Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagent Preparation
2.2. Ge Enrichment
2.3. Carbon and Nitrogen Source Screening
2.4. Plackett–Burman Experimental Design
2.5. Box–Behnken Test Design
2.6. Determination of Mycelial Biomass
2.7. Determination of Organic Germanium Content
2.7.1. Determination of Total Germanium Content via Sample Digestion
2.7.2. Sample Preparation for Determination of Inorganic Germanium Content
2.7.3. Determination of Germanium Concentration in Samples
2.7.4. Determination of Organic Ge Content
2.8. Determination of Soluble Protein Content
2.9. Determination of Polysaccharide Content
2.10. Preparation of Aqueous Extracts
2.11. Preparation of the Solution for the Determination of Total Reducing Power
2.12. Preparation of the Solution for the Determination of Hydroxyl Scavenging Ability
2.13. Preparation of the Solution for the Determination of Superoxide Anion Free Radical Scavenging Ability
2.14. Preparation of the ABTS Stock Solution
2.15. Preparation of the Solution for the Determination of Immunomodulatory Activity
2.16. Data Statistics and Analysis
3. Results
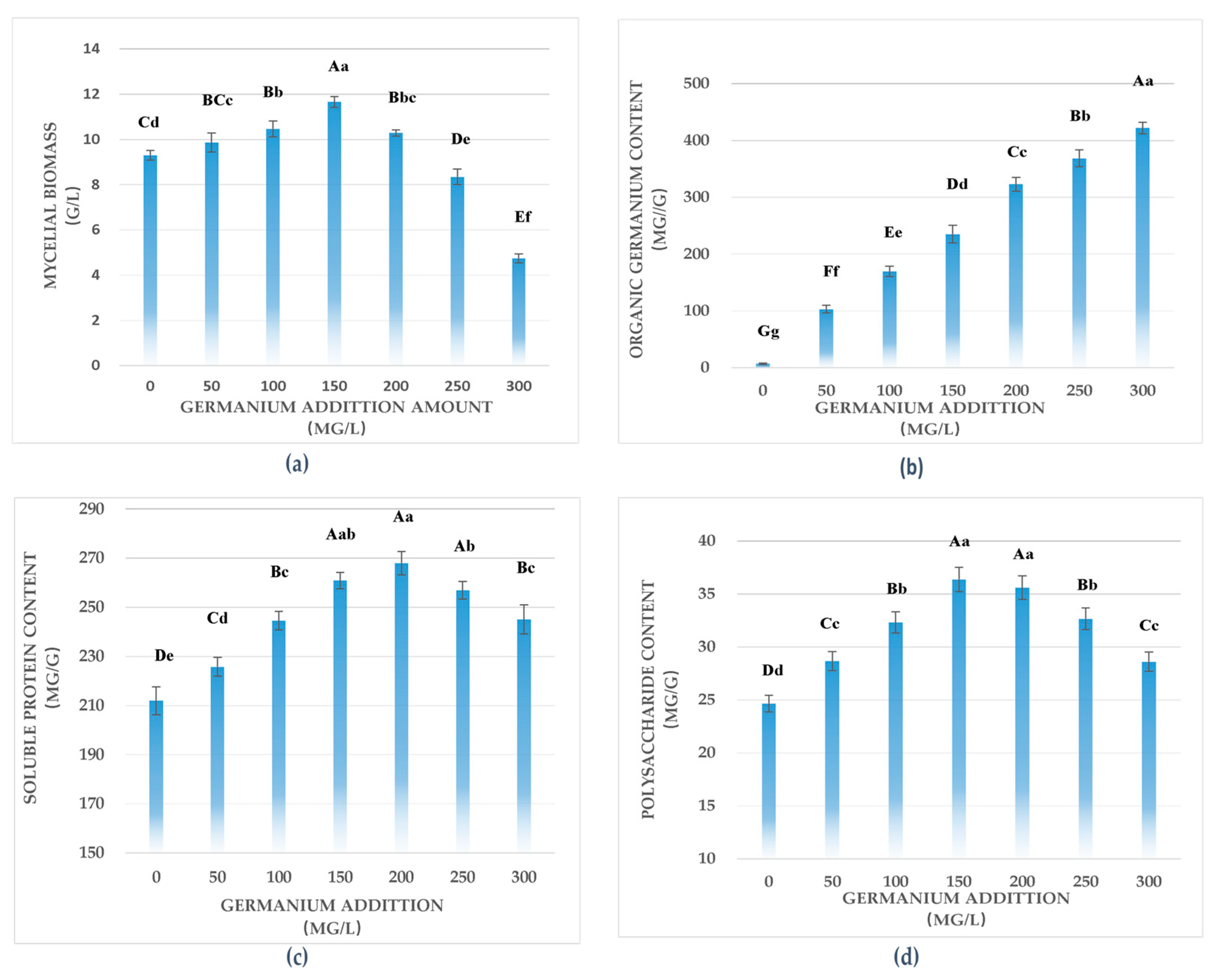
3.1. Effects of Germanium Enrichment on Mycelial Biomass
3.2. Effects of Germanium Enrichment on Organic Ge Content
3.3. Effects of Germanium Enrichment on Soluble Protein Content
3.4. Effects of Germanium Enrichment on Polysaccharide Content
3.5. Screening of Carbon and Nitrogen Sources
3.6. Plackett–Burman Design (PBD) Analysis
3.7. Box–Behnken Design (BBD) Analysis
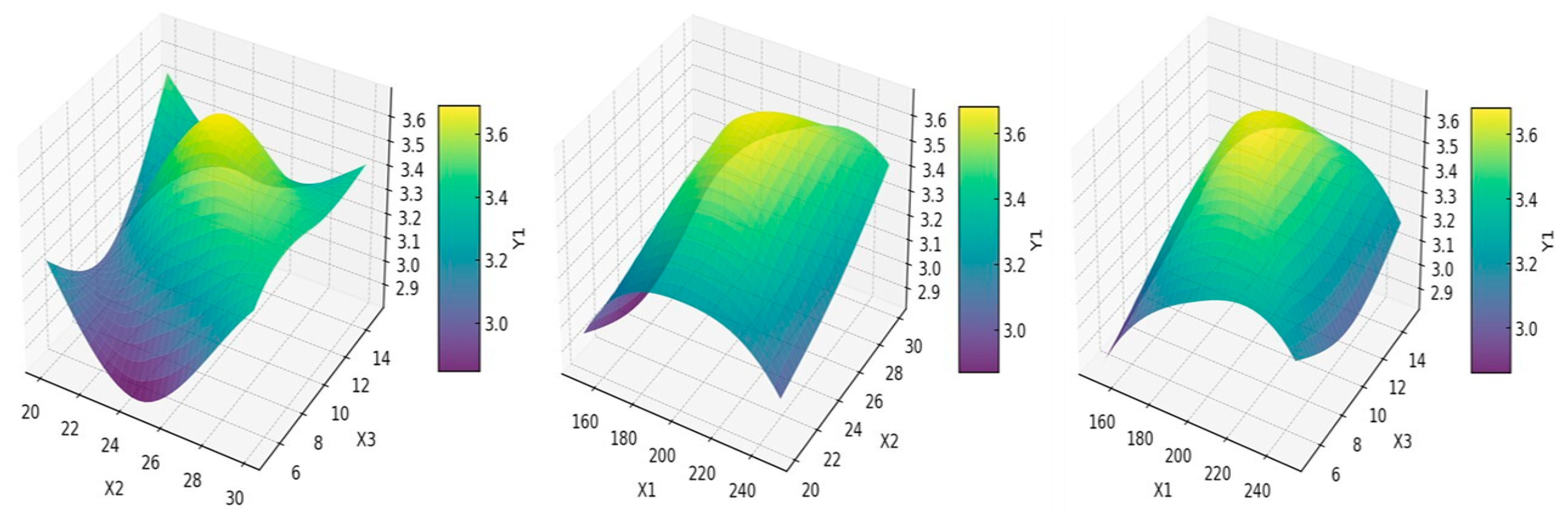
3.8. Three-Dimensional Response Surface Analysis of the Interactions of Various Key Factors
3.9. Model Validation
3.10. Effects on Ferric Reducing Power
3.11. Effects on •OH Radical Scavenging Rate
3.12. Effects on ABTS Free Radical Scavenging Rate
3.13. Effects on Superoxide Anion Free Radical Scavenging Ability
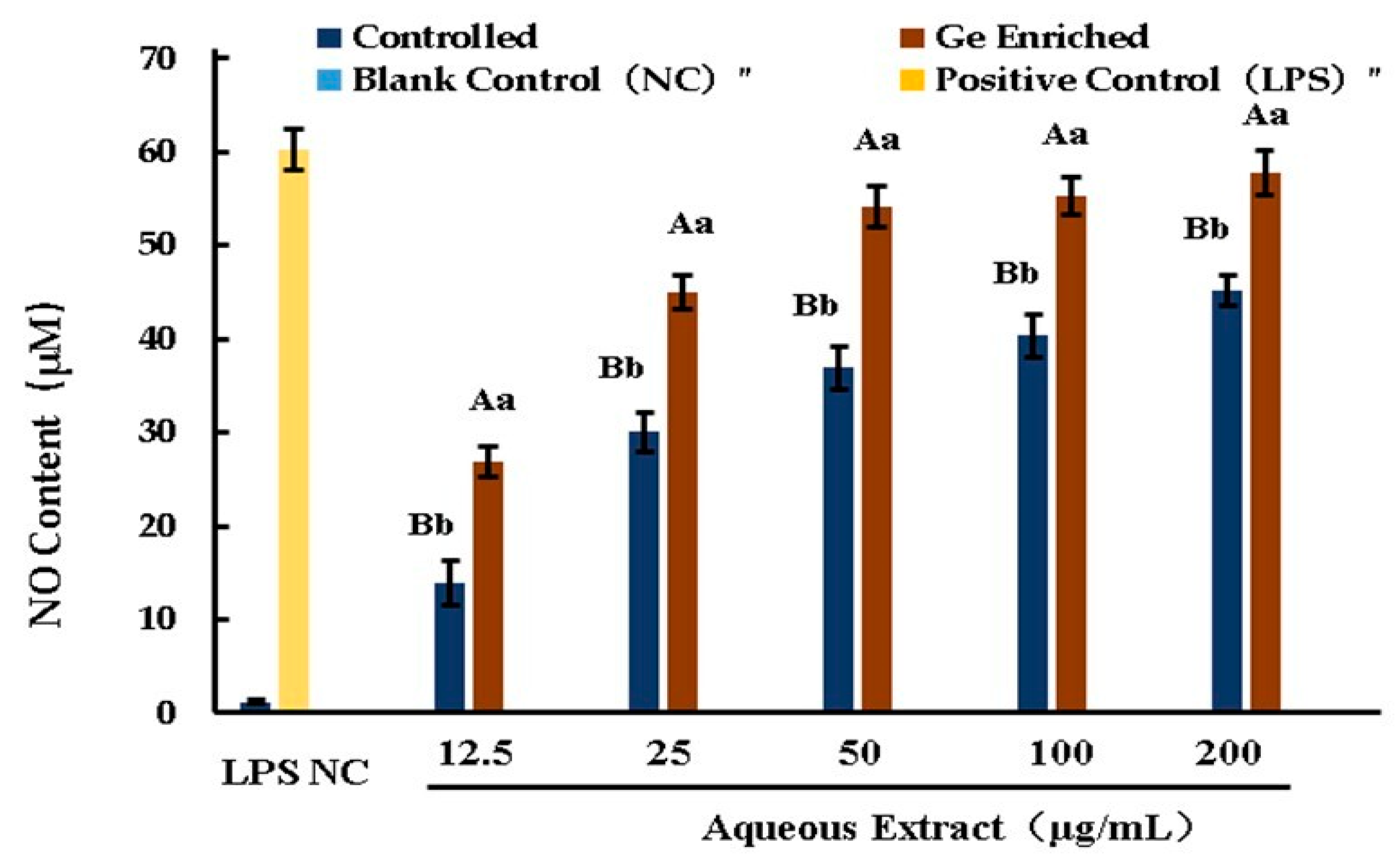
3.14. NO Release in RAW264.7 Cells
3.15. Effects on TNF-α Secretion in RAW264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Qin, J.; Guo, L.; Guo, Q.; Kang, W.; Ma, C.; Chen, L. Anti-inflammatory properties of polysaccharides from edible fungi on health-promotion: A review. Front. Pharmacol. 2024, 15, 1447677. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Itokawa, Y.; Tajima, M.; Ukawa, Y.; Cho, K.H.; Choi, J.S.; Ishida, T.; Gu, Y. Radioprotective Effect of Lyophyllum Decastes and the Effect on Immunological Functions in Irradiated Mice. J. Tradit. Chin. Med. Engl. Ed. 2007, 27, 6. [Google Scholar]
- Chen, X.; Liu, Y.; Guo, W.; Wang, M.; Zhao, J.; Zhang, X.; Zheng, W. The development and nutritional quality of Lyophyllum decastes affected by monochromatic or mixed light provided by light-emitting diode. Front. Nutr. 2024, 11, 1404138. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Hou, Y. Structure identification and biological activities of a new polysaccharide isolated from Lyophyllum decastes (Fr.) sing. Pharmacogn. Mag. 2022, 18, 112–120. [Google Scholar]
