3.2.1. Sensitivity Properties of the Al/WO3/C Energetic Formulations
The different materials were then mixed, using the sonication process described above, in various proportions to form Al/WO3/C energetic formulations. An equivalence ratio of 1.4 was set between the fuel and oxidizer ingredients and the concentration of the carbon additive was varied from about 2 to 35 vol. %.
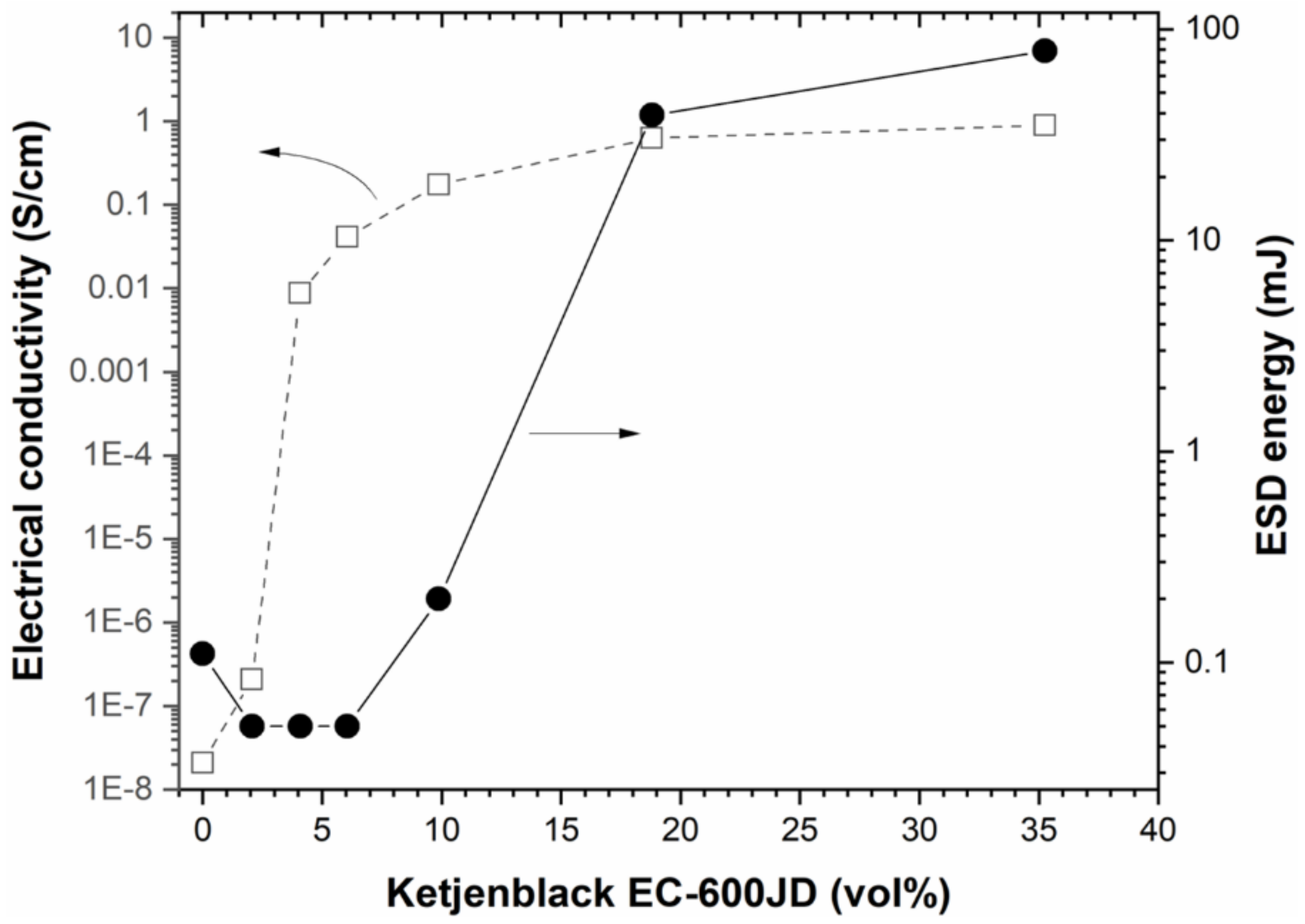
Figure 4 shows the electrical conductivity of the different as-prepared Al/WO
3/C composite materials as a function of the Ketjenblack carbon concentration. The energetic mixtures were pressed at 31–35% of the TMD except for the mixtures containing 18.80 and 34.25 vol. % of carbon, for which values of 28 and 22% were determined due to the high concentration of low-density carbon. The pristine Al/WO
3 has an insulating character (conductivity < 10
−8 S/cm) due to the insulating alumina layer surrounding the aluminum core and the non-conductive WO
3 phase. The conductivity between 0 and 4.08 vol. % of the highly conductive carbon additive increased significantly by five orders of magnitude (< 10
−8 S/cm vs. 8.79 × 10
−3 S/cm). Subsequently, the conductivity values slightly increased again and reached a plateau at 10
−1 S/cm; namely 1.75, 6.27 and 8.95 × 10
−1 S/cm for 9.88, 18.8 and 35.25 vol. % of Ketjenblack EC−600D carbon, respectively. This jump in conductivity can be interpreted by a percolation phenomenon which is due to the connectivity of the additive nanoparticles to each other. Approximately, the percolation threshold can be determined around 2.5 vol. %, i.e., the concentration for which the distribution of the highly conductive carbon is homogeneous within the Al/WO
3 nanothermite until it builds a continuous three-dimensional conductive network. Al/WO
3/C energetic mixtures were further investigated for their sensitivity to electrostatic discharge. The spark energy during the test ranged from 0.05 mJ to 10 J by varying the voltage and capacitance of the device.
Figure 4 shows the energy values, as a function of the concentration of Ketjenblack carbon in the nanothermite, for which there is no ignition of the composition. In other words, these are the values just above the energy required to ignite the energetic materials. As expected, the curve follows the same trend as that of the electrical conductivity profile as a function of the volume percentage of the Ketjenblack additive in the nanothermite; as expected, we can see a prompt increase with the conductive carbon additive until reaching values that reflect a plateau.
