Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supports Preparation
2.2. Catalysts Preparation
2.3. Materials Characterization
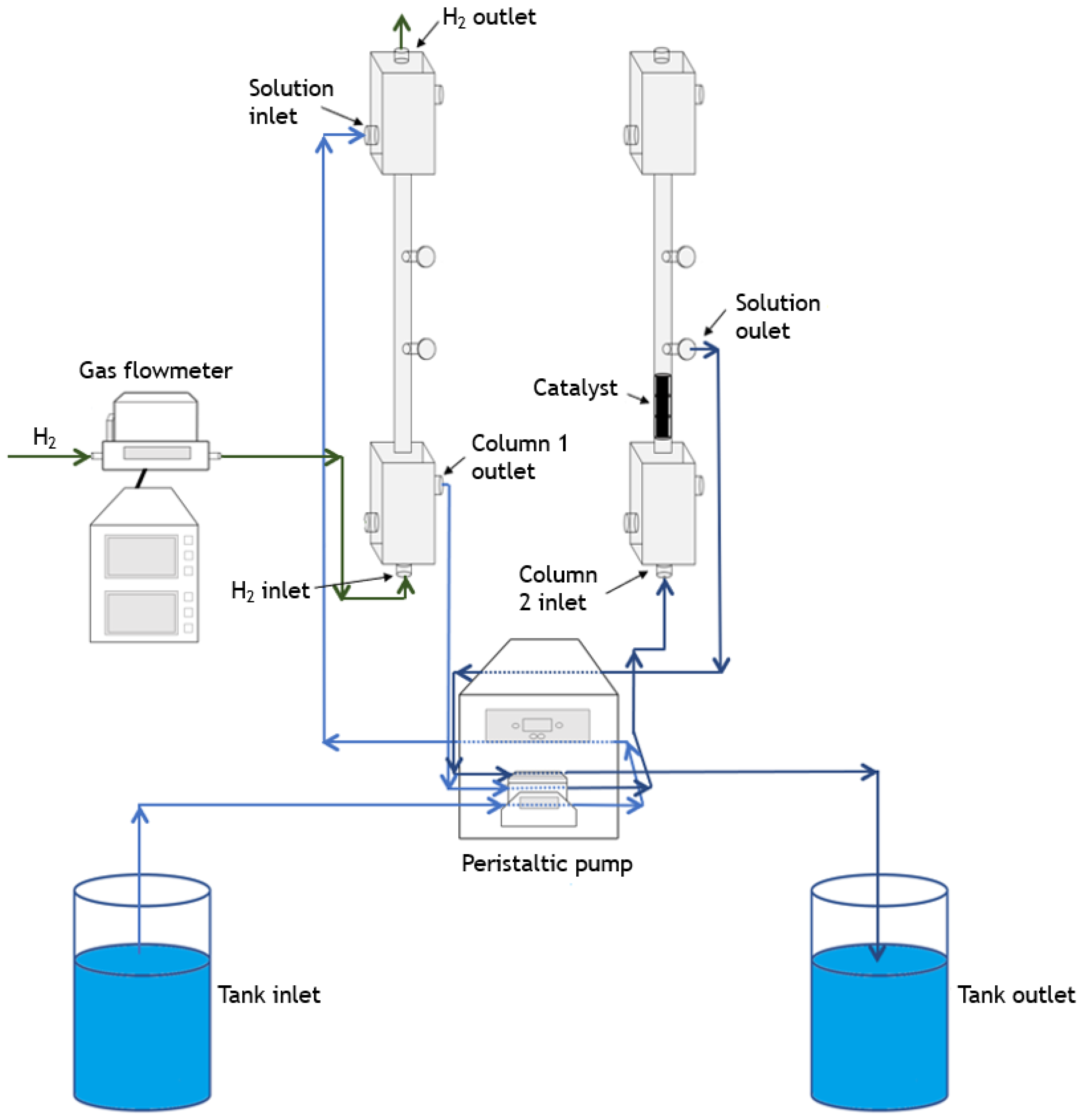
2.4. Catalytic Experiments
3. Results and Discussion
3.1. Materials Characterization
3.2. Catalytic Experiments
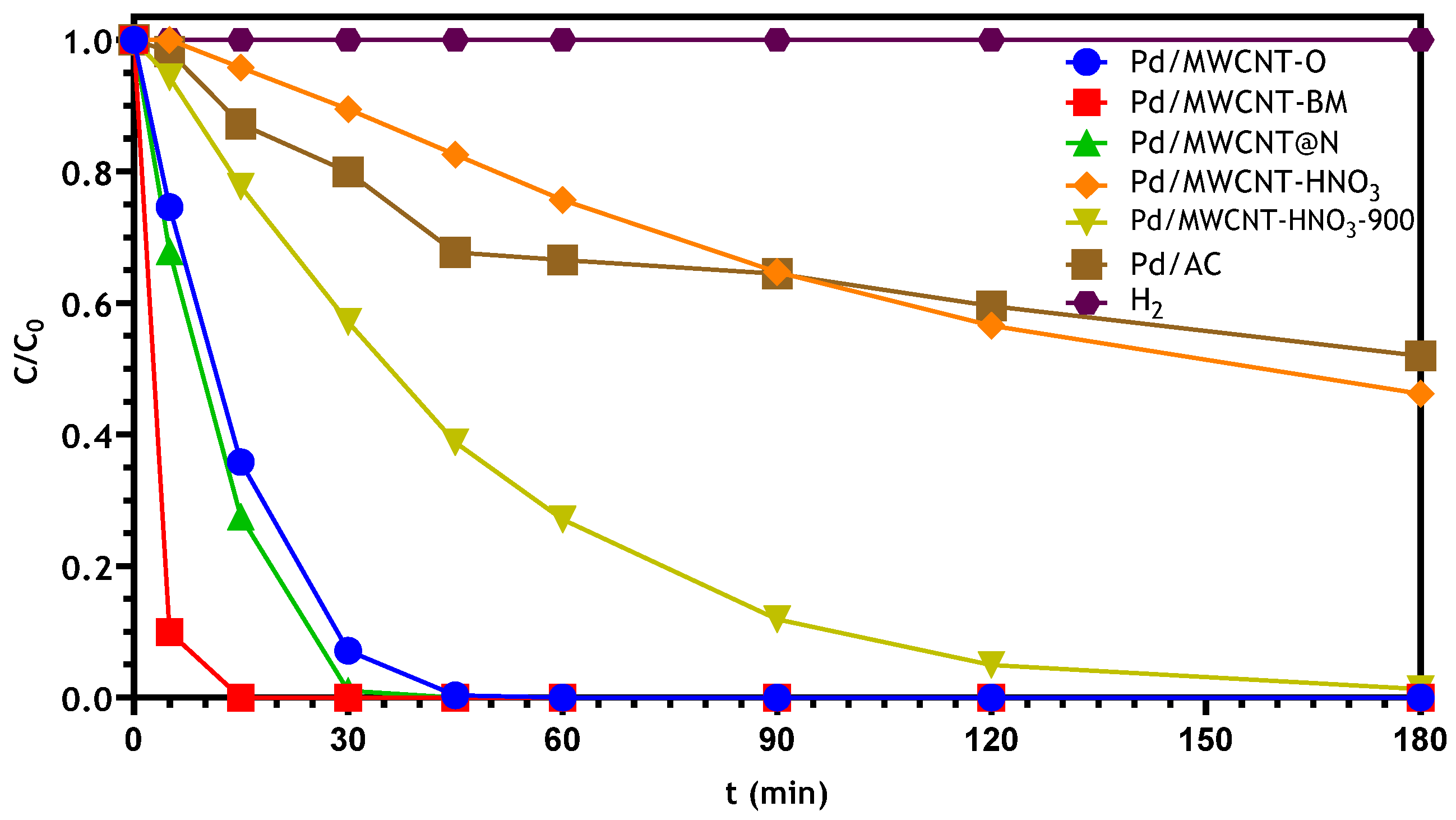
3.2.1. Bromate Catalytic Reduction in a Semi-Batch Reactor
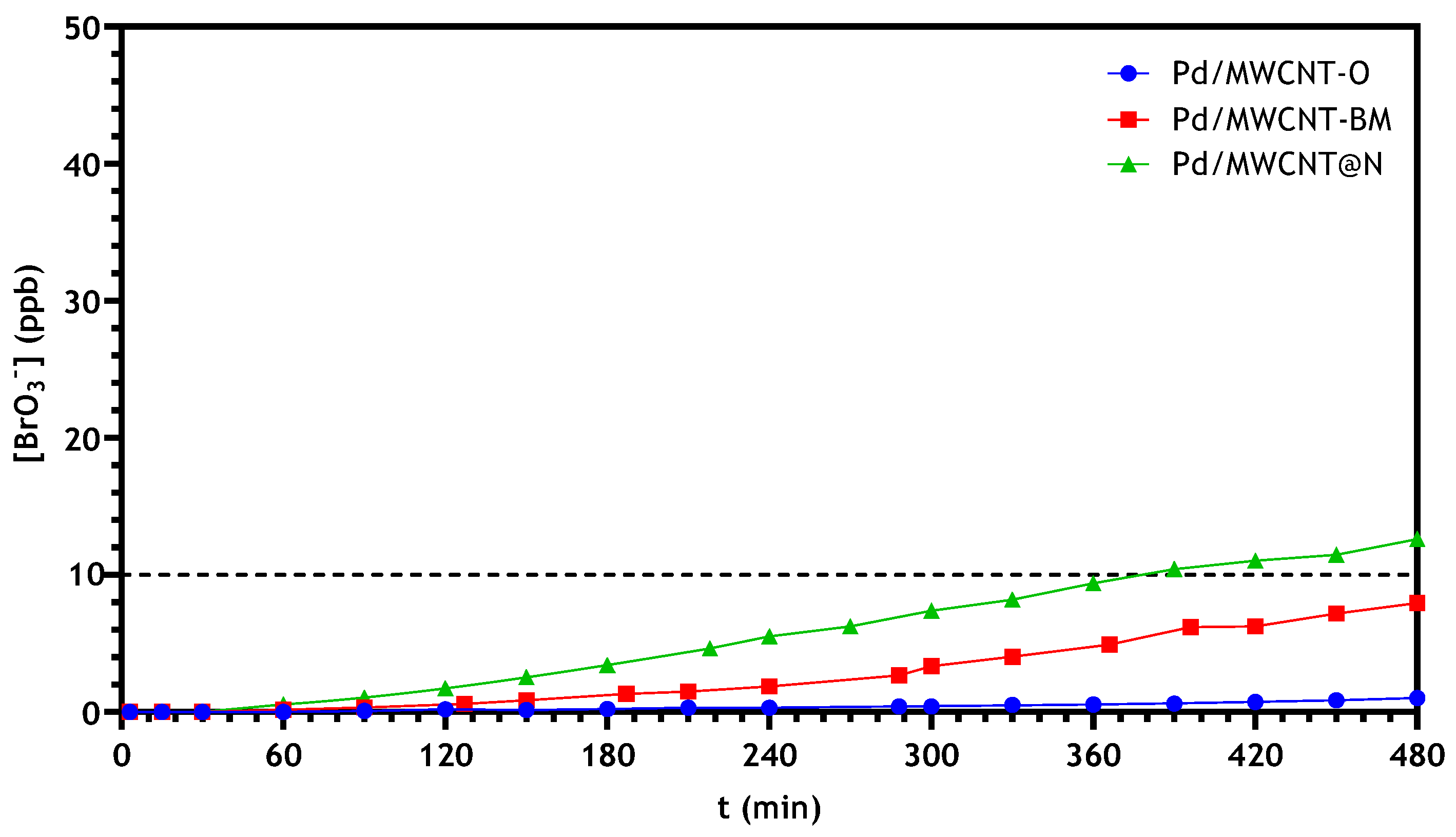
3.2.2. Bromate Catalytic Reduction in a Continuous Reactor
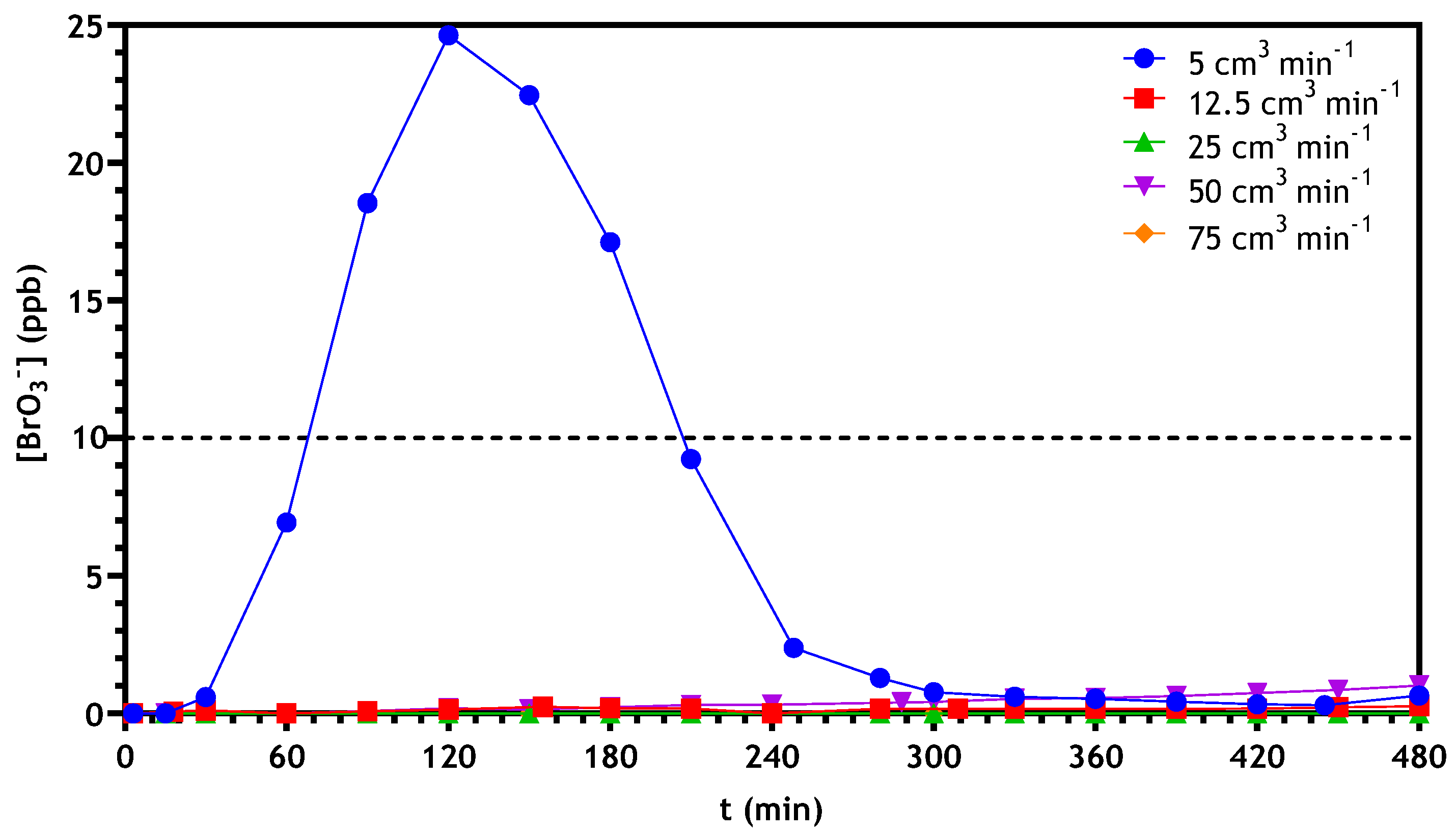
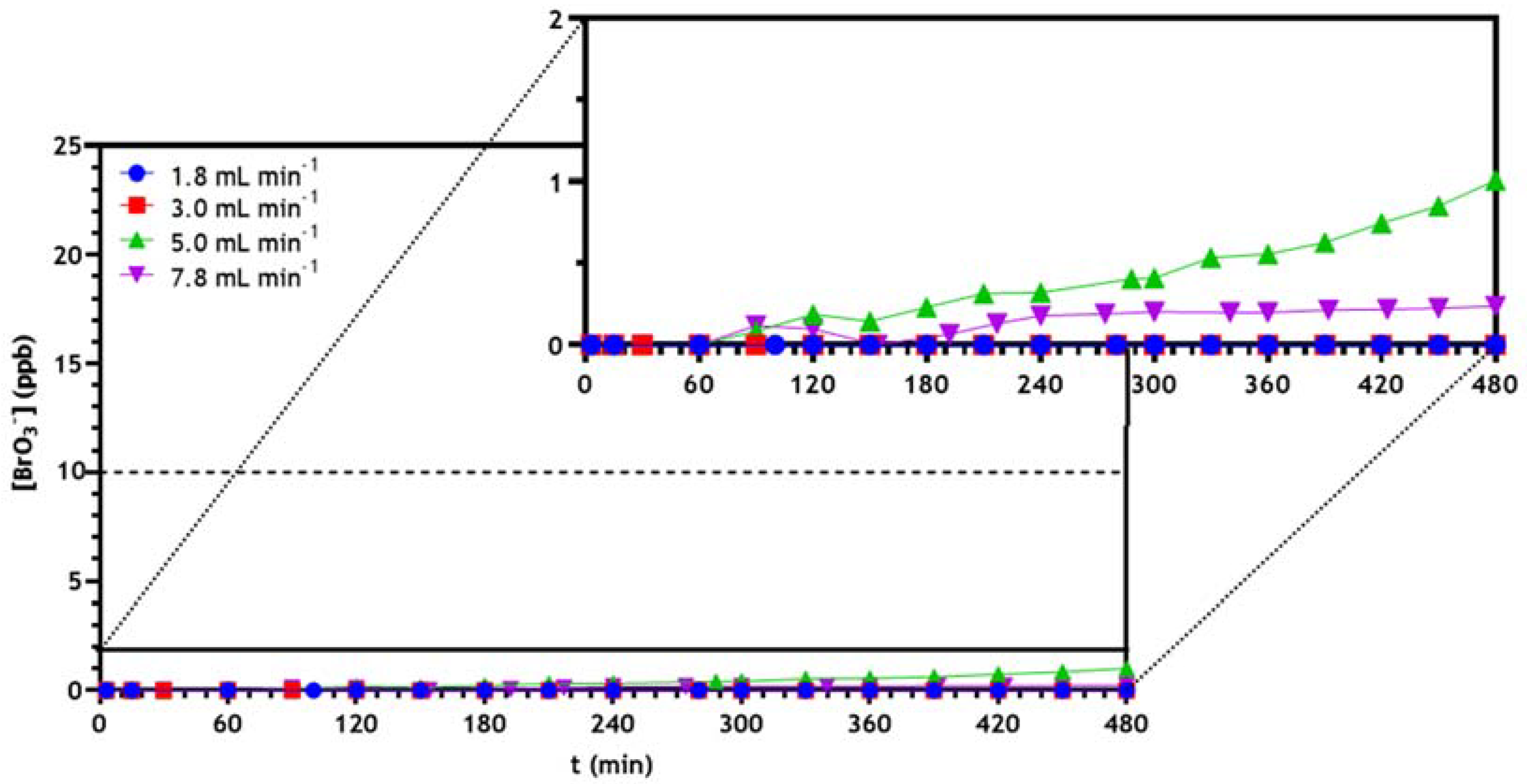
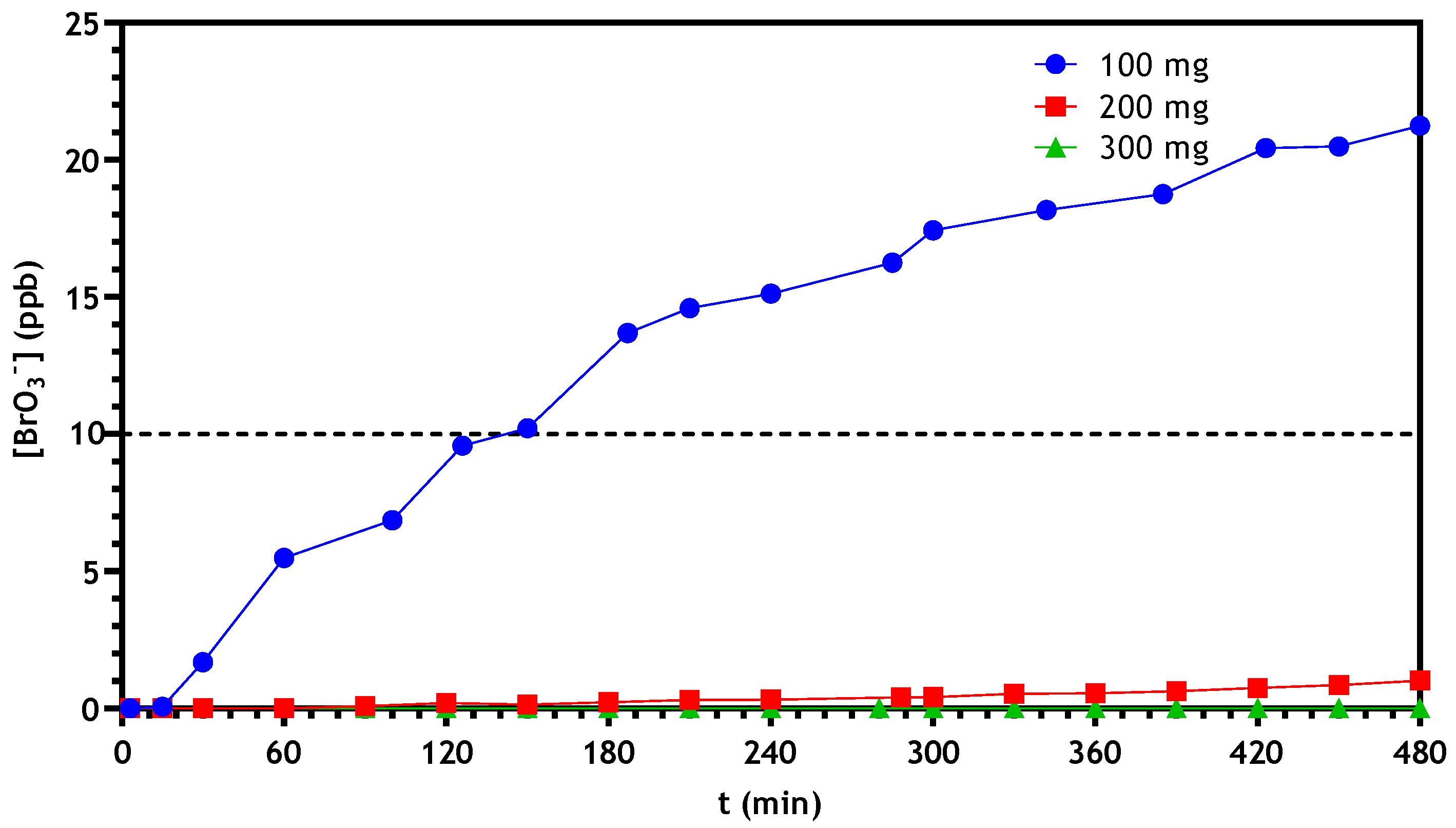
3.2.3. Optimization of Continuous Reactor Operating Conditions
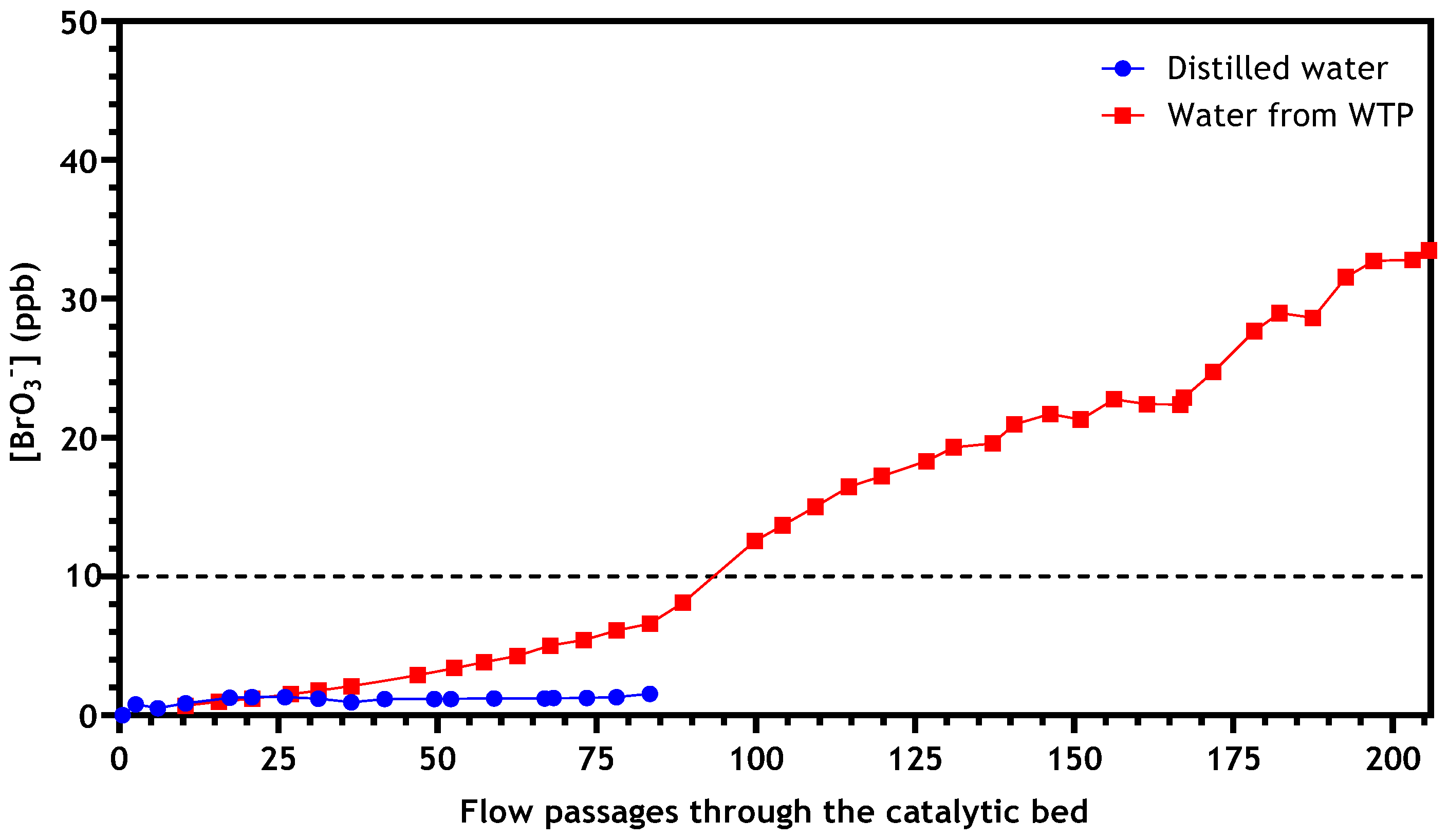
3.2.4. Application to Real Drinking Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed.; World Health Organization: Switzerland, Geneva, 2017. [Google Scholar]
- Butler, R.A.Y.; Godley, A.; Lytton, L.; Cartmell, E. Bromate Environmental Contamination: Review of Impact and Possible Treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 193–217. [Google Scholar] [CrossRef]
- Huang, W.-J.; Cheng, Y.-L. Effect of characteristics of activated carbon on removal of bromate. Sep. Purif. Technol. 2008, 59, 101–107. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhou, J.; Gao, N. Photocatalytic decomposition of bromate ion by the UV/P25-Graphene processes. Water Res. 2014, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.A.; Kabsch-Korbutowicz, M.; Łakomska, S. Removal of bromate ions from water in the processes with ion-exchange membranes. Sep. Purif. Technol. 2015, 145, 75–82. [Google Scholar] [CrossRef]