- Zhang, G.; Wang, Y.; Qin, C.; Ye, S.; Zhang, F.; Linhardt, R.J.; Zhang, A. Structural characterization of an antioxidant polysaccharide isolated from the fruiting bodies of Lyophyllum decastes. J. Mol. Struct. 2023, 1285, 135507. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lin, H.; Liu, P.; Zhang, F.; Lin, X.; Liang, J.; Tao, Y.; Jiang, Y.; Chen, B. Ultrasonic treatment decreases Lyophyllum decastes fruiting body browning and affects energy metabolism. Ultrason. Sonochem. 2022, 89, 106111. [Google Scholar] [CrossRef]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef]
- Ukawa, Y.; Ito, H.; Hisamatsu, M. Antitumor effects of (1→3)-β-d-glucan and (1→6)-β-d-glucan purified from newly cultivated mushroom, Hatakeshimeji (Lyophyllum decastes Sing.). J. Biosci. Bioeng. 2000, 90, 98–104. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.; Dai, H.; Sun, J.; Ren, J.; Wang, W.; Qiao, S.; Liu, C.; Sun, L.; Liu, S.; et al. Polysaccharides from Lyophyllum decastes reduce obesity by altering gut microbiota and increasing energy expenditure. Carbohydr. Polym. 2022, 295, 119862. [Google Scholar] [CrossRef]
- Laverde, D.; Armiento, S.; Molinaro, A.; Huebner, J.; De Castro, C.; Romero-Saavedra, F. Identification of a capsular polysaccharide from Enterococcus faecium U0317 using a targeted approach to discover immunogenic carbohydrates for vaccine development. Carbohydr. Polym. 2024, 330, 121731. [Google Scholar] [CrossRef] [PubMed]
- Siwulski, M.; Budzyńska, S.; Rzymski, P.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Mleczek, M. The effects of germanium and selenium on growth, metalloid accumulation and ergosterol content in mushrooms: Experimental study in Pleurotus ostreatus and Ganoderma lucidum. Eur. Food Res. Technol. 2019, 245, 1799–1810. [Google Scholar] [CrossRef]
- Patel, M.; Karamalidis, A.K. Germanium: A review of its US demand, uses, resources, chemistry, and separation technologies. Sep. Purif. Technol. 2021, 275, 118981. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Qi, X.; Zhang, S.; Zhi, C.; Meng, H.; Cheng, Z. Effects of exogenous germanium and effective microorganisms on germanium accumulation and nutritional qualities of garlic (Allium sativum L.). Sci. Hortic. 2021, 283, 110114. [Google Scholar] [CrossRef]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant activity of Ge-132, a synthetic organic germanium, on cultured mammalian cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Popov, A.V. Physiological activity of trace element germanium including anticancer properties. Biomedicines 2023, 11, 1535. [Google Scholar] [CrossRef]
