. Development and Validation of a New Method for Detecting Acetic Bacteria in Wine. Foods 2023, 12, 3734. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Hu, K.; Li, J.; Durán-Guerrero, E.; Liu, S.; Guo, M.; Liu, A. Microbial Characterization of Sichuan Baoning Vinegar: Lactic Acid Bacteria, Acetic Acid Bacteria and Yeasts. Arch. Microbiol. 2024, 206, 59. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyama, H.; Adachi, O. Respiratory Chains and Bioenergetics of Acetic Acid Bacteria. Adv. Microb. Physiol. 1994, 36, 247–301. [Google Scholar]
- Ano, Y.; Toyama, H.; Adachi, O.; Matsushita, K. Energy Metabolism of a Unique Acetic Acid Bacterium, Asaia bogorensis, That Lacks Ethanol Oxidation Activity. Biosci. Biotechnol. Biochem. 2008, 72, 989–997. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Microorganisms, 14th ed.; Pearson Education: London, UK, 2018; ISBN 9781292235103. [Google Scholar]
- Matsushita, K.; Matsutani, M. Distribution, Evolution, and Physiology of Oxidative Fermentation. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 159–178. [Google Scholar]
- Hanke, T.; N�h, K.; Noack, S.; Polen, T.; Bringer, S.; Sahm, H.; Wiechert, W.; Bott, M. Combined Fluxomics and Transcriptomics Analysis of Glucose Catabolism via a Partially Cyclic Pentose Phosphate Pathway in Gluconobacter oxydans 621H. Appl. Environ. Microbiol. 2013, 79, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, R.; Yin, H.; Bai, X.; Chang, Y.; Xia, M.; Wang, M. Acetobacter pasteurianus Metabolic Change Induced by Initial Acetic Acid to Adapt to Acetic Acid Fermentation Conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7007–7016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, P.; Lei, Q.; Li, B.; Sun, Y.; Li, S.; Lei, H.; Xie, N. Metabolic Adaptability Shifts of Cell Membrane Fatty Acids of Komagataeibacter Hansenii HDM1-3 Improve Acid Stress Resistance and Survival in Acidic Environments. J. Ind. Microbiol. Biotechnol. 2019, 46, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; Ehrenreich, A.; Liebl, W.; García-Martínez, T.; Mauricio, J.C. Combining Omics Tools for the Characterization of the Microbiota of Diverse Vinegars Obtained by Submerged Culture: 16S RRNA Amplicon Sequencing and MALDI-TOF MS. Front. Microbiol. 2022, 13, 1055010. [Google Scholar] [CrossRef]
- Adachi, O.; Miyagawa, E.; Shinagawa, E.; Matsushita, K.; Ameyama, M. Purification and Properties of Particulate Alcohol Dehydrogenase from Acetobacter Aceti. Agric. Biol. Chem. 1978, 42, 2331–2340. [Google Scholar] [CrossRef]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic Acid Bacteria: A Group of Bacteria with Versatile Biotechnological Applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef]
- Ameyama, M.; Adachi, O. Membrane-Bound. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; pp. 450–457. [Google Scholar]
- Adachi, O.; Tayama, K.; Shinagawa, E.; Matsushita, K.; Ameyama, M. Purification and Characterization of Membrane-Bound Aldehyde Dehydrogenase from Gluconobacter suboxydans. Agric. Biol. Chem. 1980, 44, 503–515. [Google Scholar] [CrossRef]
- Saeki, A.; Taniguchi, M.; Matsushita, K.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Microbiological Aspects of Acetate Oxidation by Acetic Acid Bacteria, Unfavorable Phenomena in Vinegar Fermentation. Biosci. Biotechnol. Biochem. 1997, 61, 317–323. [Google Scholar] [CrossRef]
- Saeki, A.; Matsushita, K.; Takeno, S.; Taniguchi, M.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Enzymes Responsible for Acetate Oxidation by Acetic Acid Bacteria. Biosci. Biotechnol. Biochem. 1999, 63, 2102–2109. [Google Scholar] [CrossRef][Green Version]
- Yakushi, T.; Matsushita, K. Alcohol Dehydrogenase of Acetic Acid Bacteria: Structure, Mode of Action, and Applications in Biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 1257–1265. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.; Zhang, H.; Liebl, W.; Toyama, H.; Chen, F. Oxidative Fermentation of Acetic Acid Bacteria and Its Products. Front. Microbiol. 2022, 13, 879246. [Google Scholar] [CrossRef]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780429091483. [Google Scholar]