- Kishimoto, N.; Matsuda, N. Bromate Ion Removal by Electrochemical Reduction Using an Activated Carbon Felt Electrode. Environ. Sci. Technol. 2009, 43, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Restivo, J.; Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Metal assessment for the catalytic reduction of bromate in water under hydrogen. Chem. Eng. J. 2015, 263, 119–126. [Google Scholar] [CrossRef]
- Perez-Coronado, A.M.; Soares, O.S.G.P.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A.; Pereira, M.F.R. Catalytic reduction of bromate over catalysts based on Pd nanoparticles synthesized via water-in-oil microemulsion. Appl. Catal. B Environ. 2018, 237, 206–213. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Ramalho, P.S.F.; Fernandes, A.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic bromate reduction in water: Influence of carbon support. J. Environ. Chem. Eng. 2019, 7, 103015–103023. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Palomares, A.E. A Review on the Catalytic Hydrogenation of Bromate in Water Phase. Catalysts 2021, 11, 365. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Lopes, C.W.; Rey, F.; Palomares, A.E. The Influence of the Support Nature and the Metal Precursor in the Activity of Pd-based Catalysts for the Bromate Reduction Reaction. ChemCatChem 2021, 13, 1230–1238. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Morais, D.F.S.; Boaventura, R.A.R.; Moreira, F.C.; Vilar, V.J.P. Bromate removal from water intended for human consumption by heterogeneous photocatalysis: Effect of major dissolved water constituents. Chemosphere 2021, 263, 128111–128119. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tang, Y.; Yuan, X.; Qiang, Z. Reduction of bromate by zero valent iron (ZVI) enhances formation of brominated disinfection by-products during chlorination. Chemosphere 2021, 268, 129340–129347. [Google Scholar] [CrossRef]
