Antibacterial Efficacy of Feline-Derived Lactic Acid Bacteria against Enteropathogenic Escherichia coli: A Comprehensive In Vitro Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and LAB Isolation
2.2. Preliminary Identification of Strains
2.2.1. Morphological Characteristics
2.2.2. Physiological Experiments
2.2.3. 16 S rRNA Gene Analysis
2.3. Screening of LAB with Strong Antibacterial Activity against EPEC
2.4. Physiological and Biochemical Characteristics of Selected LAB Isolates
2.5. Hydrophobicity and Auto-Aggregation Ability of Selected LAB Isolates
2.6. Survival of Representative Strains in GI Fluids
2.7. Safety Evaluation
2.7.1. Hemolytic Activity
2.7.2. Antibiotic Susceptibility
2.7.3. PCR Screening of Strains for Virulence Factors, Biogenic Amines, and Antibiotic Resistance Genes
2.8. Broad-Spectrum Antibacterial Activity and Antibacterial Substances
2.8.1. Broad-Spectrum Antibacterial Activity
2.8.2. Antimicrobial Substance Exploration
2.8.3. Growth Curve and Acid Production Curve
2.8.4. Determination of Organic Acid Content by High-Performance Liquid Chromatography (HPLC)
2.9. Statistical Analyses
3. Results
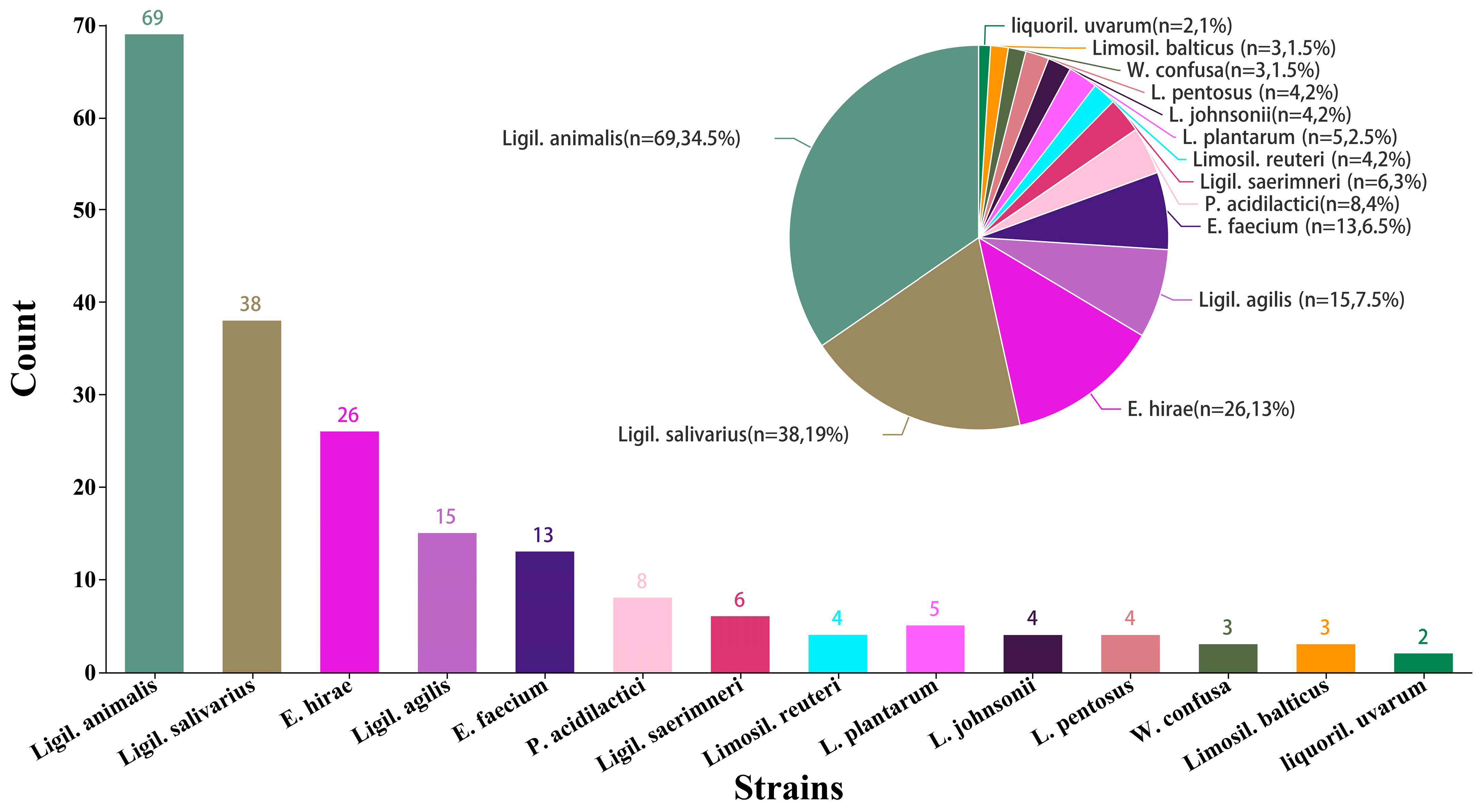
3.1. Isolation and Screening of Lactic Acid Bacteria in Healthy Cat Feces
3.2. Inhibitory Activity against EPEC of LAB Strains Isolated
3.3. Physiological and Biochemical Characteristics
3.4. Cell Surface Hydrophobicity and Auto-Aggregation Ability of Selected LAB Isolates
3.5. 16S DNA Gene Sequence Analysis of Selected LAB Isolates
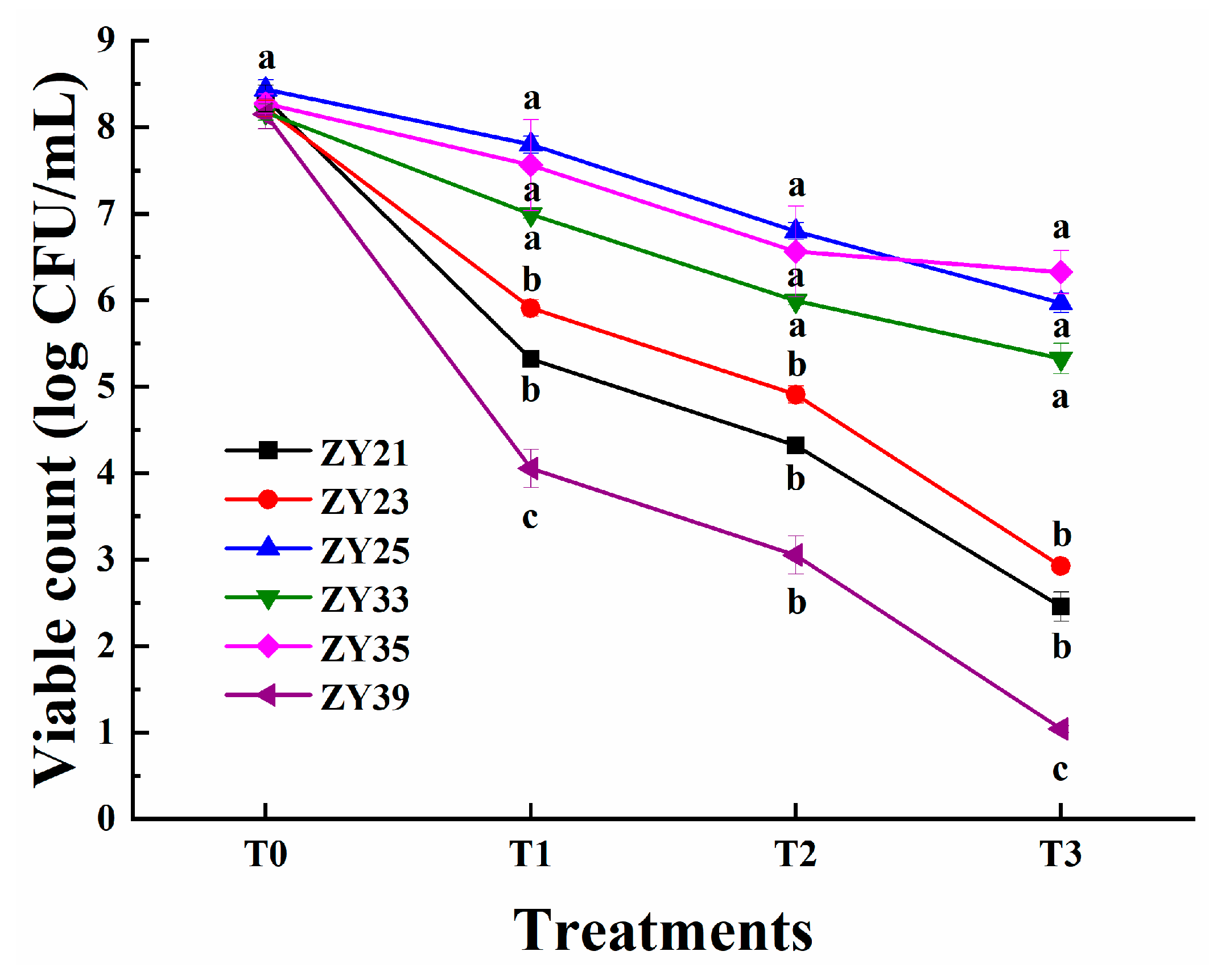
3.6. Resistance to Simulated GIT Conditions of Selected LAB Isolates
3.7. Safety Evaluation of Selected LAB Isolates
3.7.1. Hemolytic Activity of ZY25, ZY33, and ZY35
3.7.2. Profiles of Antibiotic Susceptibility of ZY25 and ZY35
3.7.3. Virulence Factor, Biogenic Amine, and Antibiotic Resistance Genes of ZY25 and ZY35
3.8. Broad-Spectrum Antibacterial Activity and Antibacterial Substances of ZY25 and ZY35
3.8.1. Antibacterial Spectra of ZY25 and ZY35
3.8.2. The Influences of pH, Protease, and Catalase on Antimicrobial Activity
3.8.3. Growth Curves and Acid Production Capacity of ZY25 and ZY35
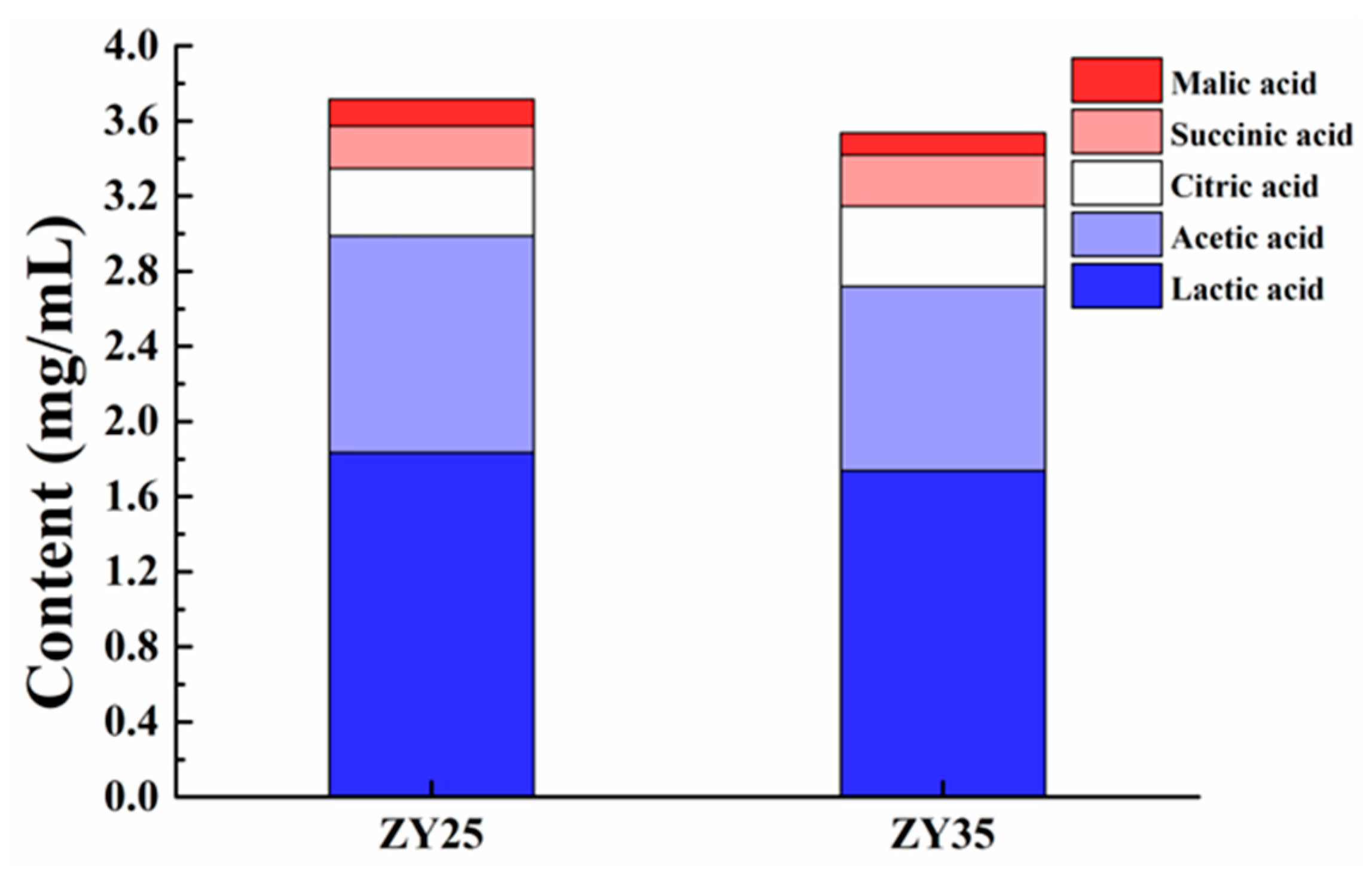
3.8.4. Organic Acid Produced by Fermentation of ZY25 and ZY35
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Watson, V.; Jacob, M.; Flowers, J.; Strong, S.; DebRoy, C.; Gookin, J. Association of atypical enteropathogenic Escherichia coli with diarrhea and related mortality in kittens. J. Clin. Microbiol. 2017, 55, 2719–2735. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, A.; Carr, A.; Gaunt, M. Enteropathogenic Escherichia coli (EPEC) infection in association with acute gastroenteritis in 7 dogs from Saskatchewan. Can. Vet. J. 2016, 57, 964–968. [Google Scholar]