- Drysdale, G.S.; Fleet, G.H. The Effect of Acetic Acid Bacteria upon the Growth and Metabolism of Yeasts during the Fermentation of Grape Juice. J. Appl. Bacteriol. 1989, 67, 471–481. [Google Scholar] [CrossRef]
- Adams, M.; Moss, M.O.; McClure, P. Food Microbiology, 4th ed.; The Royal Society of Chemistry: London, UK, 2015; ISBN 978-1-84973-960-3. [Google Scholar]
- Ebner, H.; Sellmer, H.F.S. Vinegar. In Biotechnology Set; Wiley: Hoboken, NJ, USA, 2001; pp. 579–591. [Google Scholar]
- De Ley, J.; Gillis, M.; Swings, J.; Family, V.I. Acetobacteriaceae, Bergey’s Manual of Systematic Bacteriology, 1st ed.; Krieg, N.R., Holt, J.G., Eds.; Williams and Wilkins Co: Baltimore, MD, USA, 1984; Volume 1. [Google Scholar]
- Drysdale, G.S.; Fleet, G.H. Acetic Acid Bacteria in Winemaking: A Review. Am. J. Enol. Vitic. 1988, 39, 143–154. [Google Scholar] [CrossRef]
- White, G.; Wang, C. The Dissimilation of Glucose and Gluconate by Acetobacter xylinum. 1. The Origin and the Fate of Triose Phosphate. Biochem. J. 1964, 90, 408–423. [Google Scholar] [CrossRef]
- Pedraza, R.O. Recent Advances in Nitrogen-Fixing Acetic Acid Bacteria. Int. J. Food Microbiol. 2008, 125, 25–35. [Google Scholar] [CrossRef]
- García-García, I.; Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-Martínez, T.; Mauricio, J.C. Biotechnologically Relevant Features of Gluconic Acid Production by Acetic Acid Bacteria. Acetic Acid. Bact. 2017, 6, 7–10. [Google Scholar] [CrossRef]
- Andrés-Barrao, C.; Barja, F. Acetic Acid Bacteria Strategies Contributing to Acetic Acid Resistance During Oxidative Fermentation. In Acetic Acid Bacteria; CRC Press: Boca Raton, FL, USA, 2017; pp. 92–119. [Google Scholar]
- Nakano, S.; Fukaya, M.; Horinouchi, S. Putative ABC Transporter Responsible for Acetic Acid Resistance in Acetobacter Aceti. Appl. Environ. Microbiol. 2006, 72, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Fukaya, M. Analysis of Proteins Responsive to Acetic Acid in Acetobacter: Molecular Mechanisms Conferring Acetic Acid Resistance in Acetic Acid Bacteria. Int. J. Food Microbiol. 2008, 125, 54–59. [Google Scholar] [CrossRef]
- Nakano, S.; Ebisuya, H. Physiology of Acetobacter and Komagataeibacter Spp.: Acetic Acid Resistance Mechanism in Acetic Acid Fermentation. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 223–234. [Google Scholar]
- Kourouma, M.C.; Mbengue, M.; Sarr, N.C.D.; Sarr, K.; Kane, C.T. Thermoresistant, Ethanol-Resistant and Acid-Resistant Properties of Acetic Acid Bacteria Isolated from Fermented Mango Alcohol. Adv. Microbiol. 2022, 12, 177–191. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Li, D.; Wang, C.; Xu, N.; Wu, S.; He, S.; Hu, Y. Correlation between Ethanol Resistance and Characteristics of PQQ-Dependent ADH in Acetic Acid Bacteria. Eur. Food Res. Technol. 2016, 242, 837–847. [Google Scholar] [CrossRef]
- Kanchanarach, W.; Theeragool, G.; Yakushi, T.; Toyama, H.; Adachi, O.; Matsushita, K. Characterization of Thermotolerant Acetobacter pasteurianus Strains and Their Quinoprotein Alcohol Dehydrogenases. Appl. Microbiol. Biotechnol. 2010, 85, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, H.; Wang, S.; Zhao, A.; Qu, L.; Xiong, W.; Alam, A.; Ma, W.; Lv, Y.; Xu, J. Screening a Panel of Acid-Producing Strains by Developing a High-Throughput Method. Biotechnol. Bioprocess. Eng. 2022, 27, 810–817. [Google Scholar] [CrossRef]
- Es-sbata, I.; Lakhlifi, T.; Yatim, M.; El-Abid, H.; Belhaj, A.; Hafidi, M.; Zouhair, R. Screening and Molecular Characterization of New Thermo- and Ethanol-tolerant Acetobacter malorum Strains Isolated from Two Biomes Moroccan Cactus Fruits. Biotechnol. Appl. Biochem. 2021, 68, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Tonouchi, N. Cellulose and Other Capsular Polysaccharides of Acetic Acid Bacteria. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 299–320. [Google Scholar]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of Bacterial Cellulose in Food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of Bacterial Cellulose Addition on Physical Properties of WPI Emulsions. Comparison with Common Thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Vigentini, I.; Fabrizio, V.; Dellacà, F.; Rossi, S.; Azario, I.; Mondin, C.; Benaglia, M.; Foschino, R. Set-Up of Bacterial Cellulose Production From the Genus Komagataeibacter and Its Use in a Gluten-Free Bakery Product as a Case Study. Front. Microbiol. 