- Chen, X.; Huo, X.; Liu, J.; Wang, Y.; Werth, C.J.; Strathmann, T.J. Exploring beyond palladium: Catalytic reduction of aqueous oxyanion pollutants with alternative platinum group metals and new mechanistic implications. Chem. Eng. J. 2017, 313, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Restivo, J.; Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic reduction of bromate over monometallic catalysts on different powder and structured supports. Chem. Eng. J. 2017, 309, 197–205. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Yuranova, T.; Kiwi-Minsker, L.; García-Bordeje, E.; Derrouiche, S. The use of Pd catalysts on carbon-based structured materials for the catalytic hydrogenation of bromates in different types of water. Appl. Catal. B Environ. 2014, 146, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Marco, Y.; García-Bordejé, E.; Franch, C.; Palomares, A.E.; Yuranova, T.; Kiwi-Minsker, L. Bromate catalytic reduction in continuous mode using metal catalysts supported on monoliths coated with carbon nanofibers. Chem. Eng. J. 2013, 230, 605–611. [Google Scholar] [CrossRef]
- Thakur, D.B.; Tiggelaar, R.M.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Silicon based microreactors for catalytic reduction in aqueous phase: Use of carbon nanofiber supported palladium catalyst. Chem. Eng. J. 2013, 227, 128–136. [Google Scholar] [CrossRef]
- Höller, V.; Yuranov, I.; Kiwi-Minsker, L.; Renken, A. Structured multiphase reactors based on fibrous catalysts: Nitrite hydrogenation as a case study. Catal. Today 2001, 69, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Palomares, A.E.; Franch, C.; Corma, A. A study of different supports for the catalytic reduction of nitrates from natural water with a continuous reactor. Catal. Today 2011, 172, 90–94. [Google Scholar] [CrossRef]