- Luo, X.; Sun, J.; Kong, D.; Lei, Y.; Gong, F.; Zhang, T.; Shen, Z.; Wang, K.; Luo, H.; Xu, Y. The role of germanium in diseases: Exploring its important biological effects. J. Transl. Med. 2023, 21, 795. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Kim, S.U.; Seo, D.C.; Chang, N.I.; Lee, J.B.; Park, J.H.; Kim, K.S.; Kim, S.D.; Kim, H.T.; Heo, J.-S. Effect of inorganic and organic germanium treatments on the growth of lettuce (Lactuca sativa). J. Korean Soc. Appl. Biol. Chem. 2009, 52, 389–396. [Google Scholar] [CrossRef]
- Kaiser, S.; Wagner, S.; Moschner, C.; Funke, C.; Wiche, O. Accumulation of germanium (Ge) in plant tissues of grasses is not solely driven by its incorporation in phytoliths. Biogeochemistry 2020, 148, 49–68. [Google Scholar] [CrossRef]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in effect of germanium or germanium compounds on animals—A review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Babakhanyan, M.; Simonyan, K.; Darbinyan, L.; Ghalachyan, L.; Zaqaryan, S.; Gulnazaryan, S.; Hovhannisyan, L. Agricultural and health-related perspectives of Lycium barbarum L. introduction. Electron. J. Nat. Sci. 2024, 42, 4–8. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, S.; Li, Y.; Xu, S.; Shi, G.; Ding, Z. Effect of selenium on mushroom growth and metabolism: A review. Trends Food Sci. Technol. 2021, 118, 328–340. [Google Scholar] [CrossRef]
- Kora, A.J. Nutritional and antioxidant significance of selenium-enriched mushrooms. Bull. Natl. Res. Cent. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- de Souza, D.F.; da Silva, M.d.C.S.; de Souza, T.C.; Rocha, G.C.; Kasuya, M.C.M.; Eller, M.R. Effect of selenium-enriched substrate on the chemical composition, mineral bioavailability, and yield of edible mushrooms. Biol. Trace Elem. Res. 2023, 201, 3077–3087. [Google Scholar] [CrossRef]
- Ogra, Y.; Ishiwata, K.; Ruiz Encinar, J.; Łobiński, R.; Suzuki, K.T. Speciation of selenium in selenium-enriched shiitake mushroom, Lentinula edodes. Anal. Bioanal. Chem. 2004, 379, 861–866. [Google Scholar] [CrossRef]
- Le Tham, X.; Matsuhashi, S.; Kume, T. Growth and fruitbody formation of Ganoderma lucidum on media supplemented with vanadium, selenium and germanium. Mycoscience 1999, 40, 87–92. [Google Scholar] [CrossRef]
- Zhukovetska, O.; Fizer, M.; Mariychuk, R.; Guzenko, O.; Snigur, D. Spectrophotometric and DFT study of Germanium (IV) interaction with 6, 7-dihydroxy-2, 4-diphenyl-1-benzopyran-1-ium bromide in solutions: Room-temperature cloud point extraction and its analytical application. J. Mol. Liq. 2024, 401, 124724. [Google Scholar] [CrossRef]
- Rautenberger, R. Germanium dioxide as agent to control the biofouling diatom Fragilariopsis oceanica for the cultivation of Ulva fenestrata (Chlorophyta). Bot. Mar. 2024, 67, 93–100. [Google Scholar] [CrossRef]