2019, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-Bacterial Cellulose/Soy Protein Isolate Complex Gel as Fat Substitutes in Ice Cream Model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial Cellulose IV. Application to Processed Foods. Food Hydrocoll. 1993, 6, 503–511. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on Production, Characterization and Applications of Microbial Levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Influence of Levan-Producing Acetic Acid Bacteria on Buckwheat-Sourdough Breads. Food Microbiol. 2017, 65, 95–104. [Google Scholar] [CrossRef]
- Han, Y.W. Microbial Levan. Adv. Appl. Microbiol. 1990, 35, 171–194. [Google Scholar] [PubMed]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan Polysaccharide: From a Century of Past Experiences to Future Prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Acetic Acid Bacteria; Sengun, I.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153490. [Google Scholar]
- Srikanth, R.; Siddartha, G.; Sundhar Reddy, C.H.S.S.; Harish, B.S.; Janaki Ramaiah, M.; Uppuluri, K.B. Antioxidant and Anti-Inflammatory Levan Produced from Acetobacter xylinum NCIM2526 and Its Statistical Optimization. Carbohydr. Polym. 2015, 123, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mantovan, J.; Bersaneti, G.T.; Faria-Tischer, P.C.S.; Celligoi, M.A.P.C.; Mali, S. Use of Microbial Levan in Edible Films Based on Cassava Starch. Food Packag. Shelf Life 2018, 18, 31–36. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Dextran Dextrinase and Dextran of Gluconobacter oxydans. J. Ind. Microbiol. Biotechnol. 2005, 32, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Bottari, B.; Morreale, F.; Savo Sardaro, M.L.; Angelino, D.; Raimondi, S.; Rossi, M.; Pellegrini, N. Potential Prebiotic Effect of a Long-Chain Dextran Produced by Weissella cibaria: An in vitro Evaluation. Int. J. Food Sci. Nutr. 2020, 71, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides Enriched in Rare Sugars: Bacterial Sources, Production, and Applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential Applications of Bacterial Cellulose and Its Composites for Cancer Treatment. Int J Biol Macromol 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.M. Porto: Um Vinho Com História. Available online: https://www.ivdp.pt/pt/vinhos/vinhos-do-porto/historia/ (accessed on 1 February 2024).
- Milheiro, J.; Cosme, F.; Filipe-Ribeiro, L.; Nunes, F.M. Port Wine: Production and Ageing. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Jackson, R.S. Specific and Distinctive Wine Styles. Wine Sci. 2008, 520–576. [Google Scholar] [CrossRef]
- Instituto dos Vinhos do Douro e do Porto Processo de Vinificação Ao Longo Dos Tempos. Available online: https://www.ivdp.pt/pt/vinhos/vinhos-do-porto/enologia/ (accessed on 1 February 2024).
- TAYLOR’S Como é Feito o Vinho Do Porto? Available online: https://www.taylor.pt/pt/o-que-e-o-vinho-do-porto/como-e-feito-o-vinho-do-porto (accessed on 1 February 2024).
- Ferreira, V.; Escudero, A.; Fernández, P.; Cacho, J.F. Changes in the Profile of Volatile Compounds in Wines Stored under Oxygen and Their Relationship with the Browning Process. Z. Für Leb. Und -Forsch. A 1997, 205, 392–396. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Falqué, E.; Castro, M.; E Silva, H.O.; Machado, B.; Ferreira, A.C.S. Further Insights into the Floral Character of Touriga Nacional Wines. J. Food Sci. 2007, 72, S396–S401. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Wine Vinegar: Technology, Authenticity and Quality Evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Sellmer-Wilsberg, S. Wine and Grape Vinegars. In Vinegars of the World; Springer: Milano, Italy, 2009; pp. 145–156. [Google Scholar]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Milano, Italy, 2009; ISBN 978-88-470-0865-6. [Google Scholar]
- European Commission Regulation (EU) 2016/263; European Parliament: Brussels, Belgium, 2016.