- Yuranova, T.; Franch, C.; Palomares, A.E.; Garcia-Bordejé, E.; Kiwi-Minsker, L. Structured fibrous carbon-based catalysts for continuous nitrate removal from natural water. Appl. Catal. B Environ. 2012, 123, 221–228. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon 2010, 48, 4369–4381. [Google Scholar] [CrossRef]
- Soares, S.; Rocha, R.P.; Gonçalves, A.; Figueiredo, J.; Órfão, J.; Pereira, F. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation. C—J. Carbon Res. 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar] [PubMed] [Green Version]
- Rocha, R.P.; Soares, O.S.G.P.; Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Different methodologies for synthesis of nitrogen doped carbon nanotubes and their use in catalytic wet air oxidation. Appl. Catal. A Gen. 2017, 548, 62–70. [Google Scholar] [CrossRef]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric Acid Purification of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Nitrate Reduction Catalyzed by Pd–Cu and Pt–Cu Supported on Different Carbon Materials. Catal. Lett. 2010, 139, 97–104. [Google Scholar] [CrossRef]
- Kasaini, H.; Goto, M.; Furusaki, S. Selective Separation of Pd(II), Rh(III), and Ru(III) Ions from a Mixed Chloride Solution Using Activated Carbon Pellets. Sep. Sci. Technol. 2000, 35, 1307–1327. [Google Scholar] [CrossRef]
- Watanabe, S.; Arunajatesan, V. Influence of Acid Modification on Selective Phenol Hydrogenation Over Pd/Activated Carbon Catalysts. Top. Catal. 2010, 53, 1150–1152. [Google Scholar] [CrossRef]
