A Review on van der Waals Boron Nitride Quantum Dots
Abstract
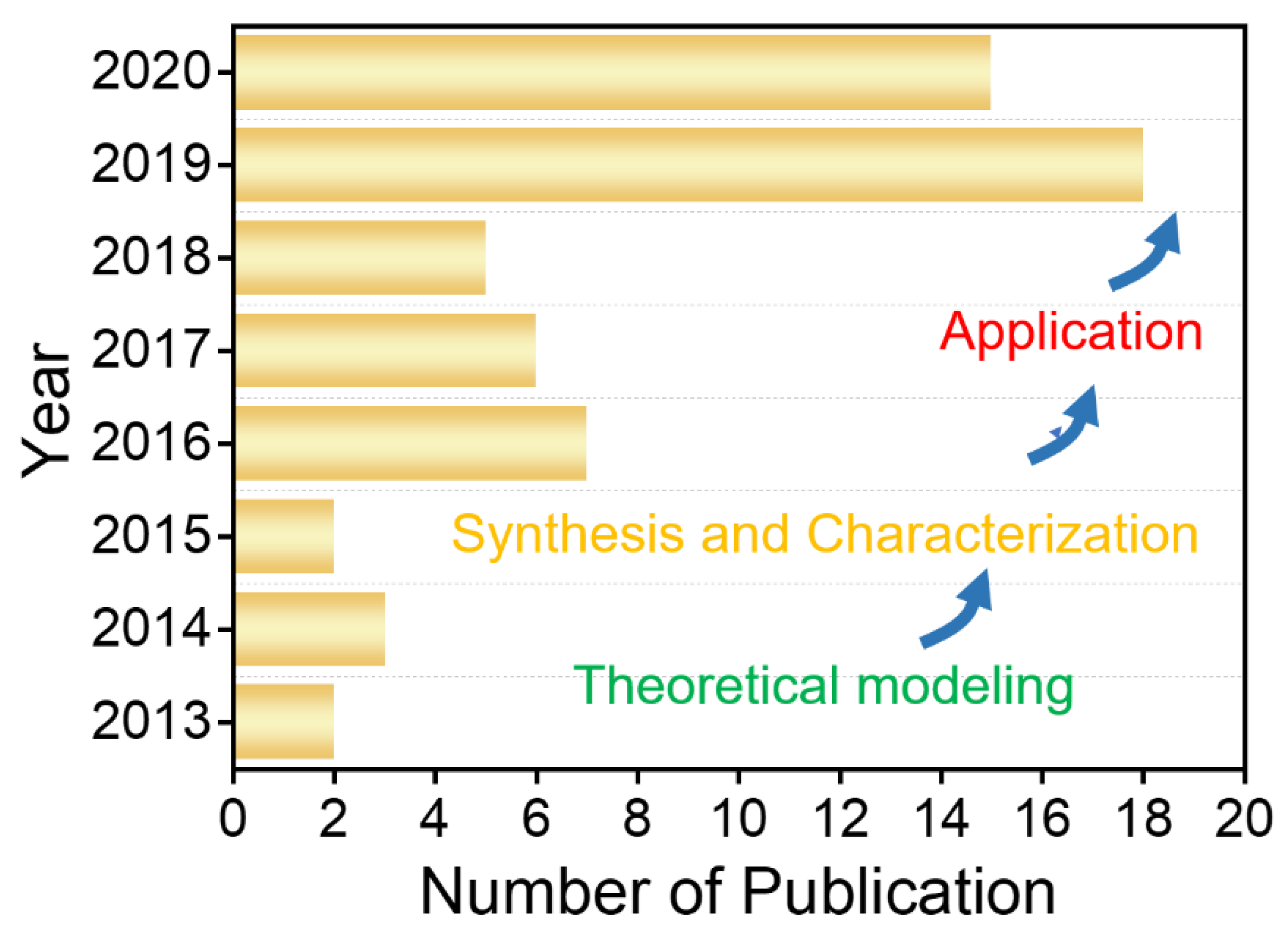
:1. Introduction
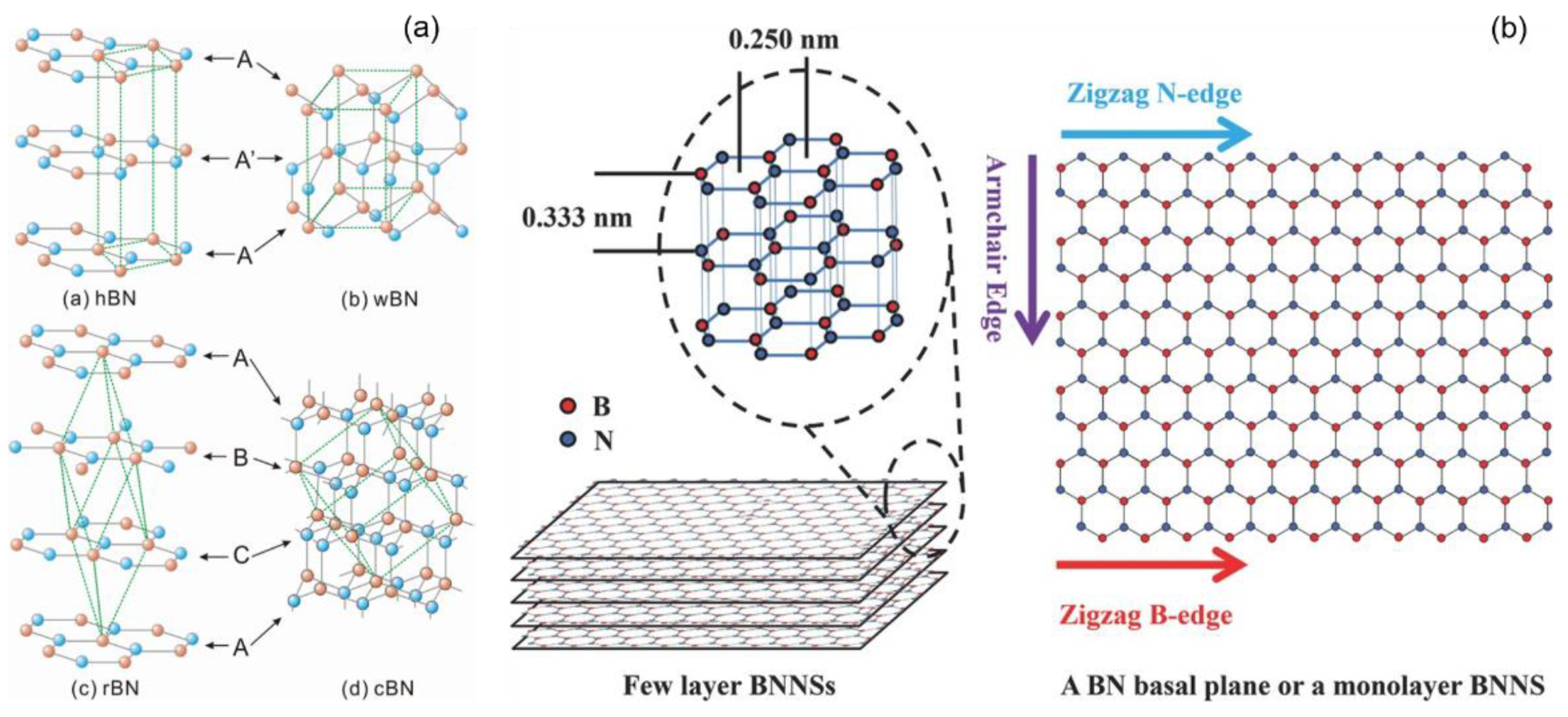
2. Crystal Structures
3. Synthesis of BNQDs
3.1. Top-Down Approaches
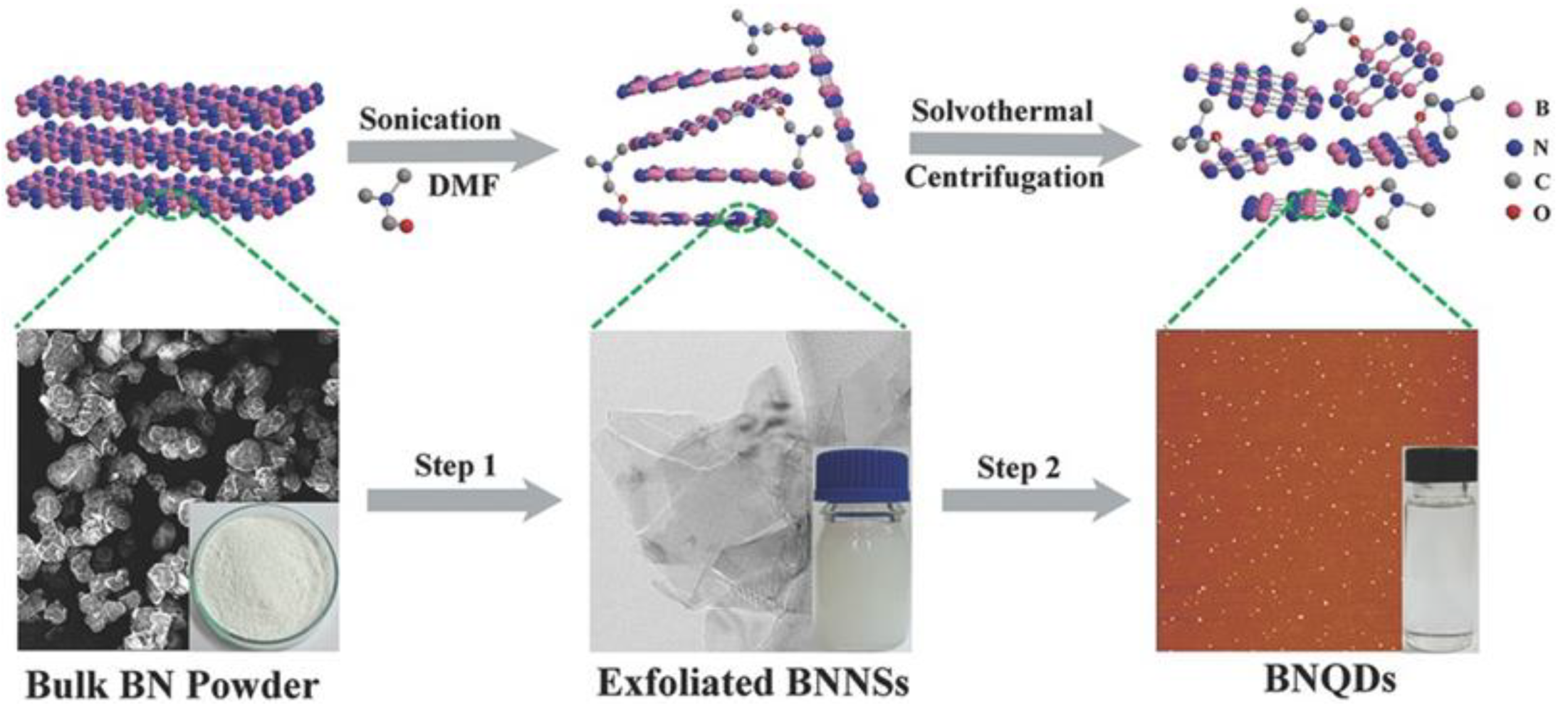
3.1.1. Sonication-Assisted Liquid Exfoliation Method
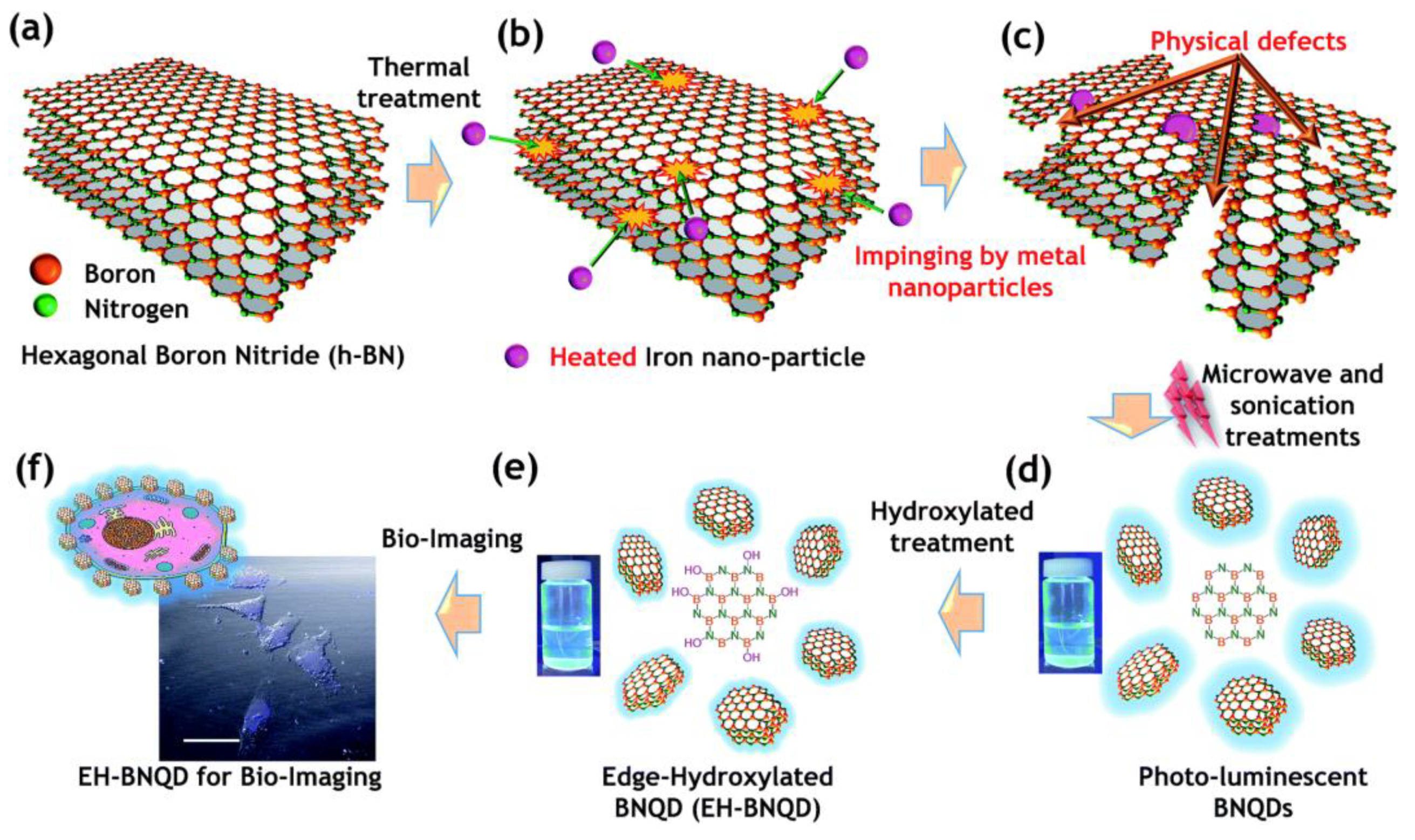
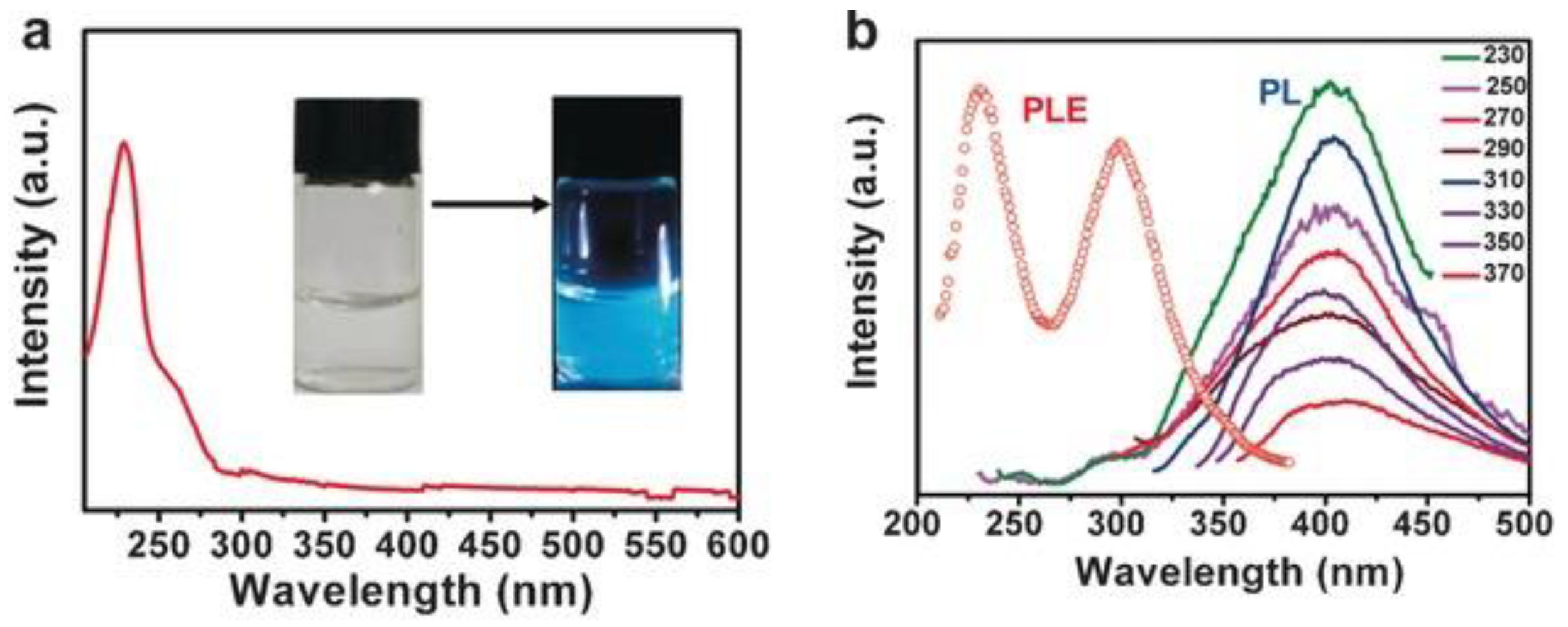
3.1.2. Defect Engineering Method
3.1.3. Intercalation Method
3.1.4. Other Methods
3.2. Bottom-Up Approaches
3.2.1. Hydrothermal Methods
3.2.2. Carbothermal Reduction Reaction
3.2.3. Other Methods
3.3. Combined Method
4. Applications of BNQDs
4.1. Biological Application
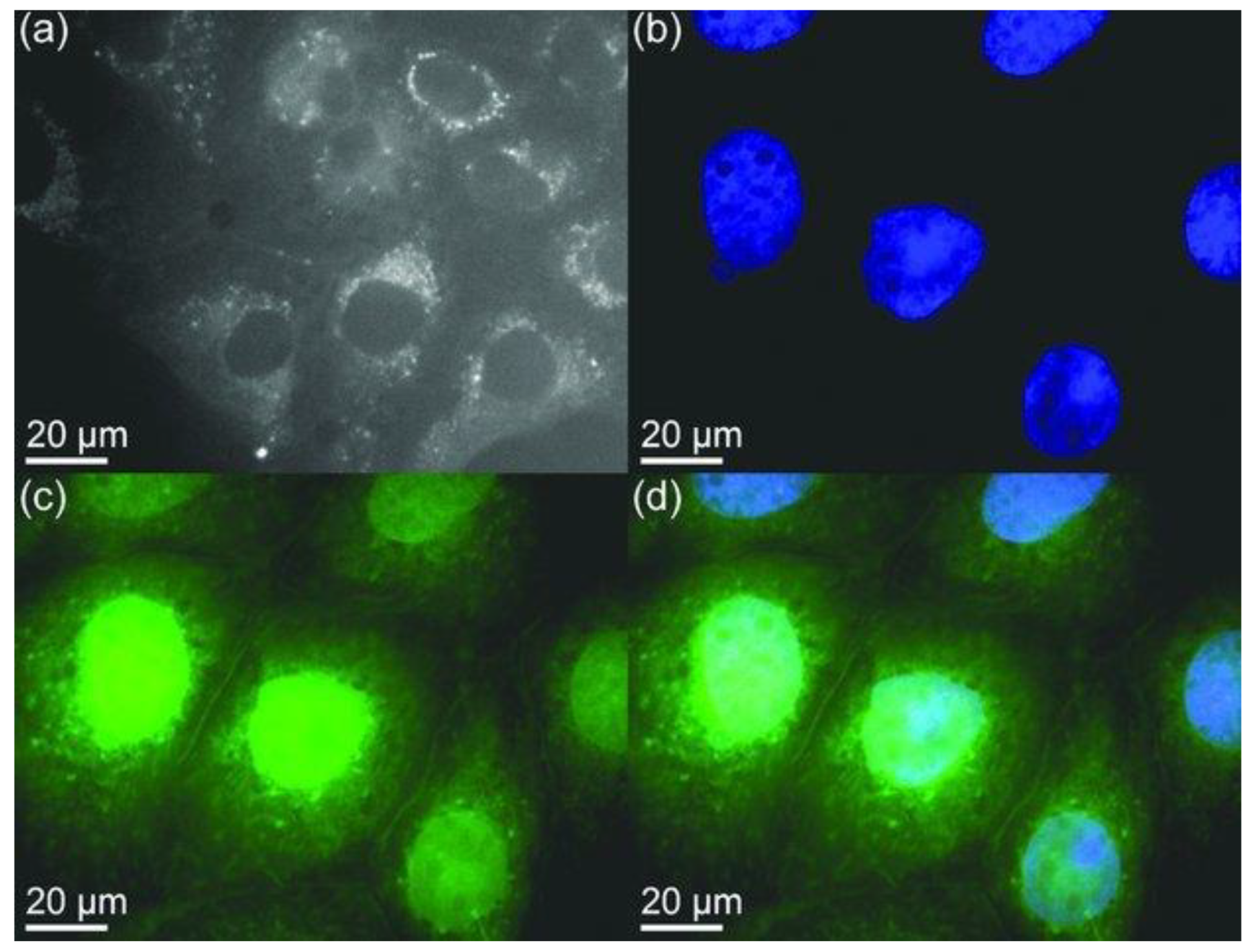
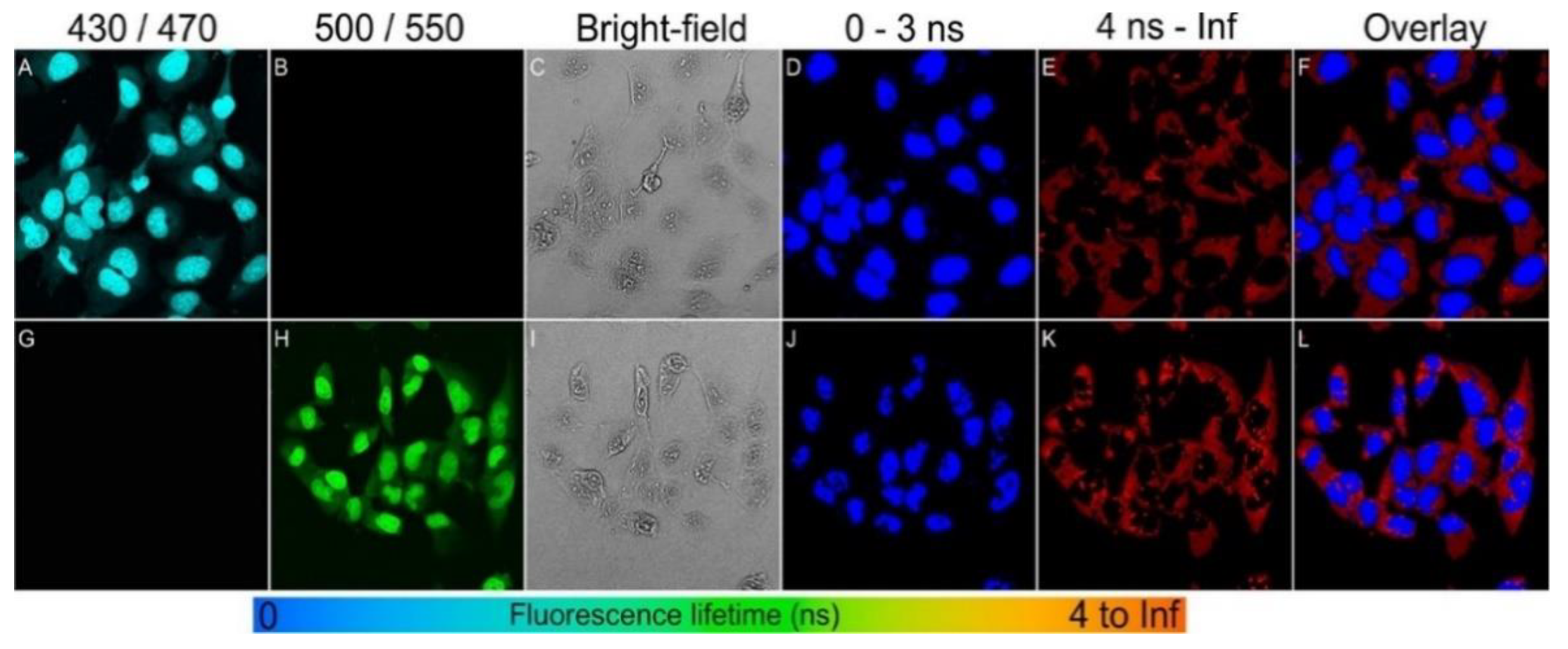
4.1.1. Bioimaging
4.1.2. Biosensing
4.2. Chemical Sensing
4.3. Gas Sensing
4.4. Temperature Sensing
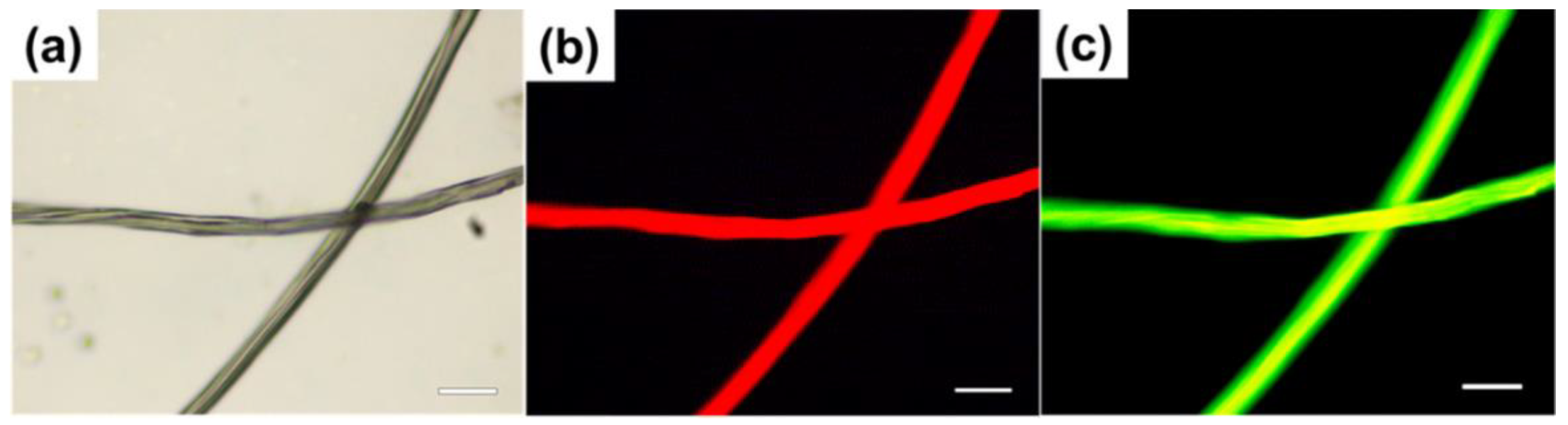
4.5. Fluorescent Staining
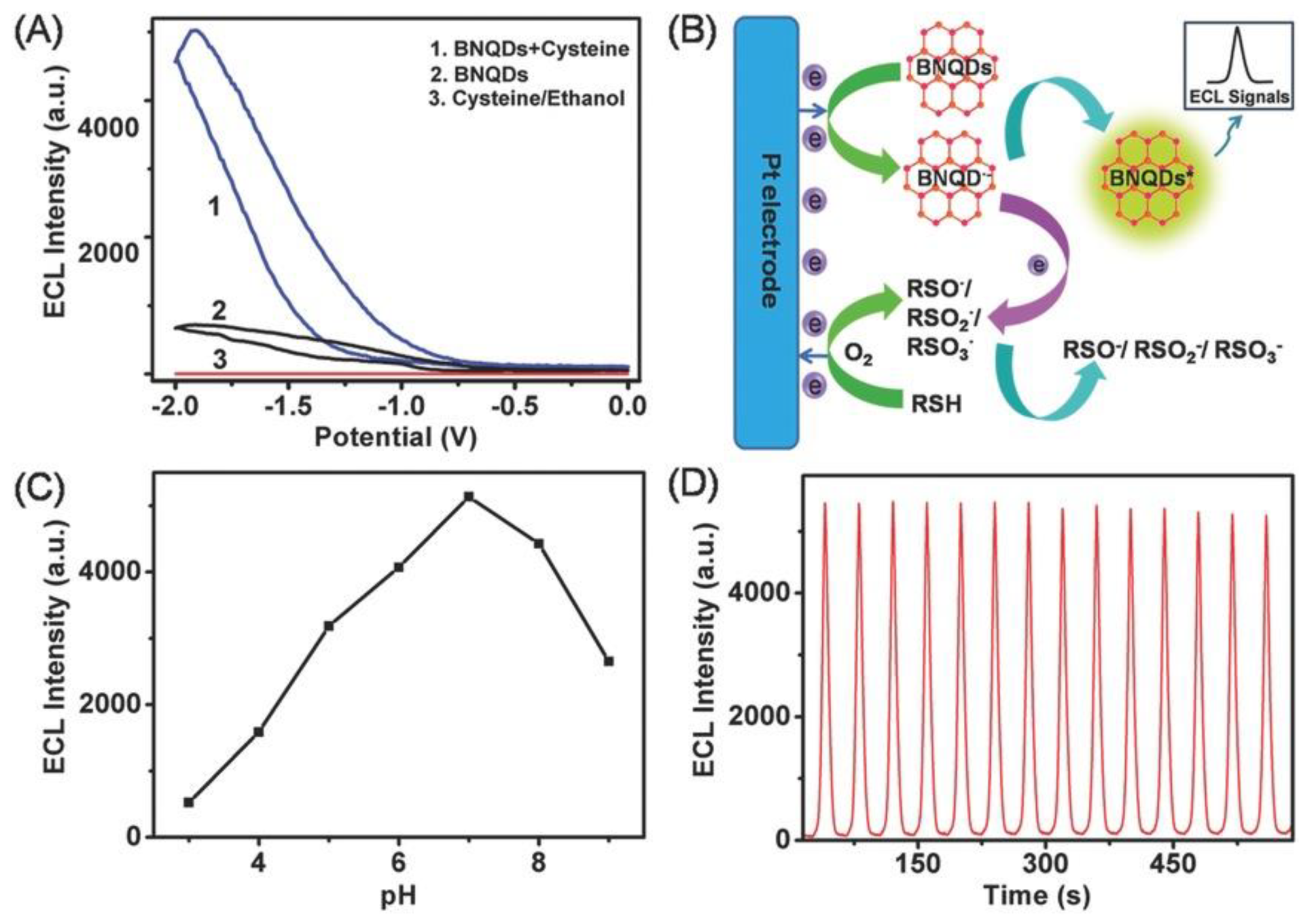
4.6. Electrochemiluminescence (ECL) Responses
4.7. Photocatalyst
4.8. Other Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, C.; Xu, Y.; Bando, Y.; Golberg, D. Highly thermo-conductive fluid with boron nitride nanofillers. ACS Nano 2011, 5, 6571–6577. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Santos, E.J.G.; Xing, T.; Cappelluti, E.; Roldán, R.; Chen, Y.; Watanabe, K.; Taniguchi, T. Dielectric screening in atomically thin boron nitride nanosheets. Nano Lett. 2014, 15, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.; Long, M.; He, J. Enhanced thermoelectric properties in boron nitride quantum-dot. Results Phys. 2017, 7, 1487–1491. [Google Scholar] [CrossRef]
- Lee, C.H.; Xie, M.; Kayastha, V.; Wang, J.; Yap, Y.K. Patterned growth of boron nitride nanotubes by catalytic chemical vapor deposition. Chem. Mater. 2010, 22, 1782–1787. [Google Scholar] [CrossRef]
- Qin, J.-K.; Liao, P.-Y.; Si, M.; Gao, S.; Qiu, G.; Jian, J.; Wang, Q.; Zhang, S.-Q.; Huang, S.; Charnas, A.; et al. Raman response and transport properties of tellurium atomic chains encapsulated in nanotubes. Nat. Electron. 2020, 3, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.; Hao, B.; Waters, K.; Lee, C.H.; Idrobo, J.C.; Zhang, D.; Pandey, R.; Yap, Y.K. Two-dimensional gold quantum dots with tunable bandgaps. ACS Nano 2019, 13, 4347–4353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, D.; Yap, Y.K. Boron nitride nanotubes: Recent advances in their synthesis, functionalization, and applications. Molecules 2016, 21, 922. [Google Scholar] [CrossRef]
- Lee, C.H.; Qin, S.; Savaikar, M.A.; Wang, J.; Hao, B.; Zhang, D.; Banyai, D.; Jaszczak, J.A.; Clark, K.W.; Idrobo, J.-C.; et al. Room-temperature tunneling behavior of boron nitride nanotubes functionalized with gold quantum dots. Adv. Mater. 2013, 25, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee, C.H.; Yap, Y.K. Recent advancements in boron nitride nanotubes. Nanoscale 2010, 2, 2028–2034. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nat. Cell Biol. 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Hitz, E.; Lin, Y.; Yang, B.; Hu, L. Solution processed boron nitride nanosheets: Synthesis, assemblies and emerging applications. Adv. Funct. Mater. 2017, 27, 1701450. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, J.; Nitta, T.; Natori, A. Electronic and magnetic properties of BNC ribbons. Phys. Rev. B 2005, 72, 205429. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, G.; Liu, Z.; Wu, J.; Duan, W.; Gu, B.L.; Zhang, S.B. Half metallicity along the edge of zigzag boron Ni-tride nanoribbons. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 78, 205415. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E. Magnetic boron nitride nanoribbons with tunable electronic properties. Nano Lett. 2008, 8, 2210–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Guo, W. Energy-gap modulation of BN ribbons by transverse electric fields: First-principles calculations. Phys. Rev. B 2008, 77, 075403. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Zhao, M.; Wang, X.; Li, S.; He, X.; Wang, Z.; Bu, H. Honeycomb-patterned quantum dots beyond graphene. J. Phys. Chem. C 2011, 115, 17743–17749. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sharma, S.R.K.C.Y.; Pati, S.K. Tuning the electronic and optical properties of graphene and Boron nitride quantum dots by molecular charge-transfer interactions: A theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 13881–13887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamijala, S.S.; Bandyopadhyay, A.; Pati, S.K. Structural stability, electronic, magnetic, and optical properties of rectangular graphene and boron nitride quantum dots: Effects of size, substitution, and electric field. J. Phys. Chem. C 2013, 117, 23295–23304. [Google Scholar] [CrossRef] [Green Version]
- Stengl, V.; Kormunda, M.; Štengl, V.; Henych, J. Self-assembled BN and BCN quantum dots obtained from high intensity ultrasound exfoliated nanosheets. Sci. Adv. Mater. 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.M.; Ong, A.C.M.; Allwood, D.A. Fabrication and luminescence of monolayered boron nitride quantum dots. Small 2013, 10, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Shtansky, D.V.; Firestein, K.L.; Golberg, D.V. Fabrication and application of BN nanoparticles, nanosheets and their nanohybrids. Nanoscale 2018, 10, 17477–17493. [Google Scholar] [CrossRef] [Green Version]
- Stagi, L.; Ren, J.; Innocenzi, P. From 2-D to 0-D boron nitride materials, the next challenge. Materials 2019, 12, 3905. [Google Scholar] [CrossRef] [Green Version]
- Paine, R.T.; Narula, C.K. Synthetic routes to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Mishra, N.S.; Saravanan, P. A review on the synergistic features of hexagonal boron nitride (white graphene) as Ad-sorbent-photo active nanomaterial. Chem. Select 2018, 3, 8023–8034. [Google Scholar]
- Zhang, W.J.; Chong, Y.M.; Bello, I.; Lee, S.T. Nucleation, growth and characterization of cubic boron nitride (cBN) films. J. Phys. D Appl. Phys. 2007, 40, 6159–6174. [Google Scholar] [CrossRef]
- Lee, C.H.; Kayastha, V.K.; Wang, J.; Yap, Y.K. Introduction to B-C-N materials. In B-C-N Nanotubes and Related Nanostructures; Springer: New York, NY, USA, 2009; Volume 6, pp. 1–22. [Google Scholar] [CrossRef]
- Hao, B.; Lee, C.H.; Wang, J.; Asthana, A.; Winslow, D.; Zhang, D.; Yap, Y.K. Controlled synthesis of functional boron nitride nanostructures for applications. In Nanotubes and Nanosheets: Functionalization and Applications of Boron Nitride and Other Materials; Ying, I.C., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 551–572. [Google Scholar]
- Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, N.; Yap, Y.K. Introduction to boron nitride nanotubes: Synthesis, properties, functionalization, and cutting. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–15. Available online: https://www.sciencedirect.com/science/article/pii/B9780323389457000018 (accessed on 26 March 2021).
- Hod, O. Graphite and hexagonal boron-nitride have the same interlayer distance. Why? J. Chem. Theory Comput. 2012, 8, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Gorbachev, R.V.; Riaz, I.; Nair, R.R.; Jalil, R.; Britnell, L.; Belle, B.D.; Hill, E.W.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Hunting for monolayer boron nitride: Optical and Raman signatures. Small 2011, 7, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Paciĺ, D.; Meyer, J.C.; Girit, Ç.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Wu, S.; Brzozowski, K.J. Surface free energy and polarity of organic pigments. J. Colloid Interface Sci. 1971, 37, 686–690. [Google Scholar] [CrossRef]
- Li, H.; Tay, R.Y.; Tsang, S.H.; Zhen, X.; Teo, E.H.T. Controllable synthesis of highly luminescent boron nitride quantum dots. Small 2015, 11, 6491–6499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphene quantum dots sur-face-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. N. J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Lei, Z.; Xu, S.; Wan, J.; Wu, P. Facile preparation and multifunctional applications of boron nitride quantum dots. Nanoscale 2015, 7, 18902–18907. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xu, Y.; Wang, Y.; Chen, X.; Ji, X.; Niu, F.; Song, Z.; Liu, J. Boron nitride quantum dots with solvent-regulated blue/green photoluminescence and electrochemiluminescent behavior for versatile applications. Adv. Opt. Mater. 2017, 5, 1600661. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of cardiac troponin-I biosensor based on boron nitride quantum dots including molecularly imprinted polymer. Biosens. Bioelectron. 2019, 126, 418–424. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, Y.; He, M.; Tong, Y.; Zhong, X.; Fang, J.; Bu, X. Facile microwave approach to controllable boron nitride quantum dots. J. Mater. Sci. 2017, 52, 13522–13532. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Yadav, S.K.; Savu, R.; Moshkalev, S.A. Mechanical pressure-induced chemical cutting of boron nitride sheets into boron nitride quantum dots and optical properties. J. Alloys Compd. 2016, 683, 38–45. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Wang, Z.; Pei, Z.; Huang, Y.; Song, X.; Zeng, H.; Zhi, C. Hydrothermal synthesis of blue-fluorescent monolayer BN and BCNO quantum dots for bio-imaging probes. RSC Adv. 2016, 6, 79090–79094. [Google Scholar] [CrossRef]
- Dehghani, A.; Ardekani, S.M.; Lesani, P.; Hassan, M.; Gomes, V.G. Two-photon active boron nitride quantum dots for multiplexed imaging, intracellular ferric ion biosensing, and pH tracking in living cells. ACS Appl. Bio Mater. 2018, 1, 975–984. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Wang, X. One-pot solvothermal synthesis of water-soluble boron nitride nanosheets and fluorescent boron nitride quantum dots. Mater. Lett. 2019, 234, 306–310. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kotal, M.; Jang, M.-H.; Lee, J.; Cho, Y.-H.; Kim, W.-J.; Oh, I.-K. Defect engineering route to boron nitride quantum dots and edge-hydroxylated functionalization for bio-imaging. RSC Adv. 2016, 6, 73939–73946. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, S. Creating high yield water-soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem. Commun. 2012, 48, 10177–10179. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Li, F.F.; Cui, W.R.; Liang, R.P.; Qiu, J.D. Facile and green approach to the synthesis of boron Ni-tride quantum dots for 2,4,6-trinitrophenol sensing. ACS Appl. Mater. Interfaces 2018, 10, 7315–7323. [Google Scholar] [CrossRef]