- Decreto-Lei n.o 174/2007 de 8 de Maio Do Ministério Da Agricultura, Do Desenvolvimento Rural e Das Pescas; Diário da República, 1.a série: Lisboa, Portugal, 2007; pp. 1–3.
- García-García, I.; Santos-Dueñas, I.M.; Jiménez-Ot, C.; Jiménez-Hornero, J.E.; Bonilla-Venceslada, J.L. Vinegar Engineering. In Vinegars of the World; Springer: Milano, Italy, 2009; pp. 97–120. [Google Scholar]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Qiu, J.; Ren, C.; Fan, J.; Li, Z. Antioxidant Activities of Aged Oat Vinegar in Vitro and in Mouse Serum and Liver. J. Sci. Food Agric. 2010, 90, 1951–1958. [Google Scholar] [CrossRef]
- Nanda, K.; Miyoshi, N.; Nakamura, Y.; Shimoji, Y.; Tamura, Y.; Nishikawa, Y.; Uenakai, K.; Kohno, H.; Tanaka, T. Extract of Vinegar “Kurosu” from Unpolished Rice Inhibits the Proliferation of Human Cancer Cells. J. Exp. Clin. Cancer Res. 2004, 23, 69–75. [Google Scholar]
- María Luzón-Quintana, L.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Singh, A.K. Overview of Vinegar Production. Palarch’s J. Archaeol. Egypt/Egyptol. 2020, 17, 4027–4037. [Google Scholar]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of Major Volatile Compounds during the Production of Fruit Vinegars by Static Headspace Gas Chromatography–Mass Spectrometry Method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Dabija, A.; Hatnean, C.A. Study Concerning the Quality of Apple Vinegar Obtained through Classical Method. J. Agroaliment. Process. Technol. 2014, 20, 304–310. [Google Scholar]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á. Bio-Produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Morales, L.; González, G.; Casas, J.; Troncoso, A. Multivariate Analysis of Commercial and Laboratory Produced Sherry Wine Vinegars: Influence of Acetification and Aging. Eur. Food Res. Technol. 2001, 212, 676–682. [Google Scholar] [CrossRef]
- Andreou, V.; Giannoglou, M.; Xanthou, M.Z.; Metafa, M.; Katsaros, G. Aging Acceleration of Balsamic Vinegar Applying Micro-Oxygenation Technique. Food Chem. 2023, 419, 136077. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.; Xing, J.; Chao, J.; Qin, X.; Li, Z. Comparison of Two Types of Vinegar with Different Aging Times by NMR-based Metabolomic Approach. J. Food Biochem. 2019, 43, e12835. [Google Scholar] [CrossRef]
- Li, H.; Ming, X.; Liu, Z.; Xu, L.; Xu, D.; Hu, L.; Mo, H.; Zhou, X. Accelerating Vinegar Aging by Combination of Ultrasonic and Magnetic Field Assistance. Ultrason. Sonochem. 2021, 78, 105708. [Google Scholar] [CrossRef] [PubMed]
- Tonello, M.J. Acetification of Porto Wine. A Preliminary Step for Vinegar Making. Ph.D. Thesis, University of Porto, Porto, Portugal, 2015. [Google Scholar]
- Durán-Guerrero, E.; Castro, R. Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products. Foods 2021, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Torija, M.J.; Mas, A.; Morales, M.L.; Troncoso, A.M. Changes of Volatile Compounds in Wine Vinegars during Their Elaboration in Barrels Made from Different Woods. Food Chem. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Callejón, R.M.; Tesfaye, W.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Volatile Compounds in Red Wine Vinegars Obtained by Submerged and Surface Acetification in Different Woods. Food Chem. 2009, 113, 1252–1259. [Google Scholar] [CrossRef]
- Callejón, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the Typical Aroma of Sherry Vinegar: Sensory and Chemical Approach. J. Agric. Food Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef] [PubMed]
- García-Parrilla, M.C.; González, G.A.; Heredia, F.J.; Troncoso, A.M. Differentiation of Wine Vinegars Based on Phenolic Composition. J. Agric. Food Chem. 1997, 45, 3487–3492. [Google Scholar] [CrossRef]