- Contreras, R.C.; Guicheret, B.; Machado, B.F.; Rivera-Cárcamo, C.; Alvarez, M.A.C.; Salas, B.V.; Ruttert, M.; Placke, T.; Réguillon, A.F.; Vanoye, L.; et al. Effect of mesoporous carbon support nature and pretreatments on palladium loading, dispersion and apparent catalytic activity in hydrogenation of myrcene. J. Catal. 2019, 372, 226–244. [Google Scholar] [CrossRef]
- Simonov, P.A.; Troitskii, S.Y.; Likholobov, V.A. Preparation of the Pd/C catalysts: A molecular-level study of active site formation. Kinet. Catal. 2000, 41, 255–269. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R.; et al. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna Kumar, M.; Ramaprabhu, S. Palladium dispersed multiwalled carbon nanotube based hydrogen sensor for fuel cell applications. Int. J. Hydrog. Energy 2007, 32, 2518–2526. [Google Scholar] [CrossRef]
- Santos, A.S.G.G.; Restivo, J.; Orge, C.A.; Pereira, M.F.R.; Soares, O.S.G.P. Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization. C—J. Carbon Res. 2020, 6, 78. [Google Scholar] [CrossRef]
- Solhy, A.; Machado, B.F.; Beausoleil, J.; Kihn, Y.; Gonçalves, F.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Faria, J.L.; Serp, P. MWCNT activation and its influence on the catalytic performance of Pt/MWCNT catalysts for selective hydrogenation. Carbon 2008, 46, 1194–1207. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Yuranova, T.; Kiwi-Minsker, L.; Franch, C.; Palomares, A.E.; Armenise, S.; García-Bordejé, E. Nanostructured Catalysts for the Continuous Reduction of Nitrates and Bromates in Water. Ind. Eng. Chem. Res. 2013, 52, 13930–13937. [Google Scholar] [CrossRef]
- Alsolami, B.H.; Berger, R.J.; Makkee, M.; Moulijn, J.A. Catalyst Performance Testing in Multiphase Systems: Implications of Using Small Catalyst Particles in Hydrodesulfurization. Ind. Eng. Chem. Res. 2013, 52, 9069–9085. [Google Scholar] [CrossRef]
