Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies
Abstract
1. Introduction
1.1. Fully Hydrogenated and Semi-Hydrogenated Bilayer Graphenes—Theoretical Studies
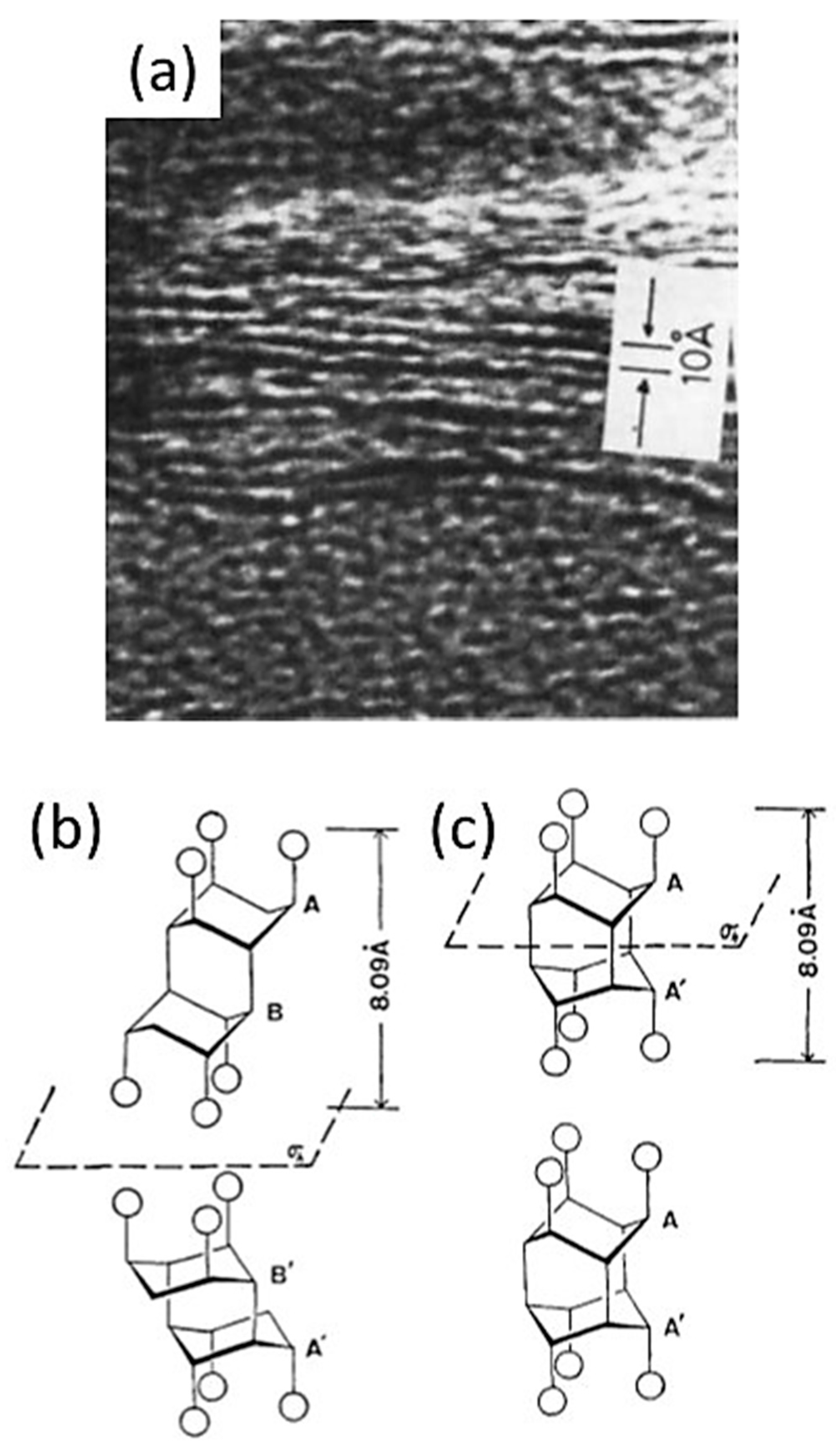
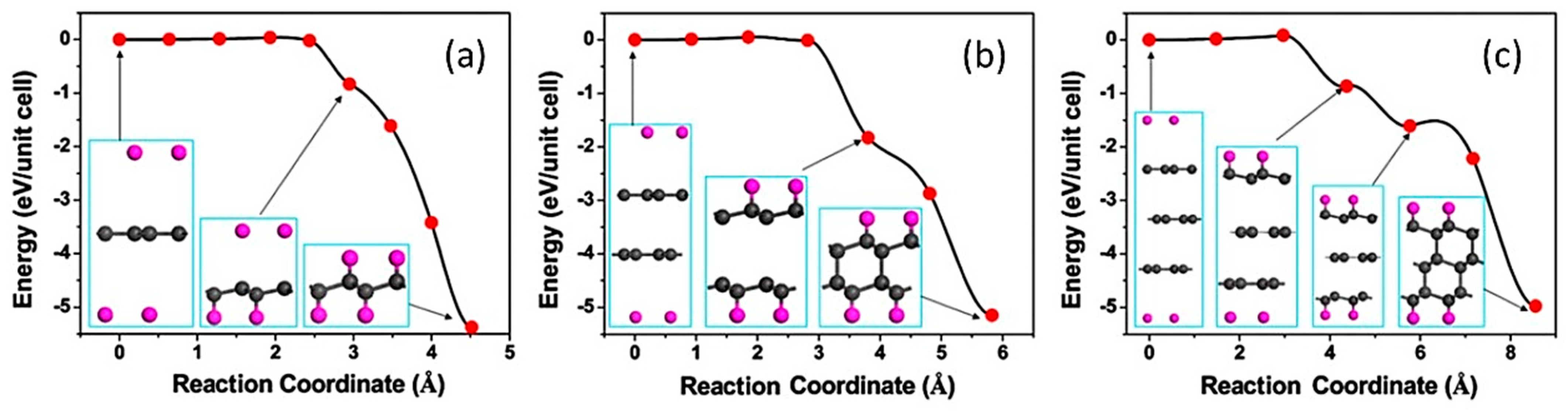
1.2. Experimental Evidence of