- Oliva, C.; Scaletsky, I.; Morais, M.; Neto, U. Severe acute diarrhea associated to classic enteropathogenic Escherichia coli (EPEC): Clinical features and fecal losses in hospitalized infants. Rev. Assoc. Med. Bras. 1997, 43, 283–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Legge, S.; Taggart, P.; Dickman, C.; Read, J.; Woinarski, J. Cat-dependent diseases cost Australia AU$6 billion per year through impacts on human health and livestock production. Wildl. Res. 2020, 47, 731–746. [Google Scholar] [CrossRef]
- Spitznagel, M.; Cox, M.; Jacobson, D.; Albers, A.; Carlson, M. Assessment of caregiver burden and associations with psychosocial function, veterinary service use, and factors related to treatment plan adherence among owners of dogs and cats. J. Am. Vet. Med. Assoc. 2019, 254, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S. Atypical enteropathogenic Escherichia coli: From kittens to humans and beyond! Infect. Immun. 2021, 89, e00752-20. [Google Scholar] [CrossRef]
- Jafari, E.; Mostaan, S.; Bouzari, S. Characterization of antimicrobial susceptibility, extended-spectrum β-lactamase genes and phylogenetic groups of enteropathogenic Escherichia coli isolated from patients with diarrhea. Osong Public Health Res. Perspect. 2020, 11, 327. [Google Scholar] [CrossRef]
- Yuan, L.; Chu, B.; Chen, S.; Li, Y.; Liu, N.; Zhu, Y.; Zhou, D. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms 2021, 9, 2363. [Google Scholar] [CrossRef]
- Wang, W.; Ma, H.; Yu, H.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Screening of Lactobacillus plantarum subsp. plantarum with Potential Probiotic Activities for Inhibiting ETEC K88 in Weaned Piglets. Molecules 2020, 25, 4481. [Google Scholar] [CrossRef]
- Fernandes, M.; Rios, J.; Vasconcelos, B.; Lourenço, M.; Matos, M.; Cavalcante, R.; de Almeida, M.V.A.; Costa, R.A.; Carneiro, V. Effect of Lactobacillus spp. cell-free supernatant against planktonic growth and biofilm formation of foodborne Escherichia coli isolates. Lett. Appl. Microbiol. 2023, 76, 1. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Krauss, H.; Ciechelska-Rybarczyk, A.; Bernatek, M.; Wojtyla-Buciora, P.; Sommermeyer, H. In-Vitro Growth Inhibition of Bacterial Pathogens by Probiotics and a Synbiotic: Product Composition Matters. Int. J. Environ. Res. Public Health 2020, 17, 3332. [Google Scholar] [CrossRef] [PubMed]