- Yaseneva, P.; Marti, C.F.; Palomares, E.; Fan, X.; Morgan, T.; Perez, P.S.; Ronning, M.; Huang, F.; Yuranova, T.; Kiwi-Minsker, L.; et al. Efficient reduction of bromates using carbon nanofibre supported catalysts: Experimental and a comparative life cycle assessment study. Chem. Eng. J. 2014, 248, 230–241. [Google Scholar] [CrossRef]
- Gašparovičová, D.; Králik, M.; Hronec, M.; Vallušová, Z.; Vinek, H.; Corain, B. Supported Pd–Cu catalysts in the water phase reduction of nitrates: Functional resin versus alumina. J. Mol. Catal. A Chem. 2007, 264, 93–102. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J. Catalytic hydrogenation of aqueous nitrate solutions in fixed-bed reactors. Catal. Today 1999, 53, 35–50. [Google Scholar] [CrossRef]
- Durkin, D.P.; Ye, T.; Choi, J.; Livi, K.J.T.; Long, H.C.D.; Trulove, P.C.; Fairbrother, D.H.; Haverhals, L.M.; Shuai, D. Sustainable and scalable natural fiber welded palladium-indium catalysts for nitrate reduction. Appl. Catal. B Environ. 2018, 221, 290–301. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Ruiz-Martínez, J.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Pereira, M.F.R. Pd–Cu/AC and Pt–Cu/AC catalysts for nitrate reduction with hydrogen: Influence of calcination and reduction temperatures. Chem. Eng. J. 2010, 165, 78–88. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Inglezakis, V.J.; Moustakas, K.G.; Malamis, S.P.; Loizidou, M.D. Removal of Cu(II) in fixed bed and batch reactors using natural zeolite and exfoliated vermiculite as adsorbents. Desalination 2007, 215, 133–142. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Gallegos-Suarez, E.; Castillejos, E.; Rodríguez-Ramos, I.; Pereira, M.F.R. Nitrate reduction over a Pd-Cu/MWCNT catalyst: Application to a polluted groundwater. Environ. Technol. 2012, 33, 2353–2358. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Yin, D.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B Environ. 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, F.; Chen, H. Enhanced catalytic hydrogenation of aqueous bromate over Pd/mesoporous carbon nitride. Chem. Eng. J. 2013, 234, 195–202. [Google Scholar] [CrossRef]
- Santos, A.S.G.G.; Restivo, J.; Orge, C.A.; Pereira, M.F.R.; Soares, O.S.G.P. Influence of organic matter formed during oxidative processes in the catalytic reduction of nitrate. J. Environ. Chem. Eng. 2021, 9, 105545–105556. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Roundy, E.; Guy, K.A.; Shapley, J.R.; Werth, C.J. Effects of Natural Water Ions and Humic Acid on Catalytic Nitrate Reduction Kinetics Using an Alumina Supported Pd−Cu Catalyst. Environ. Sci. Technol. 2006, 40, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.P.; Shapley, J.R.; Werth, C.J. Regeneration of Sulfur-Fouled Bimetallic Pd-Based Catalysts. Environ. Sci. Technol. 2007, 41, 5491–5497. [Google Scholar] [CrossRef] [PubMed]

| “Well” | “WTP” | |
|---|---|---|
| pH | 6.59 | 6.62 |
| Conductivity (µS cm−1) | 423 | 416 |
| TOC (mg L−1) | 3.6 | 3.7 |
| TC (mg L−1) | 40.8 | 39.9 |
| IC (mg L−1) | 37.1 | 36.1 |
| BrO3− (mg L−1) | - | - |
| Br− (mg L−1) | 0.32 | 5.33 |
| NO3− (mg L−1) | 1.30 | 0.33 |
| NO2− (mg L−1) | 0.07 | - |
| Cl− (mg L−1) | 5.16 | 0.42 |
| SO42− (mg L−1) | 0.17 | - |
| Na+ (mg L−1) | 7.17 | 0.10 |
| NH4+ (mg L−1) | 1.11 | 0.63 |
| Ca2+ (mg L−1) | 17.7 | 1.35 |
| Mg2+ (mg L−1) | 3.35 | - |
| Support (Catalyst) | SBET (±5 m2 g−1) | SMESO (±5 m2 g−1) | VP,P/P0=0.95 (±0.005 cm3 g−1) | VMICRO (±0.005 cm3 g−1) |
|---|---|---|---|---|
| AC (1% Pd/AC) | 897 (855) | 117 (120) | 0.455 (0.442) | 0.321 (0.303) |