- Abibu, W.A.; Karapinar, I. Effect of metals on simultaneous ABE fermentation and biohydrogen production from fig (Ficus carica) via Plackett-Burman experimental design. Biomass Convers. Biorefin. 2024, 14, 1305–1315. [Google Scholar] [CrossRef]
- Lima, E.P.; Gonçalves, G.V.; Souza, M.K.; Silva, F.L.; Silva, S.M.; Fook, M.V. Optimizing Sr-Doped CaSiO3 Synthesis for Enhanced Bioceramic Properties: A Plackett–Burman Approach. Ceram. Int. 2024, 50, 29233–29243. [Google Scholar] [CrossRef]
- Larabi, O.; Amara-Rekkab, A.; Didi, A.; Didi, M.A. Plackett-Burman and Box-Wilson designs for the removal of mercury (II) by cypress pines and leaves, optimizing the surrounding conditions. Desalination Water Treat. 2024, 318, 100342. [Google Scholar] [CrossRef]
- Aziri, S.; Bozetine, H.; Meziane, S.; Allam, D.; Berkane, N.; Bakri, R.; Amrane, A. Screening and optimization of the most influencing factors during the photodegradation of Rhodamine B by zinc oxide photocatalyst: Application of Plackett–Burman and central composite designs. React. Kinet. Mech. Catal. 2024, 137, 2395–2414. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, T.; Hou, J.; Yu, H.; Wang, X.; Gao, X.; Kang, L.; Li, L.; Liu, P.; Xu, L. Box-Behnken response surface method to optimize the liquid fermentation culture medium of lotus leaf aphrodisiac. J. Qingdao Agric. Univ. Nat. Sci. Ed. 2023, 40, 182–187. [Google Scholar]
- Pendón, M.D.; Zapiola, J.M.; Mañana, B.; Rumbo, M.; Garrote, G.L. Obtention of pelleted Kluyveromyces marxianus CIDCA 9121 with immunomodulatory properties: Selection of protective agents using Plackett–Burman and Box–Behnken experimental designs. Food Biosci. 2024, 60, 104284. [Google Scholar] [CrossRef]
- Bouzid, T.; Grich, A.; Naboulsi, A.; Regti, A.; El Himri, M.; El Haddad, M. Optimizing Eriochrome Black T adsorption through In-Situ polymerization of Poly (aniline-co-formaldehyde) on biochar: Multivariate approach using full factorial Design, Box-Behnken, AI, and DFT. Sep. Purif. Technol. 2024, 351, 128107. [Google Scholar] [CrossRef]
- El-Desouky, M.G.; Alayyafi, A.A.; Al-Hazmi, G.A.; El-Bindary, A.A. Effect of metal organic framework alginate aerogel composite sponge on adsorption of tartrazine from aqueous solutions: Adsorption models, thermodynamics and optimization via Box-Behnken design. J. Mol. Liq. 2024, 399, 124392. [Google Scholar] [CrossRef]
- Ahmad, E.A.; Dhamra, M.Y. Coomassie brilliant blue staining used in spectrophotometric assay for dopamine hydrochloride and methyldopa determination. Kim. Probl. 2024, 22, 52–67. [Google Scholar] [CrossRef]
- Abou-Melha, K.S. Effective elimination of Coomassie Brilliant Blue dye from aqueous solutions using Cerium Metal-Organic Frameworks: Synthesis, characterization, and optimization of adsorption process utilizing Box-Behnken design. J. Water Process Eng. 2024, 63, 105406. [Google Scholar] [CrossRef]