- Tarkhani, R.; Imani, A.; Hoseinifar, S.H.; Ashayerizadeh, O.; Moghanlou, K.S.; Manaffar, R.; Van Doan, H.; Reverter, M. Comparative study of host-associated and commercial probiotic effects on serum and mucosal immune parameters, intestinal microbiota, digestive enzymes activity and growth performance of roach (Rutilus rutilus caspicus) fingerlings. Fish Shellfish Immunol. 2020, 98, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Buhnik-Rosenblau, K.; Matsko-Efimov, V.; Jung, M.; Shin, H.; Danin-poleg, Y.; Kashi, Y. Indication for co-evolution of Lactobacillus johnsonii with its hosts. BMC Microbiol. 2012, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Patel, M.; Hadi, S. Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 2017, 5, e3085. [Google Scholar] [CrossRef] [PubMed]
- Sirichokchatchawan, W.; Pupa, P.; Praechansri, P.; Am-In, N.; Tanasupawat, S.; Sonthayanon, P.; Prapasarakul, N. Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb. Pathog. 2018, 119, 208–215. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Cui, M.; Wang, Y.; Jiao, Z.; Tan, Z. Ensilage of oats and wheatgrass under natural alpine climatic conditions by indigenous lactic acid bacteria species isolated from high-cold areas. PLoS ONE 2018, 13, e0192368. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Tan, Z.; Li, Z.; Jiao, Z.; Huang, Q. Screening of probiotic activities of Lactobacilli strains isolated from traditional Tibetan Qula, a raw yak milk cheese. Anim. Biosci. 2016, 29, 1490–1499. [Google Scholar] [CrossRef]
- Niu, K.M.; Kothari, D.; Cho, S.B.; Han, S.G.; Song, I.G.; Kim, S.C.; Kim, S.K. Exploring the probiotic and compound feed fermentative applications of Lactobacillus plantarum SK1305 isolated from Korean green chili pickled pepper. Probiotics Antimicrob. Proteins. 2019, 11, 801–812. [Google Scholar] [CrossRef]
- De Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Ni, K.; Yang, H.; Hua, W.; Wang, Y.; Pang, H. Selection and characterisation of lactic acid bacteria isolated from different origins for ensiling Robinia pseudoacacia and Morus alba L. leaves. J. Integr. Agric. 2016, 15, 2353–2362. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Babar, A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 2. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Lima, N.C.S.; Taborda, R.L.M.; Esquerdo, R.P.; Gama, A.; Nogueira, P.; Orlandi, P.; Matos, N.B. Antibiotic resistance and biofilm formation in children with Enteropathogenic Escherichia coli (EPEC) in Brazilian Amazon. J. Infect. Dev. Ctries. 2019, 13, 698–705. [Google Scholar] [CrossRef]