This result is justified by the fact that adding electrically conductive elements in the nanothermite allows for the rapid evacuation of electrostatic charges and thus avoids the ignition of the composition by the Joule effect. The more conductive carbon particles there are in the mixture, the easier it is to dissipate the charges, leading to an increase in the ESD value. The conductive carbon nanoparticles tend to create a continuous conductive phase such that the electrostatic current moves through the formulated mixture bypassing the sensitive energetic ingredients. However, a shift of the percolation threshold towards a higher level of carbon nanoparticles rate was observed. Indeed, the percolation threshold was recorded around 12.5 vol. % in this case, which is five times higher than that determined from the curve characterizing the evolution of the electrical conductivity of the energetic formulations (i.e., 2.5 vol. %). This difference can be explained by the following fact: in electrical conductivity measurements, in contrast to ESD tests, the powder is pressed to a density close to 30–40% of the theoretical maximum density (TMD). The shaping of the powder sample under the test conditions promotes connectivity between the particles, which increases the contact area between them. As a result, the charge carriers can self-assemble more easily to create a conductive path and thus facilitate the flow of current (higher electrical conductivity). In ESD sensitivity testing, bulk powders are examined (TMD < 10%) and therefore the contact area between the particles is not as high and the conductivity properties may be lower. This result is not entirely consistent with previous works dedicated to the spark-desensitization of nanothermites by means of conductive component additives [
10,
12]. When conductive polymers (polyaniline, polypyrrole) are used, the curves are superimposed. The fact that the oxidizer/polymer composites are synthesized, before being mixed with the fuel, probably leads to more intimate and homogeneous fuel/oxidizer/polymer systems, allowing similar percolation thresholds to be obtained between the electrical conductivity and ESD sensitivity curves as a function of the additive concentration.