| MWCNT-O (1% Pd/MWCNT-O) | 255 (201) | - | 0.498 (0.475) | - |
| MWCNT-BM (1% Pd/MWCNT-BM) | 318 (227) | - | 0.569 (0.428) | - |
| MWCNT@N (1% Pd/MWCNT@N) | 225 (177) | - | 0.494 (0.401) | - |
| MWCNT-HNO3 (1% Pd/MWCNT-HNO3) | 310 (263) | - | 0.695 (0.557) | - |
| MWCNT-HNO3-900 (1% Pd/MWCNT-HNO3-900) | 316 (328) | - | 0.653 (0.727) | - |
| Sample | Carbon (%m/m) | Hydrogen (%m/m) | Nitrogen (%m/m) | Sulfur (%m/m) | Oxygen (%m/m) |
|---|---|---|---|---|---|
| AC | 84.6 | 0.86 | 0.01 | 0.67 | 5.90 |
| MWCNT-O | 88.4 | 0.25 | 0.00 | 0.00 | 1.10 |
| MWCNT@N | 83.1 | 0.39 | 4.90 | 0.00 | 2.20 |
| MWCNT-HNO3 | 87.9 | 0.35 | 0.01 | 0.00 | 5.10 |
| MWCNT-HNO3-900 | 95.9 | 0.20 | 0.01 | 0.00 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.M.C.B.d.; Barbosa, J.R.M.; Restivo, J.; Orge, C.A.; Nogueira, A.; Castro-Silva, S.; Pereira, M.F.R.; Soares, O.S.G.P. Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water. C 2022, 8, 21. https://doi.org/10.3390/c8020021
Costa JMCBd, Barbosa JRM, Restivo J, Orge CA, Nogueira A, Castro-Silva S, Pereira MFR, Soares OSGP. Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water. C. 2022; 8(2):21. https://doi.org/10.3390/c8020021
Chicago/Turabian StyleCosta, João M. Cunha Bessa da, José R. Monteiro Barbosa, João Restivo, Carla A. Orge, Anabela Nogueira, Sérgio Castro-Silva, Manuel F. Ribeiro Pereira, and Olívia S. Gonçalves Pinto Soares. 2022. "Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water" C 8, no. 2: 21. https://doi.org/10.3390/c8020021
APA StyleCosta, J. M. C. B. d., Barbosa, J. R. M., Restivo, J., Orge, C. A., Nogueira, A., Castro-Silva, S., Pereira, M. F. R., & Soares, O. S. G. P. (2022). Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water. C, 8(2), 21. https://doi.org/10.3390/c8020021