- Shen, J.; Zhi, S.; Guo, D.; Jiang, Y.; Xu, X.; Zhao, L.; Lv, J. Prevalence, antimicrobial resistance, and whole genome sequencing analysis of Shiga toxin-producing Escherichia coli (STEC) and Enteropathogenic Escherichia coli (EPEC) from imported foods in China during 2015–2021. Toxins 2022, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Ghane, M.; Babaeekhou, L.; Ketabi, S.S. Antibiofilm Activity of Kefir Probiotic Lactobacilli against Uropathogenic Escherichia coli (UPEC). Avicenna J. Med. Biotechnol. 2020, 12, 221–229. [Google Scholar] [PubMed]
- Piatek, J.; Sommermeyer, H.; Ciechelska-Rybarczyk, A.; Bernatek, M. In-vitro pathogen inhibition: Comparing the inhibitory effects of a complex multistrain synbiotic with simple probiotics containing the yeast Saccharomyces boulardii or Lactobacillus rhamnosus bacteria. bioRxiv 2019. [Google Scholar] [CrossRef]
- Peng, X.; Ed-dra, A.; Yue, M. Whole genome sequencing for the risk assessment of probiotic lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2022, 63, 11244–11262. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef]
- Huligere, S.; Kumari, V.; Alqadi, T.; Kumar, S.; Cull, C.; Amachawadi, R.; Ramu, R. Isolation and characterization of lactic acid bacteria with potential probiotic activity and further investigation of their activity by α-amylase and α-glucosidase inhibitions of fermented batters. Front. Microbiol. 2023, 13, 1042263. [Google Scholar] [CrossRef]
- Alameri, F.; Tarique, M.; Osaili, T.; Obaid, R.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Fernandez-Cabezudo, M.; Liu, S.; al-Ramadi, B.; et al. Lactic acid bacteria isolated from fresh vegetable products: Potential probiotic and postbiotic characteristics including immunomodulatory effects. Microorganisms 2022, 10, 389. [Google Scholar] [CrossRef]
- Celandroni, F.; Vecchione, A.; Cara, A.; Mazzantini, D.; Lupetti, A.; Ghelardi, E. Identification of Bacillus species: Implication on the quality of probiotic formulations. PLoS ONE. 2019, 14, e0217021. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yang, S.; Lim, B.; Park, S.; Rackerby, B.; Kim, H. Simultaneous detection of 37 Lactobacillus species using a real-time PCR assay based on whole-genome sequence analysis. Preprints 2020. [Google Scholar] [CrossRef]