- Zhu, L.; Guan, L.; Wang, K.; Ren, C.; Gao, Y.; Li, J.; Yan, S.; Zhang, X.; Yao, X.; Zhou, Y. Recent trends in extraction, purification, structural characterization, and biological activities evaluation of Perilla frutescens (L.) Britton polysaccharide. Front. Nutr. 2024, 11, 1359813. [Google Scholar] [CrossRef]
- Ye, S.; Gao, Y.; Hu, X.; Cai, J.; Sun, S.; Jiang, J. Research progress and future development potential of Flammulina velutipes polysaccharides in the preparation process, structure analysis, biology, and pharmacology: A review. Int. J. Biol. Macromol. 2024, 267, 131467. [Google Scholar] [CrossRef]
- Ji, R.; Wang, Z.; Kuang, H. Extraction, purification, structural characterization, and biological activity of polysaccharides from Schisandra chinensis: A review. Int. J. Biol. Macromol. 2024, 271, 132590. [Google Scholar] [CrossRef] [PubMed]
- Tabtimmai, L.; Jongruksavongkul, C.; Wisetsai, A.; Sonklin, C.; Aiamsung, M.; Chamsodsai, P.; Choowongkomon, K.; Sedtananun, S. Three-phase partitioning technique for the green separation of crude polysaccharides from Schizophyllum commune and its effect on macrophage activation. Food Biosci. 2024, 58, 103735. [Google Scholar] [CrossRef]
- Jana, G.; Sing, S.; Das, A.; Basu, A. Interaction of food colorant indigo carmine with human and bovine serum albumins: A multispectroscopic, calorimetric, and theoretical investigation. Int. J. Biol. Macromol. 2024, 259, 129143. [Google Scholar] [CrossRef] [PubMed]
- Tworek, P.; Rakowski, K.; Szota, M.; Lekka, M.; Jachimska, B. Changes in Secondary Structure and Properties of Bovine Serum Albumin as a Result of Interactions with Gold Surface. ChemPhysChem 2024, 25, e202300505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-L.; Wang, W.-J.; Hu, Z.-Y.; Zhang, R.-J.; Shi, J.-H. Comprehending the intermolecular interaction of JAK inhibitor fedratinib with bovine serum albumin (BSA)/human alpha-1-acid glycoprotein (HAG): Multispectral methodologies and molecular simulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123277. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, S.; Wang, L.; Niu, D.; Gao, W.; Zeng, X.-A.; Woo, M.; Han, Z.; Wang, R. Pulsed electric field enhances glucose glycation and emulsifying properties of bovine serum albumin: Focus on polarization and ionization effects at a high reaction temperature. Int. J. Biol. Macromol. 2024, 257, 128509. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef]
- Qiu, L.; Li, X.; Xu, D.; Shao, D.; Du, G.; Deng, S. Comparison of the corrosion inhibition property on cold rolled steel in sulfuric acid media between reflux and ultrasound extracts from rapeseed meal. Ind. Crops Prod. 2024, 216, 118809. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, R.; Huang, G.; Ou, Z.; Shen, Z. Effects of honokiol combined with resveratrol on bacteria responsible for oral malodor and their biofilm. J. Oral Microbiol. 2024, 16, 2361402. [Google Scholar] [CrossRef]