- Thangasamy, P.; Santhanam, M.; Sathish, M. Supercritical fluid facilitated disintegration of hexagonal boron nitride nanosheets to quantum dots and its application in cells imaging. ACS Appl. Mater. Interfaces 2016, 8, 18647–18651. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, D.; Sone, K.; Wang, M.; Gautam, U.K.; Golberg, D.; Itoh, H.; Ichihara, M.; Honma, I. Rapid and direct conversion of graphite crystals into high-yielding, good-quality graphene by supercritical fluid exfoliation. Chem. A Eur. J. 2010, 16, 6488–6494. [Google Scholar] [CrossRef]
- Thangasamy, P.; Sathish, M. Supercritical fluid processing: A rapid, one-pot exfoliation process for the production of surfactant-free hexagonal boron nitride nanosheets. CrystEngComm 2015, 17, 5895–5899. [Google Scholar] [CrossRef]
- Sathish, M.; Mitani, S.; Tomai, T.; Honma, I. Supercritical fluid assisted synthesis of N-doped graphene nanosheets and their capacitance behavior in ionic liquid and aqueous electrolytes. J. Mater. Chem. A 2014, 2, 4731–4738. [Google Scholar] [CrossRef]
- Thangasamy, P.; Sathish, M. Rapid, one-pot synthesis of luminescent MoS2 nanoscrolls using supercritical fluid processing. J. Mater. Chem. C 2016, 4, 1165–1169. [Google Scholar] [CrossRef]
- Angizi, S.; Hatamie, A.; Ghanbari, H.; Simchi, A.A. Mechanochemical green synthesis of exfoliated edge-functionalized boron nitride quantum dots: Application to vitamin C sensing through hybridization with gold electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28819–28827. [Google Scholar] [CrossRef]
- Angizi, S.; Shayeganfar, F.; Azar, M.H.; Simchi, A. Surface/edge functionalized boron nitride quantum dots: Spectroscopic fingerprint of bandgap modification by chemical functionalization. Ceram. Int. 2020, 46, 978–985. [Google Scholar] [CrossRef]
- Cai, R.; Nie, M.; Xu, F. Ultrafast turbulence-induced disintegration of BN and WS2 quantum dots for potential multifunctional nanotheranostics. Mater. Des. 2019, 181, 107925. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Park, J.H.; Neupane, R.; Reyes, C.A.D.L.; Sudeep, P.M.; Paulose, M.; Martí, A.A.; Tiwary, C.S.; Khabashesku, V.N.; Varghese, O.K.; et al. Fluorinated boron nitride quantum dots: A new 0D material for energy conversion and detection of cellular metabolism. Part. Part. Syst. Charact. 2019, 36, 1800346. [Google Scholar] [CrossRef]
- Duong, N.M.H.; Glushkov, E.; Chernev, A.; Navikas, V.; Comtet, J.; Nguyen, M.A.P.; Toth, M.; Radenovic, A.; Tran, T.T.; Aharonovich, I. Facile production of hexagonal boron nitride nanoparticles by cryogenic exfoliation. Nano Lett. 2019, 19, 5417–5422. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.T.; Firestein, K.L.; Steinman, A.E.; Kovalskii, A.M.; Sukhorukova, I.V.; Lebedev, O.I.; Shtansky, D.V.; Golberg, D. Synthesis of boron nitride nanostructures from borates of alkali and alkaline earth metals. J. Mater. Chem. A 2015, 3, 20749–20757. [Google Scholar] [CrossRef]