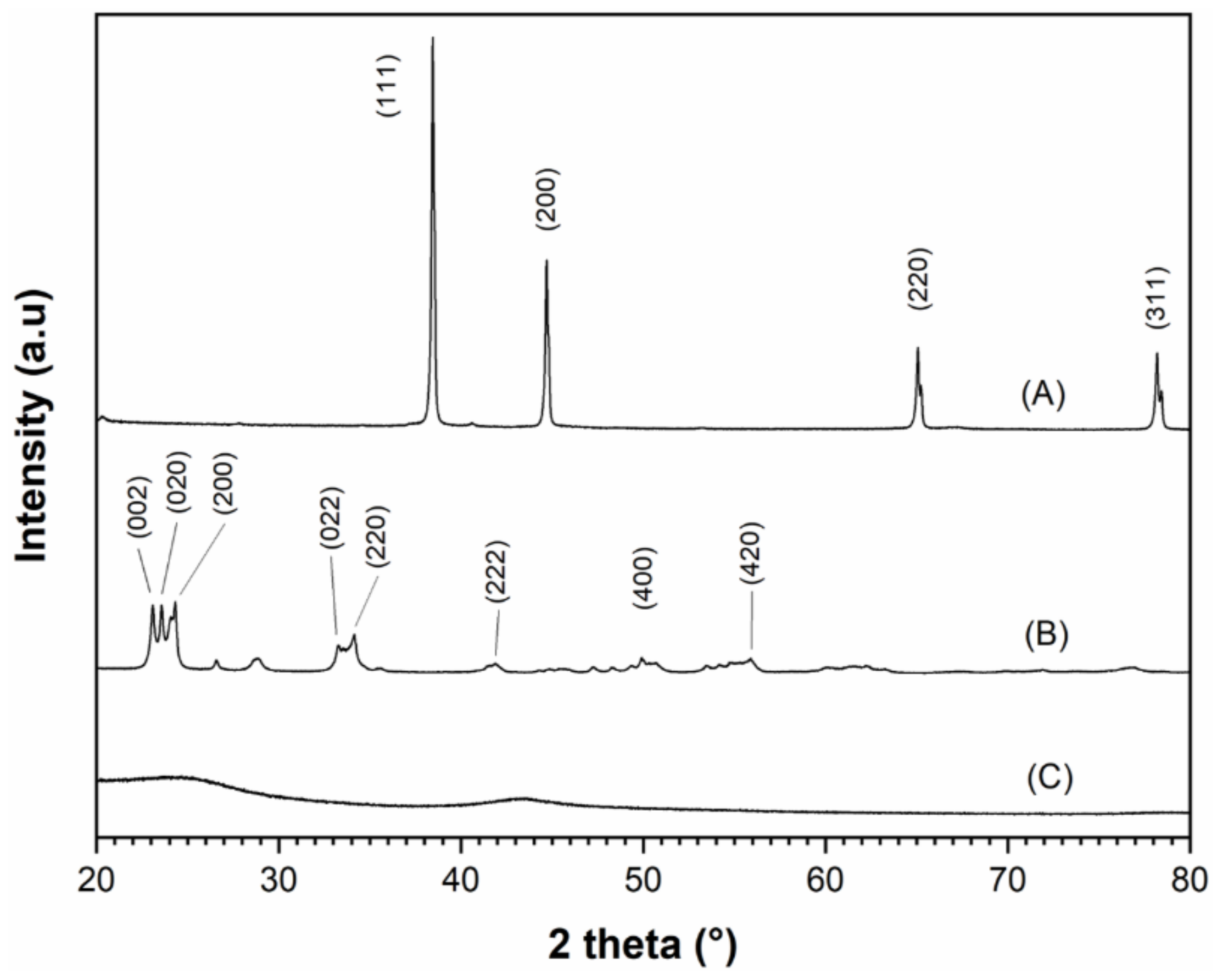
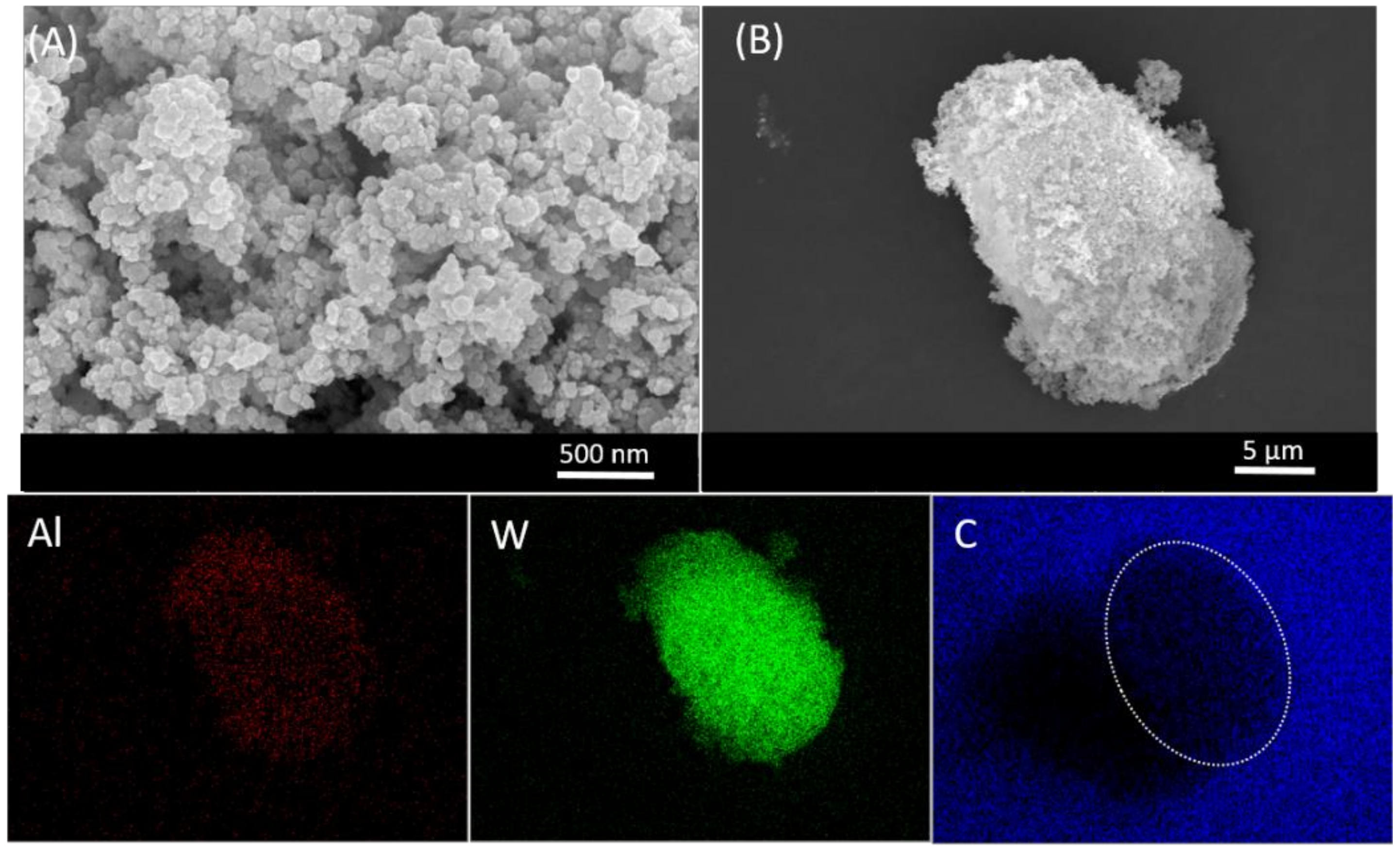
A scanning electron microscopy (SEM) analysis was performed to determine the conductive carbon-additive dispersion quality within Al/WO
3 nanothermites. Representative SEM pictures of the Al/WO
3/carbon with a concentration of 3 wt. % (6.05 vol. %) are presented in
Figure 5.
It can be noted that it is difficult to know which particles represent a given component because the particles have relatively similar sizes and shapes. At best, particles with a quasi-spherical morphology can be attributed to aluminum (Al) in agreement with the previous TEM analysis. An energy dispersive X-ray spectroscopy (EDX) analysis was performed to verify the chemical composition of the nanothermite (
Supplementary Information). Only aluminum (Al), tungsten (W), and carbon (C) were identified. The gold (Au) element observed on the spectrum comes from a layer of Au applied to the surface of the particles to facilitate observation under the electron microscope. To distinguish the particles from each other, elemental mappings of aluminum, tungsten, and carbon were performed (
Figure 5). Clearly, Al and W can be observed in the volume of the aggregate studied and seem to be randomly but homogeneously and uniformly distributed. Regarding the carbon element, as the sample was deposited on a carbon-rich adhesive tape, it is observed throughout the picture. However, from a geometric dotted line representation of the location of the aggregate in the image, we can see that the carbon is also present in this specific area and that its distribution is optimal, i.e., over the entire surface of the aggregate. Based on this electron microscope analysis, the homogeneous distribution and intimacy of the Al, WO
3, and C nanoparticles were evidenced.
To complement the spark ignition sensitivity of nanothermites (the crucial and probably most important criterion in terms of sensitivity), mechanical tests such as friction and impact tests were also carried out on Al/WO
3/Ketjenblack energetic formulations. The values have been collected in
Table 1.