- Heckmann, M.; Stadlbauer, V.; Drotarova, I.; Gramatte, T.; Feichtinger, M.; Arnaut, V.; Atzmüller, S.; Schwarzinger, B.; Röhrl, C.; Blank-Landeshammer, B. Identification of oxidative-stress-reducing plant extracts from a novel extract library—Comparative analysis of cell-free and cell-based in vitro assays to quantitate antioxidant activity. Antioxidants 2024, 13, 297. [Google Scholar] [CrossRef]
- Dubnov, G.; Kohen, R.; Berry, E.M. Diet restriction in mice causes differential tissue responses in total reducing power and antioxidant compounds. Eur. J. Nutr. 2000, 39, 18–30. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.P.; Shetty, K. Phenolic antioxidant mobilization during yogurt production from soymilk using Kefir cultures. Process Biochem. 2005, 40, 1791–1797. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, J.-J.; Zhou, N.; Zhang, R.; Fang, L.; Yang, Y.; Gao, X.; Chen, C.; Yan, X.; Fan, K. A natural biogenic nanozyme for scavenging superoxide radicals. Nat. Commun. 2024, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Liu, Y.; Yang, H.; Yan, X.; Zhang, Y.; Zhong, Z. Preparation, characterization, antioxidant, and antifungal activity of phenyl/indolyl-acyl chitooligosaccharides. Carbohydr. Res. 2024, 538, 109077. [Google Scholar] [CrossRef]
- Yan, H.; Hou, W.; Lei, B.; Liu, J.; Song, R.; Hao, W.; Ning, Y.; Zheng, M.; Guo, H.; Pan, C. Ultrarobust stable ABTS radical cation prepared using Spore@ Cu-TMA biocomposites for antioxidant capacity assay. Talanta 2024, 276, 126282. [Google Scholar] [CrossRef]
- Minarti, M.; Ariani, N.; Megawati, M.; Hidayat, A.; Hendra, M.; Primahana, G.; Darmawan, A. Potential Antioxidant Activity Methods DPPH, ABTS, FRAP, Total Phenol and Total Flavonoid Levels of Macaranga hypoleuca (Reichb. f. & Zoll.) Leaves Extract and Fractions. In Proceedings of the E3S Web of Conferences, Beijing, China, 14–15 October 2024; p. 07005. [Google Scholar]
- Santos, F.H.; Ferreira, D.C.; Matheus, J.R.; Fai, A.E.; Pelissari, F.M. Antioxidant Activity Assays for Food Packaging Materials. In Food Packaging Materials: Current Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 293–309. [Google Scholar]
- Bekdeser, B.; Apak, R. Colorimetric Sensing of Antioxidant Capacity via Auric Acid Reduction Coupled to ABTS Oxidation. ACS Omega 2024, 9, 11738–11746. [Google Scholar] [CrossRef]
- Rod-In, W.; You, S.; Park, W.J.; Surayot, U. Suaeda maritima polysaccharides attenuate LPS-induced inflammation of RAW264. 7 cells and antioxidative activity. Int. Immunopharmacol. 2024, 137, 112482. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, X.; Li, T.; Song, M.; Wang, S.; Wen, T.; Zhu, Z. Structural characterization of polysaccharides from Dictyophora rubrovolvata mycelium and their immunostimulatory activity in RAW264. 7 cells. Process Biochem. 2024, 139, 22–32. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Sabier, M.; Si, J.; Wang, P.; Shen, Y.; Zhang, X.; Liu, J. Guidelines for the in vitro determination of anti-inflammatory activity. eFood 2024, 5, e160. [Google Scholar] [CrossRef]