- Weng, Q.; Ide, Y.; Wang, X.; Wang, X.; Zhang, C.; Jiang, X.; Xue, Y.; Dai, P.; Komaguchi, K.; Bando, Y.; et al. Design of BN porous sheets with richly exposed (002) plane edges and their application as TiO2 visible light sensitizer. Nano Energy 2015, 16, 19–27. [Google Scholar] [CrossRef]
- Xiong, C.; Tu, W. Synthesis of water-dispersible boron nitride nanoparticles. Eur. J. Inorg. Chem. 2014, 2014, 3010–3015. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Jiang, L.; Chen, J.; Li, Y.; Guo, W.; Hu, P.; Liu, Y. Growth and etching of monolayer hexagonal boron nitride. Adv. Mater. 2015, 27, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, S.; Song, Z.; Liu, M.; Ji, X.; Yang, W.; Liu, J. One-step synthesis of boron nitride quantum Dots: Simple chemistry meets delicate nanotechnology. Chem. A Eur. J. 2016, 22, 18899–18907. [Google Scholar] [CrossRef]
- Xing, H.; Zhai, Q.; Zhang, X.; Li, J.; Wang, E. Boron nitride quantum dots as efficient coreactant for enhanced electrochemiluminescence of ruthenium(II) Tris(2,2′-Bipyridyl). Anal. Chem. 2018, 90, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.; Chen, L.; Liu, D. A novel electrochemiluminescence sensor based on resonance energy transfer system between nitrogen doped graphene quantum dots and boron nitride quantum dots for sensitive detection of folic acid. Anal. Chim. Acta 2019, 1090, 57–63. [Google Scholar] [CrossRef]
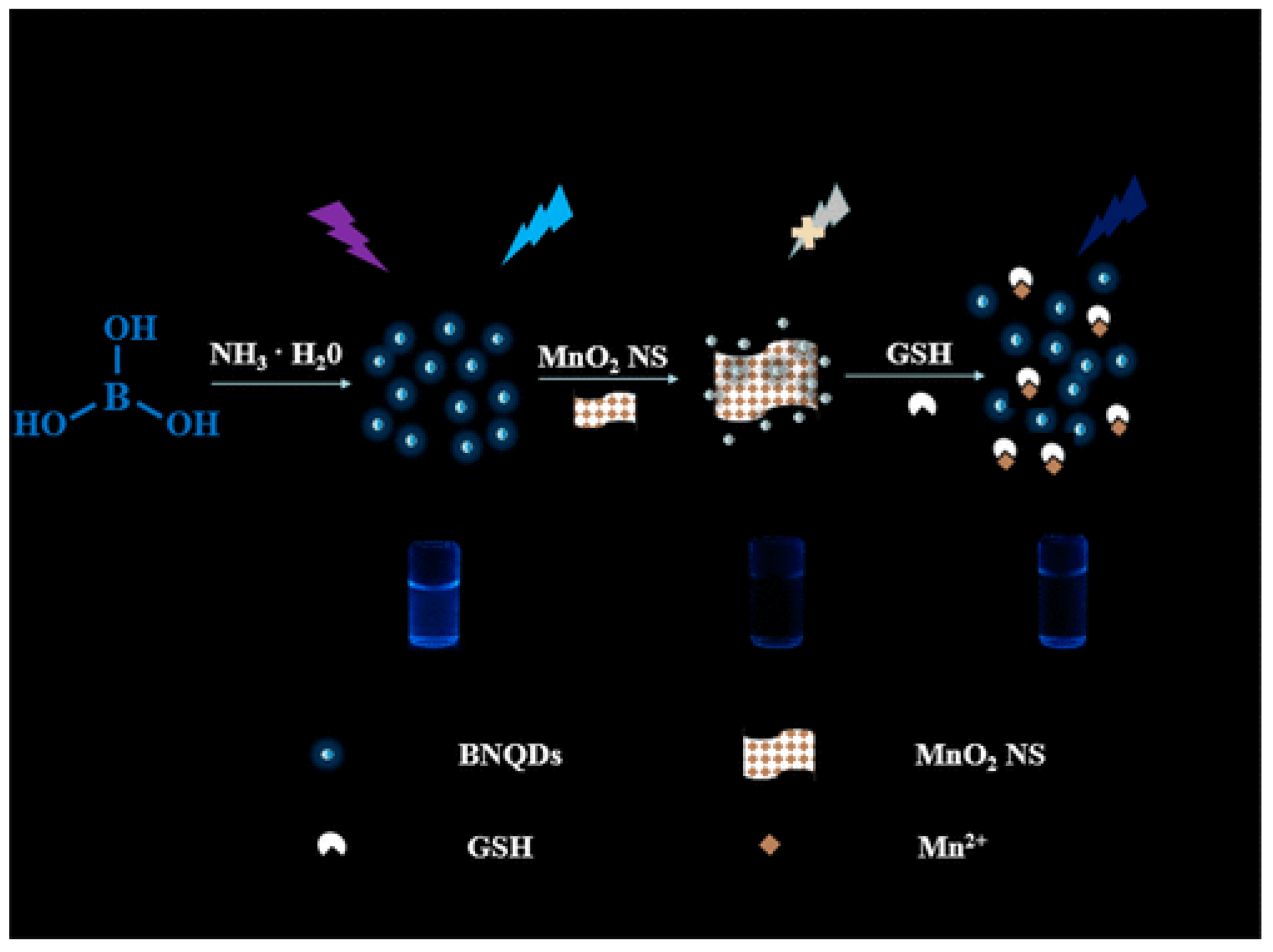
- Peng, C.; Xing, H.; Fan, X.; Xue, Y.; Li, J.; Wang, E. Glutathione regulated inner filter effect of MnO 2 nanosheets on boron nitride quantum dots for sensitive assay. Anal. Chem. 2019, 91, 5762–5767. [Google Scholar] [CrossRef]
- Qin, D.; Jiang, X.; Mo, G.; Feng, J.; Deng, B. Boron nitride quantum dots as electrochemiluminescence coreactants of RGO@Au@Ru–SiO2 for label-free detection of AFP in human serum. Electrochim. Acta 2020, 335, 135621. [Google Scholar] [CrossRef]
- Choudhury, S.P.; Feng, Z.; Gao, C.; Ma, X.; Zhan, J.; Jia, F. BN quantum dots decorated ZnO nanoplates sensor for enhanced detection of BTEX gases. J. Alloys Compd. 2020, 815, 152376. [Google Scholar] [CrossRef]
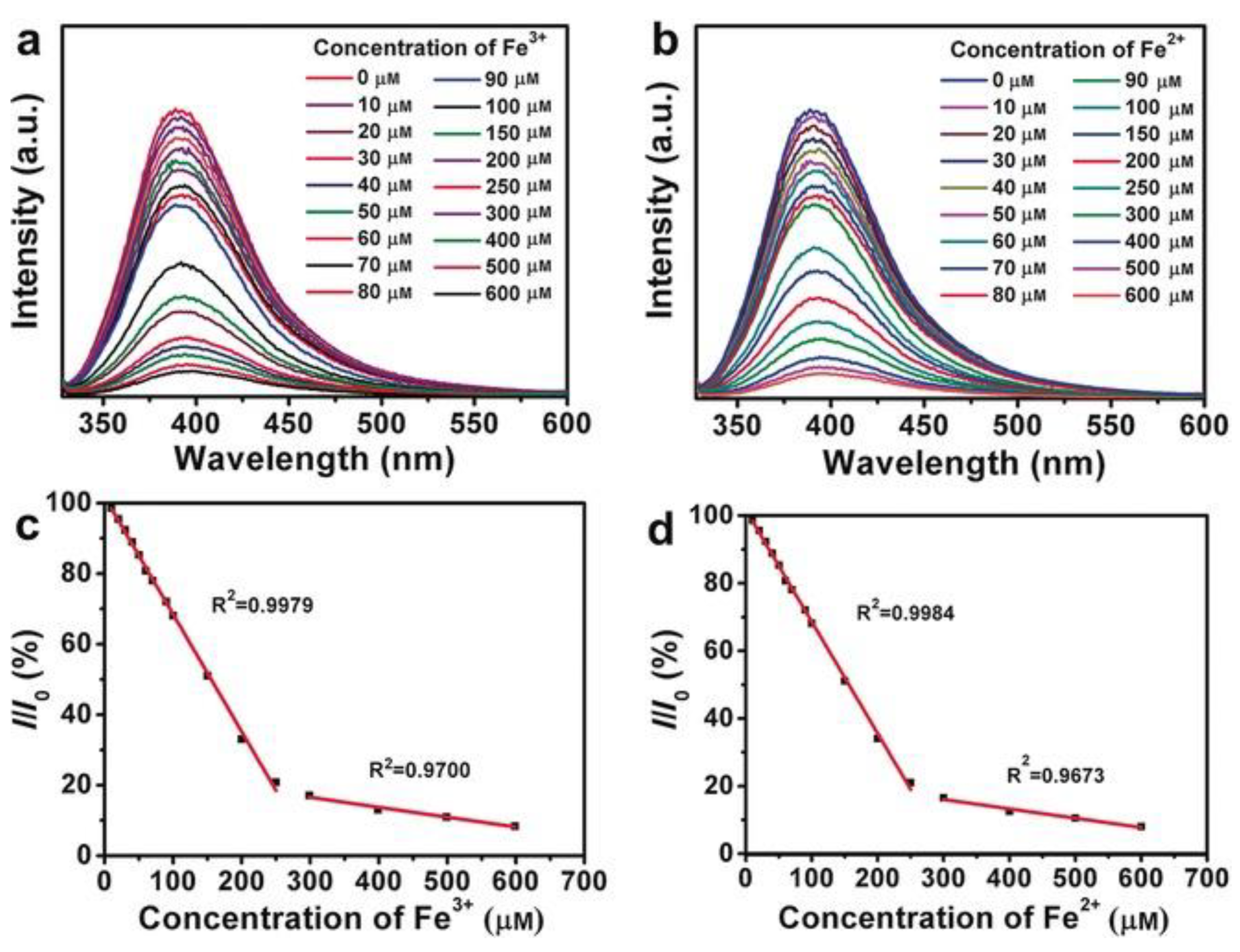
- Huo, B.; Liu, B.; Chen, T.; Cui, L.; Xu, G.; Liu, M.; Liu, J. One step synthesis of fluorescent boron nitride quantum dots via a hydrothermal strategy using melamine as nitrogen source for the detection of ferric ions. Langmuir 2017, 33, 10673–10678. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Mohanta, M.K.; Sahu, T.K.; Gogoi, D.; Peela, N.R.; Qureshi, M. Hexagonal boron nitride quantum dots as a superior hole Extractor for efficient charge separation in WO3-based photoelectrochemical water oxidation. ACS Appl. Energy Mater. 2019, 2, 7457–7466. [Google Scholar] [CrossRef]
- Sahu, T.K.; Mohanta, M.K.; Qureshi, M. Modulating water oxidation kinetics utilizing H-BN quantum dots as an efficient hole extractor on fluorine doped hematite photoanode. J. Power Sources 2020, 445, 227341. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, M.; Ma, Q. A visual electrochemiluminescence resonance energy transfer/surface plasmon coupled electrochemiluminescence nanosensor for Shiga toxin-producing Escherichia coli detection. Green Chem. 2018, 20, 5520–5527. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Nie, Y.; Zhang, Q.; Ma, Q. Sulfur regulated boron nitride quantum dots electrochemiluminescence with amplified surface plasmon coupling strategy for BRAF gene detection. Anal. Chem. 2019, 91, 6250–6258. [Google Scholar] [CrossRef]