As it can be seen, all Al/WO
3 energetic formulations w/o carbon additive have impacted the sensitivity values at least equal to 75 J. Considering NATO standards, all of them can be considered insensitive since their sensitivity thresholds are well above 40 J [
17]. As far as friction sensitivity is concerned, the threshold value increases constantly with the content of Ketjenblack EC-600JD. For example, the value increases from 42 N to over 360 N with 2.06 and 34.25 vol. % additive, respectively. As a reminder, the Al/WO3 nanothermite, i.e., without carbon, has a threshold value of less than 4.9 N. According to the sensitivity classes established by NATO, Al/WO
3/C nanothermites can be classified as sensitive, moderately sensitive, and insensitive, with values of 42 N, 80–360 N, and above 360 N, respectively [
18].
The phenomenon of desensitization by friction recorded with the introduction of a carbon component in the Al/WO3 nanothermite can be explained by a physical distancing of the fuel (Al) and oxidizer (WO3) components through the carbon nanoparticles. Thus, at a low scale, the intimacy of the mixture, the contact area between Al and WO3, and the stoichiometry of the aluminothermy reaction (1) are not maintained, and as a result, the reaction does not occur. Finally, in a global way, Al/WO3/Ketjenblack energetic composite materials can be considered as relatively safe to handle or for transport operations.
3.2.2. Reactive Behaviours of the Al/WO3/C Energetic Formulations
The reactivity properties of Al/WO
3/Ketjenblack nanothermites were recorded using a high-speed camera after ignition by a flash optical igniter. All video recordings can be seen in the
Supplementary Information (videos SI_a to SI_f) with the exception of the Al/WO
3 formulation with 34.25 vol. % (20 wt. %) of conductive carbon concentration, for which a low reactivity and an incomplete combustion reaction were observed (not shown here). All the energetic formulations showed an ease of ignition in open environments and were complete because no residues were observed at the end of the burning tests. However, longer ignition times and lower burning rates were observed for energetic samples with an increasing carbon concentration. As a pyrotechnically inert ingredient, carbon limits the thermite reaction and mitigates its reactive properties [
6,
19]. The obvious change in the reactive behaviour was observed for a carbon concentration between 9.9 and 18.8 vol. %. While vigorous reactions and dense homogeneous flames characterize energetic systems based on a low amount of carbon (videos SI_a to e), dispersed incandescent particles were observed for the Al/WO
3 nanothermite formulated with 18.8 vol. % of conductive carbon (video SI_f). In addition, the reaction time was longer in the latter case than for an amount of carbon ranging from 2 to 9.9 vol. %.