- Kim, T.; Mondal, S.; Jeong, C.; Kim, S.; Ban, O.; Jung, Y.; Yang, J.; Kim, S. Safety evaluation of Lactococcus lactis IDCC 2301 isolated from homemade cheese. Food Sci. Nutr. 2021, 10, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Tariq, M.; Saris, P.; Zaidi, A. Evaluation of the probiotic and postbiotic potential of lactic acid bacteria from artisanal dairy products against pathogens. J. Infect. Dev. Ctries. 2021, 15, 102–112. [Google Scholar] [CrossRef]
- Chen, W.; Yu, L.; Shi, Y. Safety evaluation of lactic acid bacteria. In Lactic Acid Bacteria; Springer Nature Singapore Pte Ltd. and Science Press: Singapore, 2019; pp. 371–409. [Google Scholar] [CrossRef]
- Elzeini, H.; Ali, A.; Nasr, N.; Hassan, M.; Hassan, A.; Elenany, Y. Probiotic capability of novel lactic acid bacteria isolated from worker honey bees gut microbiota. FEMS Microbiol. Lett. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Mallappa, R.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Lv, H.; Zhang, H.; Liu, Y.; Zhang, M.; Wang, Y.; Tan, Z. Probiotic potential and wide-spectrum antimicrobial activity of lactic acid bacteria isolated from infant feces. Probiotics Antimicrob. Proteins 2020, 13, 90–101. [Google Scholar] [CrossRef]
- Tkhruni, F.; Israyelyan, A. Comparative antimicrobial activity of some metabiotics synthesized by lactic acid bacteria. New Arm. Med. J. 2023, 17. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltrán-Partida, E.; Zlatev, R.; Stoytcheva, M.; González-Mendoza, D.; Salvador-Carlos, J.; Moreno-Ulloa, A.; Cheng, N. Structure-activity relationship of diameter controlled Ag@Cu nanoparticles in broad-spectrum antibacterial mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111501. [Google Scholar] [CrossRef]
- Jung, J.; Han, S.-S.; Kim, Z.; Kim, M.H.; Kang, H.; Jin, H.; Lee, M.H. In-vitro characterization of growth inhibition against the gut pathogen of potentially probiotic lactic acid bacteria strains isolated from fermented products. Microorganisms 2021, 9, 2141. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; Tian, R.; Wang, W.; Ma, J.; Gu, L.; Liu, F.; Jiang, Z.; Hou, J. Screening beneficial bacteriostatic lactic acid bacteria in the intestine and studies of bacteriostatic substances. J. Zhejiang Univ. Sci. B 2021, 22, 533–547. [Google Scholar] [CrossRef]
- Faraki, A.; Rahmani, F. The antioxidant activity of lactic acid bacteria and probiotics: A review. J. Food Saf. Hyg. 2021, 6, 168–182. [Google Scholar] [CrossRef]
- Gizachew, S.; Beeck, W.; Spacova, I.; Dekeukeleire, M.; Alemu, A.; Woldemedhin, W.; Mariam, S.; Lebeer, S.; Engidawork, E. Antibacterial and immunostimulatory activity of potential probiotic lactic acid bacteria isolated from Ethiopian fermented dairy products. Fermentation 2023, 9, 258. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, Z.; Chen, J.; Jiang, J.; Zhao, M.; Gong, D. Ligilactobacillus salivarius improve body growth and anti-oxidation capacity of broiler chickens via regulation of the microbiota-gut-brain axis. BMC Microbiol. 2023, 23, 395. [Google Scholar] [CrossRef]