- Andlauer, W.; Stumpf, C.; Fürst, P. Influence of the Acetification Process on Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; Garcia-Parrilla, M.C.; Troncoso, A.M. Improvement of Wine Vinegar Elaboration and Quality Analysis: Instrumental and Human Sensory Evaluation. Food Rev. Int. 2009, 25, 142–156. [Google Scholar] [CrossRef]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.E.; Ma, T.; Liang, Y.; Qiang, W.; Atuna, R.A.; Amagloh, F.K.; Morata, A.; Han, S. Comparison between Membrane and Thermal Dealcoholization Methods: Their Impact on the Chemical Parameters, Volatile Composition, and Sensory Characteristics of Wines. Membranes 2021, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Pilipovik, M.V.; Riverol, C. Assessing Dealcoholization Systems Based on Reverse Osmosis. J. Food Eng. 2005, 69, 437–441. [Google Scholar] [CrossRef]
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and Reverse Osmosis in Winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- Loyola García, R.; Gutiérrez-Gamboa, G.; Medel-Marabolí, M.; Díaz-Gálvez, I. Lowering Wine Alcohol Content by Reverse Osmosis and Spinning Cone Columns: Effects on Sensory Characteristics of the Beverages. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Gil, M.; Estévez, S.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Partial Dealcoholization by Reverse Osmosis on Red Wine Composition and Sensory Characteristics. Eur. Food Res. Technol. 2013, 237, 481–488. [Google Scholar] [CrossRef]
- Belisario-Sánchez, Y.Y.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Iguaz-Gainza, A.; López-Gómez, A. Aroma Recovery in Wine Dealcoholization by SCC Distillation. Food Bioproc. Tech. 2012, 5, 2529–2539. [Google Scholar] [CrossRef]
- Petrozziello, M.; Panero, L.; Guaita, M.; Prati, R.; Marani, G.; Zinzani, G.; Bosso, A. Effect of the Extent of Ethanol Removal on the Volatile Compounds of a Chardonnay Wine Dealcoholized by Vacuum Distillation. BIO Web Conf. 2019, 12, 02020. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the Physicochemical and Volatile Composition of Wine Fractions Obtained by Two Different Dealcoholization Techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The Use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Scutaraşu, E.C.; Cotea, V.V.; Luchian, C.E.; Colibaba, L.C.; Katalin, N.; Oprean, R.; Niculaua, M. Influence of Enzymatic Treatments on White Wine Composition. BIO Web Conf. 2019, 15, 02032. [Google Scholar] [CrossRef]
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological Approaches to Lowering the Ethanol Yield during Wine Fermentation. Biomolecules 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An Overview of Sensory Characterization Techniques: From Classical Descriptive Analysis to the Emergence of Novel Profiling Methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.S.Q.; Dias, L.G.; Teixeira, A. Emerging Methods for the Evaluation of Sensory Quality of Food: Technology at Service. Curr. Food Sci. Technol. Rep. 2024, 2, 77–90. [Google Scholar] [CrossRef]
- Barbe, J.-C.; Garbay, J.; Tempère, S. The Sensory Space of Wines: From Concept to Evaluation and Description. A review. Foods 2021, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Mehta, A.; Borssato, A.B. New Methods to Assess Sensory Responses: A Brief Review of Innovative Techniques in Sensory Evaluation. Curr. Opin. Food Sci. 2023, 49, 100978. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.M.; Callejón, R.M. Sensory and Spectroscopic Characterization of Argentinean Wine and Balsamic Vinegars: A Comparative Study with European Vinegars. Food Chem. 2020, 323, 126791. [Google Scholar] [CrossRef] [PubMed]
- G. Pereira, A.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; C.F.R. Ferreira, I.; Angel Prieto, M.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. An Influence of Different Yeast Species on Wine Aroma Composition. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–285. [Google Scholar]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Silva, A.P.; Rebelo, J. Port Wine, an Established Product a New Market: A Comparative Analysis of Perceptions of Firms and Consumers. Wine Econ. Policy 2019, 8, 59–68. [Google Scholar] [CrossRef]
- Vilela, A.; Ferreira, R.; Nunes, F.; Correia, E. Creation and Acceptability of a Fragrance with a Characteristic Tawny Port Wine-Like Aroma. Foods 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Gallo Worldwide Vinho de Porto—Vinagre. Available online: https://www.galloportugal.com/vinagres-pt-pt/vinho-do-porto/ (accessed on 7 February 2024).