- Yasnó, J.P.; Kiminami, R.H. Short time reaction synthesis of nano-hexagonal boron nitride. Adv. Powder Technol. 2020, 31, 4436–4443. [Google Scholar] [CrossRef]
- Jerome, R.; Sundramoorthy, A.K. Hydrothermal synthesis of boron nitride quantum dots/poly(luminol) nanocomposite for selective detection of ascorbic acid. J. Electrochem. Soc. 2019, 166, B3017–B3024. [Google Scholar] [CrossRef]
- Yang, K.; Jia, P.; Hou, J.; Bu, T.; Sun, X.; Liu, Y.; Wang, L. Innovative dual-emitting ratiometric fluorescence sensor for tetracyclines detection based on boron nitride quantum dots and europium ions. ACS Sustain. Chem. Eng. 2020, 8, 17185–17193. [Google Scholar] [CrossRef]
- Yao, Q.; Feng, Y.; Rong, M.; He, S.; Chen, X. Determination of nickel(II) via quenching of the fluorescence of boron nitride quantum dots. Microchim. Acta 2017, 184, 4217–4223. [Google Scholar] [CrossRef]
- Kong, Y.; He, Y.; Zhou, J.; Zhong, S.; Song, G. Amino acids as the nitrogen source to synthesize boron nitride quantum dots for fluorescence turn-off-on detection of ascorbic acid. ChemistrySelect 2020, 5, 3828–3834. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Ji, N.; Xu, B.; Yu, H. Boron nitride quantum dots derived from renewable lignin. ChemistrySelect 2019, 4, 3025–3030. [Google Scholar] [CrossRef]
- Wang, X.B.; Weng, Q.; Wang, X.; Li, X.; Zhang, J.; Liu, F.; Jiang, X.F.; Guo, H.; Xu, N.; Golberg, D.; et al. Biomass directed synthesis of 20 g high-quality boron nitride nanosheets for thermoconductive polymeric composites. ACS Nano 2014, 8, 9081–9088. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Muhammad, N.; Khan, G.; Rehman, F.; Khan, A.S.; Ullah, Z.; Khan, A.; Ali, H.; Ahmed, S.M.; et al. Fabrication of hexagonal boron nitride quantum dots via a facile bottom-up technique. Ceram. Int. 2019, 45, 22765–22768. [Google Scholar] [CrossRef]
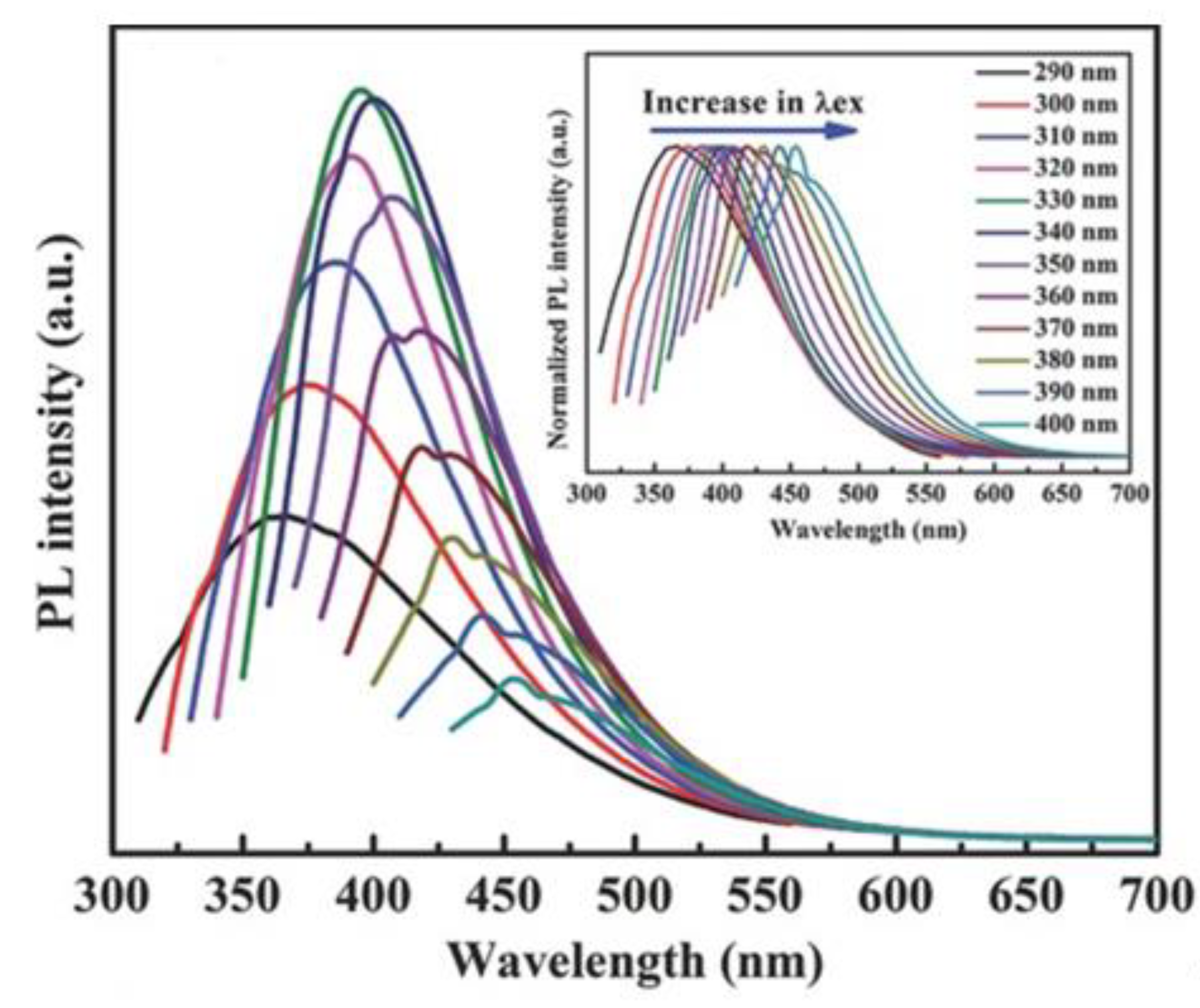
- Li, Q.; Zheng, Y.; Hou, X.; Yang, T.; Liang, T.; Zheng, J. A wide range photoluminescence intensity based temperature sensor developed with BN quantum dots and the photoluminescence mechanism. Sens. Actuators B Chem. 2020, 304, 127353. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef] [Green Version]
- Yola, M.L. Electrochemical activity enhancement of monodisperse boron nitride quantum dots on graphene oxide: Its application for simultaneous detection of organophosphate pesticides in real samples. J. Mol. Liq. 2019, 277, 50–57. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, J.; Guo, L.; Luo, F.; Qiu, B.; Hong, G.; Lin, Z. Targets regulated formation of boron nitride quantum dots—Gold nanoparticles nanocomposites for ultrasensitive detection of acetylcholinesterase activity and its inhibitors. Sens. Actuators B Chem. 2019, 279, 61–68. [Google Scholar] [CrossRef]
- Han, Y.; Niu, Y.; Liu, M.; Niu, F.; Xu, Y.; Niu, F. A rational strategy to develop a boron nitride quantum dot-based molecular logic gate and fluorescent assay of alkaline phosphatase activity. J. Mater. Chem. B 2019, 7, 897–902. [Google Scholar] [CrossRef]