- Alba, C.; Castejón, D.; Remiro, V.; Rodríguez, J.M.; Sobrino, O.J.; de María, J.; Fumanal, P.; Fumanal, A.; Cambero, M.I. Ligilactobacillus salivarius MP100 as an alternative to metaphylactic antimicrobials in swine: The impact on production parameters and meat composition. Animals 2023, 13, 1653. [Google Scholar] [CrossRef]




| Strains | Shape | Glu | Fermentation | Gram | CAT | |||
| Rod | Cocci | - | + | Homo | Hetero | |||
| 75% | 25% | 96% | 4% | 96% | 4% | + | - | |
| Isolates | Antimicrobial Activity | Isolates | Antimicrobial Activity |
|---|---|---|---|
| ZY1 | ++++ | ZY26 | +++ |
| ZY2 | ++++ | ZY27 | +++ |
| ZY3 | ++++ | ZY28 | +++ |
| ZY4 | ++++ | ZY29 | ++++ |
| ZY5 | +++ | ZY30 | +++ |
| ZY6 | +++ | ZY31 | +++ |
| ZY7 | ++++ | ZY32 | ++++ |
| ZY8 | +++ | ZY33 | ++++ |
| ZY9 | +++ | ZY34 | +++ |
| ZY10 | ++++ | ZY35 | ++++ |
| ZY11 | +++ | ZY36 | +++ |
| ZY12 | ++++ | ZY37 | ++++ |
| ZY13 | ++++ | ZY38 | +++ |
| ZY14 | ++++ | ZY39 | ++++ |
| ZY15 | +++ | ZY40 | +++ |
| ZY16 | ++++ | ZY41 | +++ |
| ZY17 | ++++ | ZY42 | ++++ |
| ZY18 | +++ | ZY43 | +++ |
| ZY19 | ++++ | ZY44 | ++++ |
| ZY20 | +++ | ZY45 | +++ |
| ZY21 | ++++ | ZY46 | ++++ |
| ZY22 | +++ | ZY47 | ++++ |
| ZY23 | ++++ | ZY48 | +++ |
| ZY24 | ++++ | ZY49 | ++++ |
| ZY25 | ++++ | ZY50 | +++ |
| Isolates | Temperature (°C) | NaCl (w/v, %) | pH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 45 | 50 | 3.0 | 6.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 9.0 | 10.0 | |
| ZY1 | − | + | + | + | + | + | − | w | + | + | + | + | + | + | + |
| ZY2 | − | + | + | + | + | − | − | − | w | + | + | + | + | + | + |
| ZY3 | − | + | + | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY4 | − | + | + | + | + | + | − | w | + | + | + | + | + | + | + |
| ZY5 | − | w | + | w | + | + | − | w | w | + | + | + | + | + | + |
| ZY6 | − | + | + | − | +. | + | − | w | w | + | + | + | + | + | + |
| ZY7 | − | + | + | + | + | + | − | w | + | + | + | + | + | + | + |
| ZY8 | − | + | + | w | + | w | − | − | − | + | + | + | + | + | + |
| ZY9 | − | + | + | w | + | − | − | − | + | + | + | + | + | + | + |
| ZY10 | w | + | w | − | + | w | − | w | + | + | + | + | + | + | + |
| ZY11 | w | + | + | − | + | − | − | w | + | + | + | + | + | + | + |