- Torri, L.; Jeon, S.-Y.; Piochi, M.; Morini, G.; Kim, K.-O. Consumer Perception of Balsamic Vinegar: A Cross-Cultural Study between Korea and Italy. Food Res. Int. 2017, 91, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.-K.; Lee, C.-S.; Chen, Y.-C.; Chiang, M.-C. Exploring Taiwanese Consumer Dietary Preferences for Various Vinegar Condiments: Novel Dietary Patterns across Diverse Cultural Contexts. Nutrients 2023, 15, 3845. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Oliveras, I.; Romero del Castillo, M.R.; Salazar, A. Influence of the Sociocultural Perspective on the Sensory Perception of Wine Consumers in Mexico and Spain. Front. Psychol. 2023, 14, 1171289. [Google Scholar] [CrossRef] [PubMed]
- Fondberg, R.; Lundström, J.N.; Seubert, J. Odor–Taste Interactions in Food Perception: Exposure Protocol Shows No Effects of Associative Learning. Chem. Senses 2021, 46, bjab003. [Google Scholar] [CrossRef]
- Spill, M.K.; Johns, K.; Callahan, E.H.; Shapiro, M.J.; Wong, Y.P.; Benjamin-Neelon, S.E.; Birch, L.; Black, M.M.; Cook, J.T.; Faith, M.S.; et al. Repeated Exposure to Food and Food Acceptability in Infants and Toddlers: A Systematic Review. Am. J. Clin. Nutr. 2019, 109, 978S–989S. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Hemingway, A.; Rajska, J.; Hartwell, H. Repeated Exposure and Conditioning Strategies for Increasing Vegetable Liking and Intake: Systematic Review and Meta-Analyses of the Published Literature. Am. J. Clin. Nutr. 2018, 108, 842–856. [Google Scholar] [CrossRef]
- Schicker, D.; Rramani, Q.; Lim, S.X.L.; Saruco, E.; Pleger, B.; Weber, B.; Schultz, J.; Freiherr, J.; Ohla, K. Taste It! 7-Day Exposure to a Protein-Enriched Milk Drink Increases Its Smell, Taste, and Flavor Familiarity and Facilitates Acquisition of Taste Familiarity of a Novel Protein Drink. Food Qual. Prefer. 2023, 106, 104808. [Google Scholar] [CrossRef]
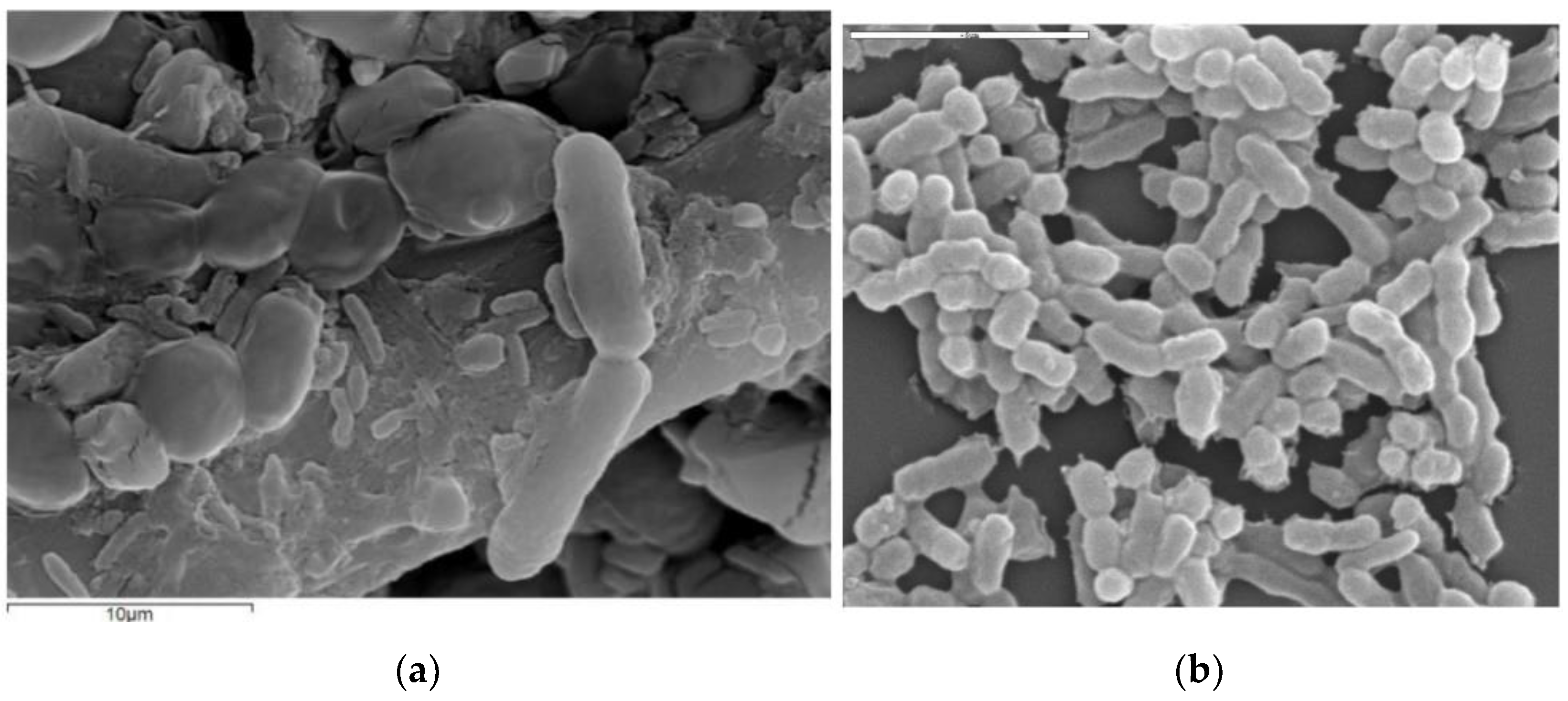

| Year | Samples/Product | AAB Species Identified/Used | Culture Media | Molecular Identification | References |
|---|---|---|---|---|---|
| 2019 | Red pitahaya and physalis fruits to produce vinegar | A. aceti, A. pasteurianus and Gluconobacter oxydans | YEPD medium | - | [37] |
| 2019 | Cocoa pods from Ivory Coast | Acetobacter pasteurianus, Acetobacter tropicalis, Acetobacter okinawensis, Acetobacter ghanensis, Acetobacter malorum and Gluconobacter oxydans | Potato medium | 16S rRNA gene sequence | [38] |
| 2020 | Cider | Acetobacter species | LMG 0404 medium | 16S rRNA gene sequence | [39] |
| 2020 | Korean rice wine vinegar | A. oryzoeni B6T | YPGDE (yeast-extract peptone glucose dextrose ethanol) medium | 16S rRNA gene sequence | [40] |
| 2020 | Soil samples from Wuhan, China | Gluconobacter oxydans FBFS97 | GYC medium | 16S rDNA gene sequence | [41] |
| 2020 | Black tea kombucha | A. indonesiensis, A. papayae, and Komagataeibacter saccharivorans | LAC and Mannitol agar medium | 16S rDNA gene sequence | [42] |
| 2020 | Kombucha | Komagataeibacter sp. DS1MA.62A, Komagataeibacter xylinus, Komagataeibacter saccharivorans, Komagataeibacter xylinus and Gluconacetobacter saccharivorans | NA and PDA medium | 16S rRNA gene sequence | [43] |