- Mares, L.C.A.; Alcazar, M.; del Carmen Lugo, E.; Mojica, L.; Velázquez, D. Phenolic Content and Antioxidant Potential of Cocoa Beans and Pod Husk From Three Endemic Varieties of South Mexico. Curr. Dev. Nutr. 2024, 8, 102600. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, J.; Wan, Q.; Wu, Y.; Wu, W.; Chen, Y. Chemerin promotes invasion of oral squamous cell carcinoma by stimulating IL-6 and TNF-α production via STAT3 activation. Mol. Biol. Rep. 2024, 51, 436. [Google Scholar] [CrossRef] [PubMed]
- Lalhminghlui, K.; Jagetia, G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA 2018, 4, FSO272. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Grabowska, A.; Chain, B. Nitric oxide up-regulates the release of inflammatory mediators by mouse macrophages. Eur. J. Immunol. 1995, 25, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y.; Li, W.; Qiu, Y.; Hua, C.; Zhang, Y.; Guo, Z.; Xie, Z. Immunomodulatory activity and active mechanisms of a low molecular polysaccharide isolated from Lanzhou lily bulbs in RAW264. 7 macrophages. J. Funct. Foods 2022, 92, 105071. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, G.; Hui, B.; Zhao, Z.; Tong, J.; Hu, X. Effect of selenium on increasing the antioxidant activity of protein extracts from a selenium-enriched mushroom species of the Ganoderma Genus. J. Food Sci. 2004, 69, FCT184–FCT188. [Google Scholar] [CrossRef]
- Ali, A.; Mac Dionys Rodrigues da Costa, E.; Magalhães, P.; Martins, A.M.C. Biological importance of vitamins and minerals. In Nutraceuticals: A Holistic Approach to Disease Prevention; De Gruyter: Berlin, Germany, 2024; p. 63. [Google Scholar]



| Factor | Coding | Level | |
|---|---|---|---|
| +1 | −1 | ||
| Ge addition amount (mg/kg) | X1 | 250 | 150 |
| Potato powder concentration (g/L) | X2 | 30 | 20 |
| Peptone concentration (g/ L) | X3 | 15 | 5 |
| Glucose concentration (g/L) | X4 | 25 | 18 |
| Potassium dihydrogen phosphate concentration (g/L) | X5 | 2 | 1.5 |
| Magnesium sulfate concentration (g/L) | X6 | 2 | 1 |
| Inoculation volume (block/bottle) | X7 | 5 | 3 |
| Initial pH of culture medium | X8 | 7 | 6 |
| Shaker speed (r/min) | X9 | 150 | 120 |
| Culture temperature (°C) | X10 | 25 | 23 |
| Level | Factor | ||
|---|---|---|---|
| Ge Oxide Addition Amount (mg/L) | Potato Extract Powder Concentration (mg/kg) | Peptone Concentration (g/L) | |
| −1 | 100 | 10 | 5 |
| 0 | 150 | 20 | 10 |
| +1 | 200 | 30 | 15 |
| Index | Biomass (g/L) | Organic Ge Content (μg/g) |
|---|---|---|
| Glucose | 11.11 ± 0.20 Aa | 263.42 ± 6.31 Aa |
| Sucrose | 10.12 ± 0.27 Bb | 240.00 ± 6.38 Bb |