- Park, Y.; Koo, C.; Chen, H.Y.; Han, A.; Son, D.H. Ratiometric temperature imaging using environment insensitive luminescence of Mn-doped core shell nanocrystals. Nanoscale 2013, 5, 4944–4950. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kong, X.; Yu, Y.; Sun, Y.; Zhang, H. Effect of annealing on upconversion luminescence of ZnO:Er3+ nano-crystals and high thermal sensitivity. J. Phys. Chem. C 2007, 111, 15119–15124. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, K.; Rai, S. Er3+/Yb3+ codoped Gd2O3 nano-phosphor for optical thermometry. Sens. Actuators A Phys. 2009, 149, 16–20. [Google Scholar] [CrossRef]
- Wang, S.; Westcott, S.; Chen, W. Nanoparticle luminescence thermometry. J. Phys. Chem. B 2002, 106, 11203–11209. [Google Scholar] [CrossRef]
- Lan, J.; Zou, H.; Liu, Z.; Gao, M.; Chen, B.; Li, Y.; Huang, C. A visual physiological temperature sensor developed with gelatin-stabilized luminescent silver nanoclusters. Talanta 2015, 143, 469–473. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, X.; Li, L.; Lou, X.; Xu, J.; Zhou, P.; Yan, M. Temperature dependent photoluminescence of composition tunable Zn_xAgInSe quantum dots and temperature sensor application. Opt. Express 2017, 25, 19065–19076. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chen, Y.-N.; Hsu, P.-C.; Shih, C.-C.; Chang, H.-T. Photoluminescent organosilane-functionalized carbon dots as temperature probes. Chem. Commun. 2013, 49, 1639–1641. [Google Scholar] [CrossRef]
- Zhai, Q.; Li, J.; Wang, E. Recent advances based on nanomaterials as electrochemiluminescence probes for the fabrication of sensors. Chem Electro Chem 2017, 4, 1639–1650. [Google Scholar] [CrossRef]
- Richter, M.M. Electrochemiluminescence. In Optical Biosensors; Elsevier: Amsterdam, The Netherlands, 2008; pp. 317–384. Available online: https://www.elsevier.com/books/optical-biosensors/ligler/978-0-444-53125-4 (accessed on 26 March 2021).
- Gao, J.; Chen, Z.; Mao, L.; Zhang, W.; Wen, W.; Zhang, X.; Wang, S. Electrochemiluminescent aptasensor based on resonance energy transfer system between CdTe quantum dots and cyanine dyes for the sensitive detection of ochratoxin A. Talanta 2019, 199, 178–183. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Zhao, P.; Nie, Z.; Yao, S. A CdTe/CdS quantum dots amplified graphene quantum dots anodic electrochemiluminescence platform and the application for ascorbic acid detection in fruits. Electrochim. Acta 2015, 178, 407–413. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence ratiometry: A new approach to DNA biosensing. Anal. Chem. 2013, 85, 5321–5325. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Label free detection of folate receptor (+) cells by molecular recognition mediated electrochemiluminescence of CdTe nanoparticles. Anal. Chem. 2014, 86, 6872–6878. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Nie, Y.; Zhang, X.; Ma, Q. Polarized-Electrochemiluminescence biosensor based on surface plasmon coupling strategy and fluorine doped BN quantum dots. Anal. Chem. 2020, 92, 9223–9229. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Wang, P.; Liu, D. An electrochemiluminescence biosensor based on boron nitride quantum dots as novel coreactant for quantitative determination of concanavalin A. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hidalgo, M.L.; Wang, F.C.; Mishchenko, A.; Schedin, F.; Nair, R.R.; Hill, E.W.; Boukhvalov, D.W.; Katsnelson, M.I.; Dryfe, R.A.W.; et al. Proton transport through one-atom-thick crystals. Nat. Cell Biol. 2014, 516, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishita, T.; Okamoto, H. Facile exfoliation and noncovalent superacid functionalization of boron nitride nanosheets and their use for highly thermally conductive and electrically insulating polymer nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 27064–27073. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Sun, B.; Jiang, P. Highly thermally conductive yet electrically insulating polymer/boron nitride nanosheets nanocomposite films for improved thermal management capability. ACS Nano 2019, 13, 337–345. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Preparation of highly thermally conductive polymer composite at low filler content via a self assembly process between polystyrene microspheres and boron nitride nanosheets. ACS Appl. Mater. Interfaces 2016, 9, 19934–19944. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, H.; Bao, H.; Jiang, P.; Huang, X. Millefeuille inspired thermally conductive polymer nanocomposites with overlapping BN nanosheets for thermal management applications. ACS Appl. Mater. Interfaces 2019, 11, 31402–31410. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase change material with anisotropically high thermal conductivity and excellent shape stability due to its robust cellulose/BNNSs skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An anisotropically high thermal conductive boron nitride/epoxy composite based on nacre-mimetic 3D network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Fu, L.; Duan, Z.; Chen, Y.; Hou, X.; Wu, Y.; Li, S.; Guo, L.; Kang, R.; et al. Enhanced thermal conductivity of polyimide composites with boron nitride nanosheets. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Qian, X.; Meng, Z.; Yang, B.; Liu, Z.-Q. Highly thermally conducting polymer based films with magnetic field assisted vertically aligned hexagonal boron nitride for flexible electronic encapsulation. ACS Appl. Mater. Interfaces 2019, 11, 17915–17924. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xu, T.; Jiang, F.; Song, N.; Shi, L.; Ding, P. High thermal conductivity property of polyamide imide/boron nitride composite films by doping boron nitride quantum dots. J. Mater. Chem. C 2019, 7, 13896–13903. [Google Scholar] [CrossRef]
- Beytur, M. Fabrication of platinum nanoparticle/boron nitride quantum dots/6-methyl-2-(3hydroxy-4-methoxybenzylidenamino)-benzothiazole (Ils) nanocomposite for electrocatalytic oxidation of methanol. J. Chil. Chem. Soc. 2020, 65, 4929–4933. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, A.; Sharma, S.; Liu, X.; Zhang, D.; Yap, Y.K. A Review on van der Waals Boron Nitride Quantum Dots. C 2021, 7, 35. https://doi.org/10.3390/c7020035
Acharya A, Sharma S, Liu X, Zhang D, Yap YK. A Review on van der Waals Boron Nitride Quantum Dots. C. 2021; 7(2):35. https://doi.org/10.3390/c7020035
Chicago/Turabian StyleAcharya, Amit, Sambhawana Sharma, Xiuling Liu, Dongyan Zhang, and Yoke Khin Yap. 2021. "A Review on van der Waals Boron Nitride Quantum Dots" C 7, no. 2: 35. https://doi.org/10.3390/c7020035
APA StyleAcharya, A., Sharma, S., Liu, X., Zhang, D., & Yap, Y. K. (2021). A Review on van der Waals Boron Nitride Quantum Dots. C, 7(2), 35. https://doi.org/10.3390/c7020035