| 2021 | Greek finishing side-stream wine to produce vinegar | A. aceti and K. europaeus | The solid medium for A. aceti comprises yeast extract (5 g/L), peptone (3 g/L), mannitol (25 g/L), and agar (12 g/L), while for K. europaeus, it consists of yeast extract (2 g/L), peptone (3 g/L), glucose (5 g/L), agar (10 g/L), with the addition of acetic acid (40 mL/L) and ethanol (30 mL/L) | - | [23] |
| 2021 | Commercial wheat flour | Acetobacter tropicalis A3 | MRS medium | DNA extraction | [44] |
| 2022 | Mature grape berries and vinegar samples from Malta | Komagateibacter spp. (strains G1 and G10), Gluconobacter spp. (strains G21 and G22), and Acetobacter spp. (strain V20) | GYC medium (grapes) AE agar medium (vinegar) | Restriction analysis of amplified 16S rRNA (ARDRA) | [20] |
| 2022 | Repositories (culture collections) | N. chiangmaiensis, Ko. Baliensis, G. cerinus, G. frateurii, G. oxydans, K. xylinus, K. hansenii, A. pasteurianus | GYC medium | - | [45] |
| 2022 | Cupei (brewing mash of Chinese cereal vinegar) | A. pasteurianus CGMCC 3089 and L. helveticus CGMCC 12062 | GY and MRS media | 16S rRNA gene sequence | [46] |
| 2022 | Fruits, juices, honey, and vinegars from biotopes of Morocco | Acetobacter fabarum and Acetobacter pasteurianus | Potato agar, CARR medium, and YPG medium | 16S rDNA gene sequence | [47] |
| 2023 | Cheese whey and olive mill wastewater | K. xylinus and K. rhaeticus | Hestrin-Schramm medium | 16S rRNA gene sequence | [48] |
| 2023 | Korean vinegar starter, plum extract, and wine | A. pasteurianus, A. orientalis, A. cibinongensi, A. pomorum, A. ascendens, A. malorum, and Gluconobacter oxydans | YGCE (yeast-extract glucose calcium carbonate ethanol) and Mannitol agar medium | 16S rRNA gene sequence | [49] |
| 2023 | Korean fruit farm-produced vinegars | A. pasteurianus and A. cerevisiae | YGC (yeast-extract glucose calcium carbonate) agar medium with 2% ethanol | 16S rRNA gene sequence | [50] |
| 2023 | Grapes from the Republic of Moldova at different stages of winemaking | A. aceti and A. pasteurianus | - | Real-Time PCR amplification | [51] |
| 2023 | Commercial Spanish wines | A. aceti, A. oeni, Gluconobacter oxidans, Komagataeibacter | GYC, G2, Kneiffel and Wallerstein medium | Quantitative PCR (qPCR) analysis | [52] |
| 2024 | Sichuan Baoning vinegar | A. pomorum, A. Pasteurianus, A. ghanensis and A. cibinongensis | GYEC medium | 16S rRNA gene sequence | [53] |
| Metabolic Characteristics | Acetobacter | Gluconobacter | References |
|---|---|---|---|
| Metabolic pathway dynamics | Hexose monophosphate pathway, Embden–Meyerhof–Parnas and Entner–Doudoroff pathways | Pentose phosphate | [17,21,34] |
| Major metabolic products | Acetate, lactate, and gluconic acid | Gluconic acid, glucono-δ-lactone, 2-ketogluconate, 2,5-diketogluconate, CO2 | [17,21,34,76] |
| Metabolism of organic acids | Efficient engagement in the tricarboxylic acid (TCA) cycle, culminating in acetate overoxidation dynamic | Deficiencies in key TCA cycle enzymes | [34,54,75] |
| Both genera | |||