| Soluble starch | 9.58 ± 0.42 Bb | 233.90 ± 8.74 Bb |
| Index | Biomass (g/L) | Organic Ge Content (μg/g) |
|---|---|---|
| Peptone | 11.20 ± 0.35 Aa | 265.83 ± 7.73 Aa |
| Yeast extract powder | 10.84 ± 0.20 Aa | 250.92 ± 5.45 Abb |
| Soy flour | 10.42 ± 0.29 Bb | 248.48 ± 6.31 BC |
| Serial # | Factor | Response | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 mg/L | X2 g/L | X3 g/L | X4 g/L | X5 g/L | X6 g/L | X7 | X8 | X9 r/min | X10 °C | Y mg/L | |
| 1 | 250 | 30 | 5 | 25 | 2 | 2 | 3 | 6 | 120 | 25 | 3.12 ± 0.12 |
| 2 | 150 | 30 | 15 | 18 | 2 | 2 | 5 | 6 | 120 | 23 | 2.95 ± 0.13 |
| 3 | 250 | 20 | 15 | 25 | 1.5 | 2 | 5 | 7 | 120 | 23 | 3.32 ± 0.08 |
| 4 | 150 | 30 | 5 | 25 | 2 | 1 | 5 | 7 | 150 | 23 | 2.80 ± 0.15 |
| 5 | 250 | 20 | 15 | 18 | 2 | 2 | 3 | 7 | 150 | 25 | 3.36 ± 0.13 |
| 6 | 150 | 20 | 5 | 25 | 1.5 | 2 | 5 | 6 | 150 | 25 | 2.49 ± 0.16 |
| 7 | 250 | 20 | 5 | 18 | 2 | 1 | 5 | 7 | 120 | 25 | 3.21 ± 0.14 |
| 8 | 150 | 30 | 5 | 18 | 1.5 | 2 | 3 | 7 | 150 | 23 | 2.59 ± 0.12 |
| 9 | 250 | 30 | 15 | 18 | 1.5 | 1 | 5 | 6 | 150 | 25 | 3.45 ± 0.13 |
| 10 | 150 | 30 | 15 | 25 | 1.5 | 1 | 3 | 7 | 120 | 25 | 2.92 ± 0.20 |
| 11 | 250 | 20 | 15 | 25 | 2 | 1 | 3 | 6 | 150 | 23 | 3.34 ± 0.12 |
| 12 | 150 | 20 | 5 | 18 | 1.5 | 1 | 3 | 6 | 120 | 23 | 2.49 ± 0.11 |
| Serial Number | X1 | X2 | X3 | Y1 (mg/L) |
|---|---|---|---|---|
| 1 | 200 | 25 | 10 | 3.68 ± 0.18 |
| 2 | 200 | 20 | 5 | 3.25 ± 0.15 |
| 3 | 150 | 25 | 5 | 2.83 ± 0.09 |
| 4 | 200 | 25 | 10 | 3.67 ± 0.08 |
| 5 | 200 | 25 | 10 | 3.60 ± 0.11 |
| 6 | 250 | 25 | 5 | 3.18 ± 0.15 |
| 7 | 200 | 30 | 15 | 3.42 ± 0.12 |
| 8 | 250 | 30 | 10 | 3.42 ± 0.20 |
| 9 | 150 | 25 | 15 | 3.12 ± 0.18 |
| 10 | 250 | 20 | 10 | 3.03 ± 0.12 |
| 11 | 150 | 20 | 10 | 2.96 ± 0.14 |
| 12 | 150 | 30 | 10 | 2.97 ± 0.09 |
| 13 | 250 | 25 | 15 | 3.19 ± 0.15 |
| 14 | 200 | 20 | 15 | 3.52 ± 0.21 |
| 15 | 200 | 25 | 10 | 3.63 ± 0.15 |
| 16 | 200 | 30 | 5 | 3.37 ± 0.21 |
| 17 | 200 | 25 | 10 | 3.63 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.; Zhao, J.; Tahir, S.; Khan, I.; Yang, G.; Zhao, B. Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity. Fermentation 2024, 10, 641. https://doi.org/10.3390/fermentation10120641
Hassan SS, Zhao J, Tahir S, Khan I, Yang G, Zhao B. Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity. Fermentation. 2024; 10(12):641. https://doi.org/10.3390/fermentation10120641
Chicago/Turabian StyleHassan, Syed Shaheer, Jinyan Zhao, Sana Tahir, Ilyas Khan, Guang Yang, and Bo Zhao. 2024. "Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity" Fermentation 10, no. 12: 641. https://doi.org/10.3390/fermentation10120641
APA StyleHassan, S. S., Zhao, J., Tahir, S., Khan, I., Yang, G., & Zhao, B. (2024). Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity. Fermentation, 10(12), 641. https://doi.org/10.3390/fermentation10120641