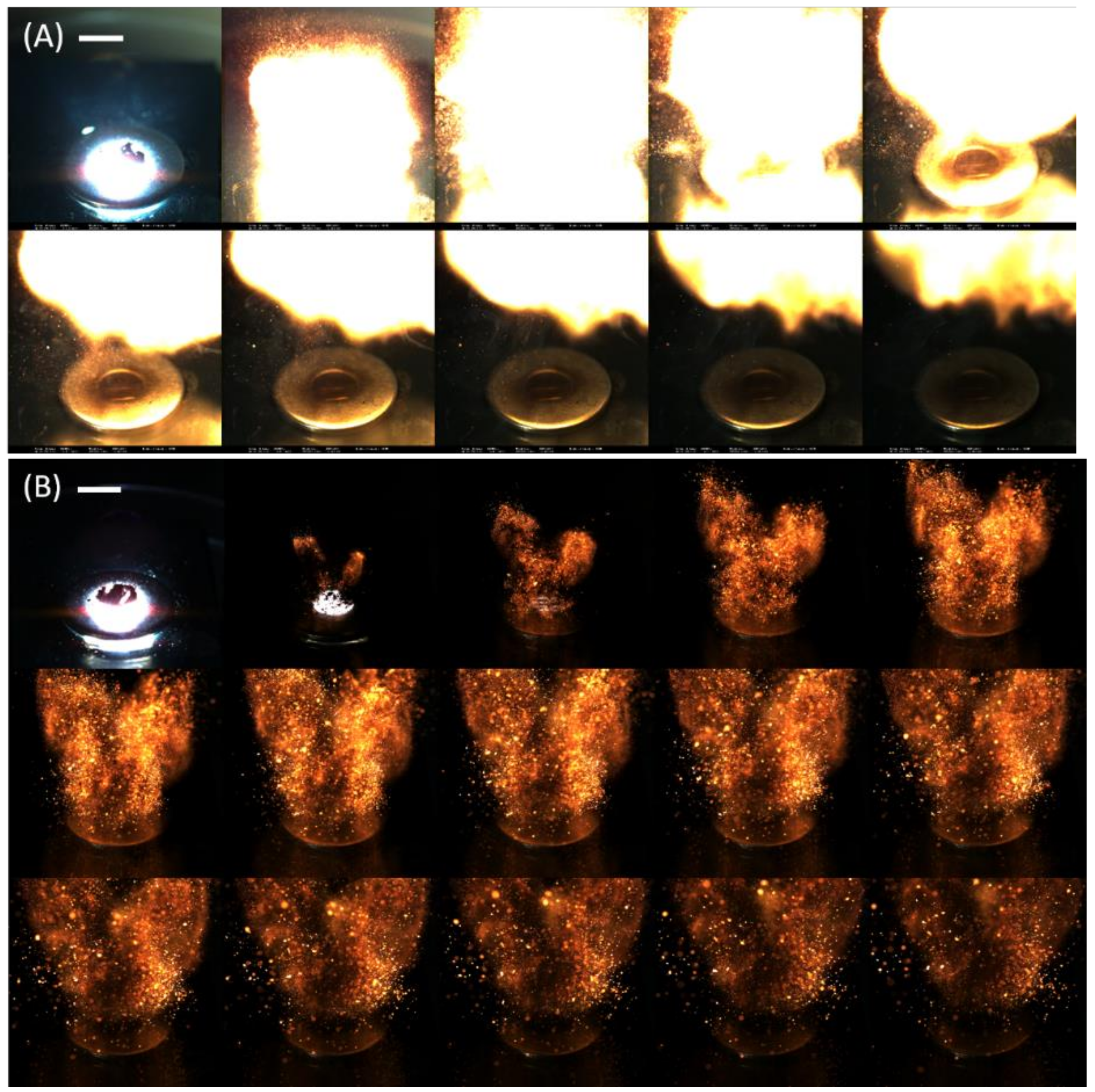
Sequential images of Al/WO
3 nanothermites without and with 18.8 vol. % of carbon additive, extracted from their flame propagation videos, are displayed in
Figure 6. The nanothermite consisting of 18.8 vol. % carbon nanoparticles was selected owing to its insensitivities toward mechanical and electrostatic discharge tests, making this handling safe for an operator. In particular, its ESD threshold value was determined to be near 40 mJ, which is two times higher than the energy than a human body can dissipate (8–20 mJ) [
2,
3,
19]. Combustion images confirmed the different reactive behaviours of both energetic systems. While a rapid formation of an intense and luminous fire ball was observed for the Al/WO
3 system (
Figure 6A), the Al/WO
3/Ketjenblack formulation can be described as a projection of uncountable incandescent particles (
Figure 6B). Moreover, the reaction time was longer for the latter than for the former since a higher number of images were required to represent the burning event. Obviously, the carbon additive, due to its inert nature, its relatively high content, and its role in suppressing the contact areas between the reactive species (Al and WO
3), significantly reduces reactive properties.
The experimental heats of explosion of the Al/WO
3/Ketjenblack energetic nanocomposites—0 and 18.8 vol. % of carbon—were determined to be equal to 2815 J/g and 1408 J/g, respectively, a loss of 50%, supporting the idea of a less powerful energetic system with carbon. The lost energy may be explained by the consumption of oxygen by the carbon particles and the fact that the corresponding oxidation reaction is less exothermic than the aluminothermal reaction [
19].
In summary, compared to previous research mentioning the use of carbon structures to desensitize nanothermites, the Ketjenblack carbon material can also be considered as a promising alternative. This view is based on several facts. First, the concentration of KB EC600JD in Al/WO
3 nanothermites (10 wt. % or 18.8 vol. %) to mitigate the spark sensitivity (ESD sensitivity > 39 mJ) is of the same order of magnitude as the addition of carbon nanotubes (13 vol. %, ESD sensitivity > 100 mJ) [
4] and graphene oxide (9.6 wt. %, ESD sensitivity > 24.5 mJ) [
6], much lower than carbon fibers (40 wt. %, ESD sensitivity 35 mJ) [
8], but slightly higher than nanodiamonds (3 wt. %, ESD sensitivity > 20 mJ) [
9]. However, the major advantage of the use of Ketjenblack carbon over the other additives mentioned is the maintenance of an acceptable reactive behavior of the studied system, whereas this one disappears with the addition of carbon fibers and nanodiamonds. For carbon nanotubes and graphene oxide, no reactivity study was conducted by the authors.