| Carbohydrate metabolism | Glucose, arabinose, fructose, galactose, mannose, ribose, sorbose, and xylose | [21,34,70,74] | |
| Polyol metabolism | Glycerol, mannitol, sorbitol, arabitol, erythritol, and meso-erythritol | [21,34,77] | |
| Role in winemaking | Influences aroma and SO2 binding in wine medium through glycerol conversion into dihydroxyacetone (DHA) | [19,21,34,75] | |
| Oxidation of lactate | Oxidizes lactate to acetoin, contributing to metabolic diversity and introducing ‘butter-like’ aromas and flavors reminiscent of spoiled wine into the microbial context | [34,75] | |
| EPS | Chemical Structure | Description | References |
|---|---|---|---|
| Bacterial cellulose | Homopolysaccharide | Linear glucan of glucose monomers linked by β-(1–4) bonds; | [14,18,29,88,89,90,91,92,93] |
| synthesized primarily by species of the Komagataeibacter genus; | |||
| synthesized primarily by species of the Komagataeibacter genus; | |||
| employed as a fat replacer in various food products, including meat, cheese, and ice cream; | |||
| serves as a carrier for enzyme and cell immobilization in food processes. | |||
| Levan | Homopolysaccharide | Polymer structure consisting of D-fructofuranosyl residues linked; by β-(2,6) bonds in the main chain and β-(2,1) bonds in the side chain; | [14,18,29,94,95,96,97,98,99,100] |
| synthesized by Acetobacter, Gluconobacter, and Gluconacetobacter genera; | |||
| exhibits non-Newtonian fluid behavior in solution, adhesive strength, and unique solubility characteristics; | |||
| it improves the rheological properties, textures, and shelf life of various bread types and is used as a fat substitute, stabilizer, and adhesive in food packaging. | |||
| Acetan | Heteropolysaccharide | Microbial polysaccharide with a complex chemical structure composed of glucose monomers linked by α-(1,6) and α-(1,3) glycosidic bonds; | [18,88,98] |
| produced by Acetobacter and related genera within AAB; | |||
| they are used as viscosifiers and emulsifiers in various industries. | |||
| Dextran | Heteropolysaccharide | Microbial polysaccharide composed of glucose monomers linked by α-(1,6) and α-(1,3) glycosidic bonds, resulting in a branched structure; | [18,101,102] |
| produced by certain strains of AAB and other bacteria; | |||
| it is used in various food products to improve their rheological properties, textures, and shelf life. It is also employed as a fat substitute, stabilizer, and emulsifier in food packaging. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Vilela, A. Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation 2024, 10, 200. https://doi.org/10.3390/fermentation10040200
Mota J, Vilela A. Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation. 2024; 10(4):200. https://doi.org/10.3390/fermentation10040200
Chicago/Turabian StyleMota, João, and Alice Vilela. 2024. "Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria" Fermentation 10, no. 4: 200. https://doi.org/10.3390/fermentation10040200
APA StyleMota, J., & Vilela, A. (2024). Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation, 10(4), 200. https://doi.org/10.3390/fermentation10040200