| ZY12 | − | + | + | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY13 | w | + | + | + | + | − | − | − | − | + | + | + | + | + | + |
| ZY14 | w | + | + | + | + | w | − | − | − | + | + | + | + | + | + |
| ZY15 | − | w | w | − | + | + | − | − | + | + | + | + | + | + | + |
| ZY16 | w | + | + | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY17 | − | + | w | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY18 | w | + | + | − | + | + | − | − | w | + | + | + | + | + | + |
| ZY19 | w | + | w | − | + | w | − | w | + | + | + | + | + | + | + |
| ZY20 | w | + | + | + | + | − | − | − | + | + | + | + | + | + | + |
| ZY21 | w | + | + | + | + | + | − | w | + | + | + | + | + | + | + |
| ZY22 | − | + | + | + | + | w | − | − | w | + | + | + | + | + | + |
| ZY23 | − | + | + | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY24 | − | + | + | + | + | + | − | w | + | + | + | + | + | + | + |
| ZY25 | w | + | + | + | + | + | w | + | + | + | + | + | + | + | + |
| ZY26 | − | + | w | w | + | + | − | − | + | + | + | + | + | + | + |
| ZY27 | w | + | + | + | + | w | − | − | w | + | + | + | + | + | + |
| ZY28 | − | + | + | − | + | − | − | − | + | + | + | + | + | + | + |
| ZY29 | w | + | + | + | + | + | w | w | + | + | + | + | + | + | + |
| ZY30 | − | + | + | w | + | + | − | − | w | + | + | + | + | + | + |
| ZY31 | − | + | + | − | + | + | − | − | − | + | + | + | + | + | + |
| ZY32 | − | + | + | − | + | w | − | − | + | + | + | + | + | + | + |
| ZY33 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ZY34 | w | + | + | − | + | − | − | − | w | w | + | + | + | + | + |
| ZY35 | w | + | + | + | + | w | + | + | + | + | + | + | + | + | + |
| ZY36 | − | + | + | w | + | + | − | − | w | + | + | + | + | + | + |
| ZY37 | w | + | + | − | + | + | − | − | + | + | + | + | + | + | + |
| ZY38 | − | + | + | + | + | − | − | − | + | + | + | + | + | + | + |
| ZY39 | w | + | + | + | + | + | w | w | + | + | + | + | + | + | + |
| ZY40 | w | + | + | − | + | − | w | w | w | + | + | + | + | + | + |
| ZY41 | − | + | + | + | − | − | w | w | w | + | + | + | + | + | + |
| ZY42 | w | w | + | w | + | + | − | − | w | + | + | + | + | + | + |
| ZY43 | − | + | + | + | + | + | − | − | w | + | + | + | + | + | + |
| ZY44 | w | + | + | + | + | − | − | w | w | + | + | + | + | + | + |
| ZY45 | − | + | + | + | + | w | − | w | w | + | + | + | + | + | + |
| ZY46 | − | + | + | w | + | + | − | − | − | + | + | + | + | + | + |
| ZY47 | w | + | + | + | + | − | + | + | + | + | + | + | + | + | + |
| ZY48 | − | + | + | − | + | − | − | − | + | + | + | + | + | + | + |
| ZY49 | − | + | + | w | + | w | − | − | w | + | + | + | + | + | + |
| ZY50 | − | + | + | + | + | w | − | − | − | + | + | + | + | + | + |
| Isolate | GEN | CIP | CTR | E | AMP | TET | SXT | C | MY | PEN |
|---|---|---|---|---|---|---|---|---|---|---|
| ZY25 | R | R | I | S | S | I | R | S | I | S |
| ZY35 | I | R | I | S | S | I | S | S | R | S |
| Isolates | Virulence Factor Genes | Biogenic Amine Genes | Antibiotic Resistance Genes | |||||
|---|---|---|---|---|---|---|---|---|
| ace | gelE | cylA | Hdc | Tdc | Odc | vanA | tetM | |
| ZY25 | - | - | - | - | - | - | - | - |
| ZY35 | - | - | - | - | - | - | - | - |
| Isolates | Indicator Bacteria | |||||
|---|---|---|---|---|---|---|
| P. aeruginosa | S. aureus | L. monocytogenes | E. coli | B. subtilis | S. dysenteriae | |
| ZY25 | − | +++ | ++++ | +++ | +++ | ++++ |
| ZY35 | +++ | +++ | +++ | +++ | +++ | +++ |
| Treatment | ZY25 Antimicrobial Activity | ZY35 Antimicrobial Activity |
|---|---|---|
| Fermentation liquid | +++ | +++ |
| Supernatant | +++ | +++ |
| Hydrogen peroxide | +++ | +++ |
| Proteinase K | − | − |
| Pepsinum | − | − |
| Trypsase | − | − |
| 2.5 | ++++ | ++++ |
| 3.5 | +++ | +++ |
| 4.5 | ++ | ++ |
| 5.5 | − | − |
| 6.5 | − | − |
| 7 | − | − |
| 10 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Dong, H.; Chen, Q.; Chang, X.; Wang, L.; Miao, C.; Chen, S.; Chen, L.; Wang, R.; Ge, S.; et al. Antibacterial Efficacy of Feline-Derived Lactic Acid Bacteria against Enteropathogenic Escherichia coli: A Comprehensive In Vitro Analysis. Fermentation 2024, 10, 514. https://doi.org/10.3390/fermentation10100514
Wang W, Dong H, Chen Q, Chang X, Wang L, Miao C, Chen S, Chen L, Wang R, Ge S, et al. Antibacterial Efficacy of Feline-Derived Lactic Acid Bacteria against Enteropathogenic Escherichia coli: A Comprehensive In Vitro Analysis. Fermentation. 2024; 10(10):514. https://doi.org/10.3390/fermentation10100514
Chicago/Turabian StyleWang, Weiwei, Hao Dong, Qianqian Chen, Xiaohan Chang, Longjiao Wang, Chengyi Miao, Shuxing Chen, Lishui Chen, Ran Wang, Shaoyang Ge, and et al. 2024. "Antibacterial Efficacy of Feline-Derived Lactic Acid Bacteria against Enteropathogenic Escherichia coli: A Comprehensive In Vitro Analysis" Fermentation 10, no. 10: 514. https://doi.org/10.3390/fermentation10100514
APA StyleWang, W., Dong, H., Chen, Q., Chang, X., Wang, L., Miao, C., Chen, S., Chen, L., Wang, R., Ge, S., & Xiong, W. (2024). Antibacterial Efficacy of Feline-Derived Lactic Acid Bacteria against Enteropathogenic Escherichia coli: A Comprehensive In Vitro Analysis. Fermentation, 10(10), 514. https://doi.org/10.3390/fermentation10100514