the First Representative of 2D Diamond-Like Structures
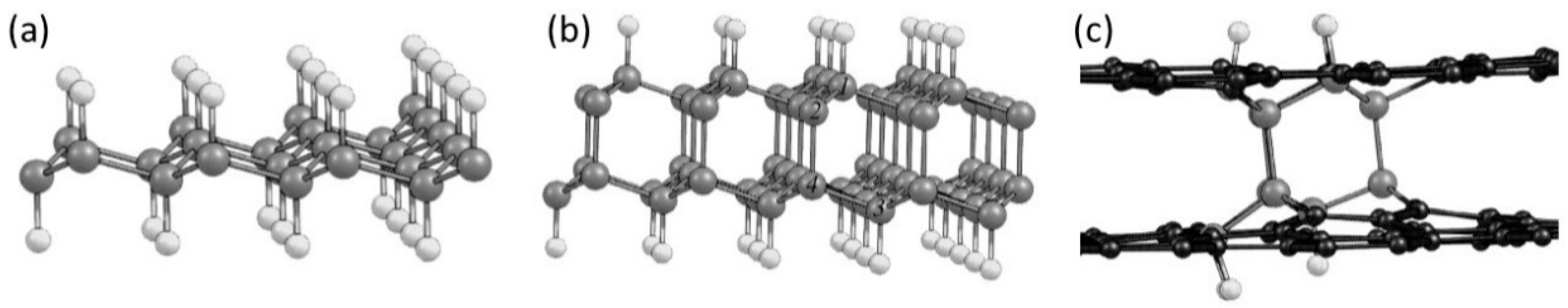
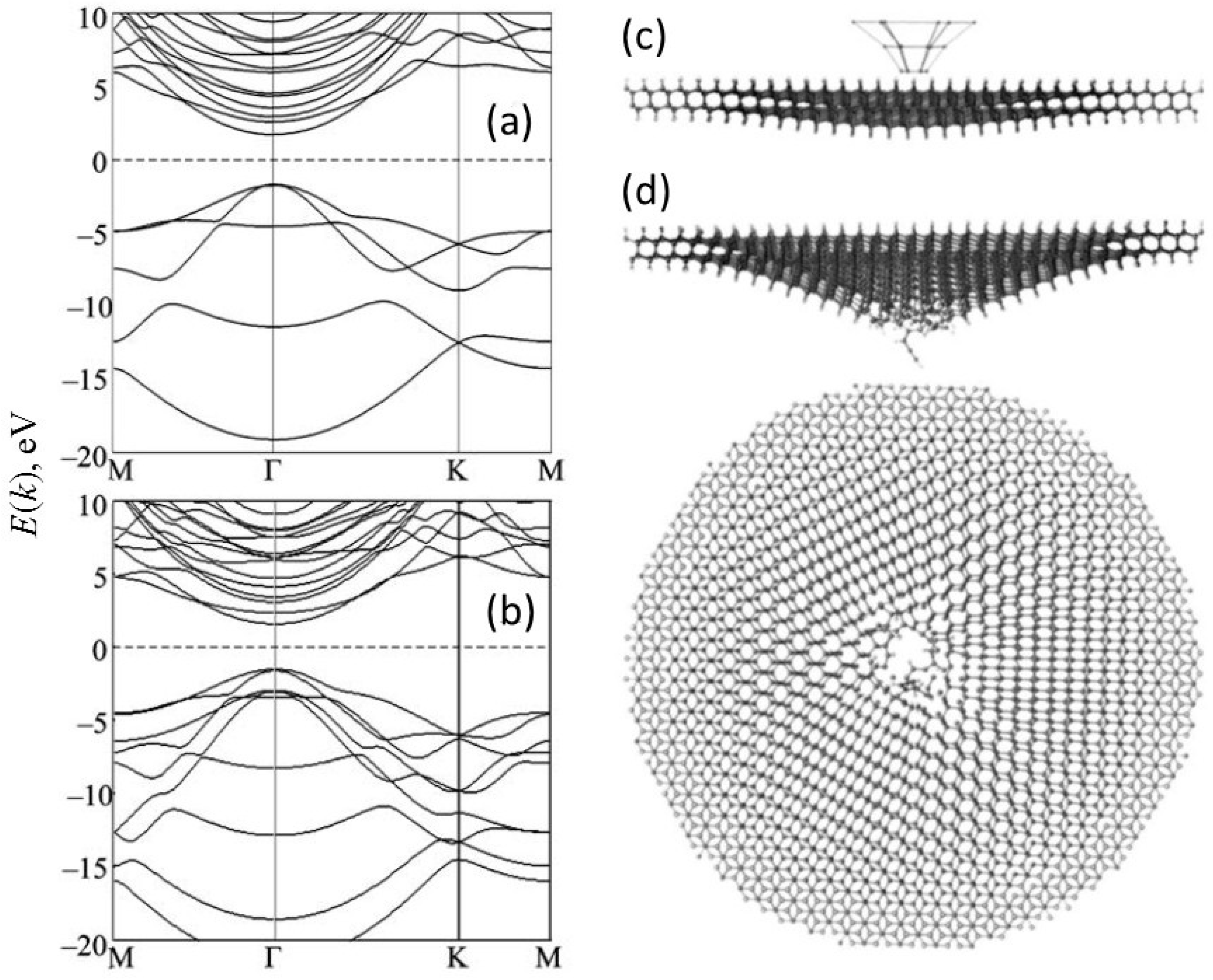
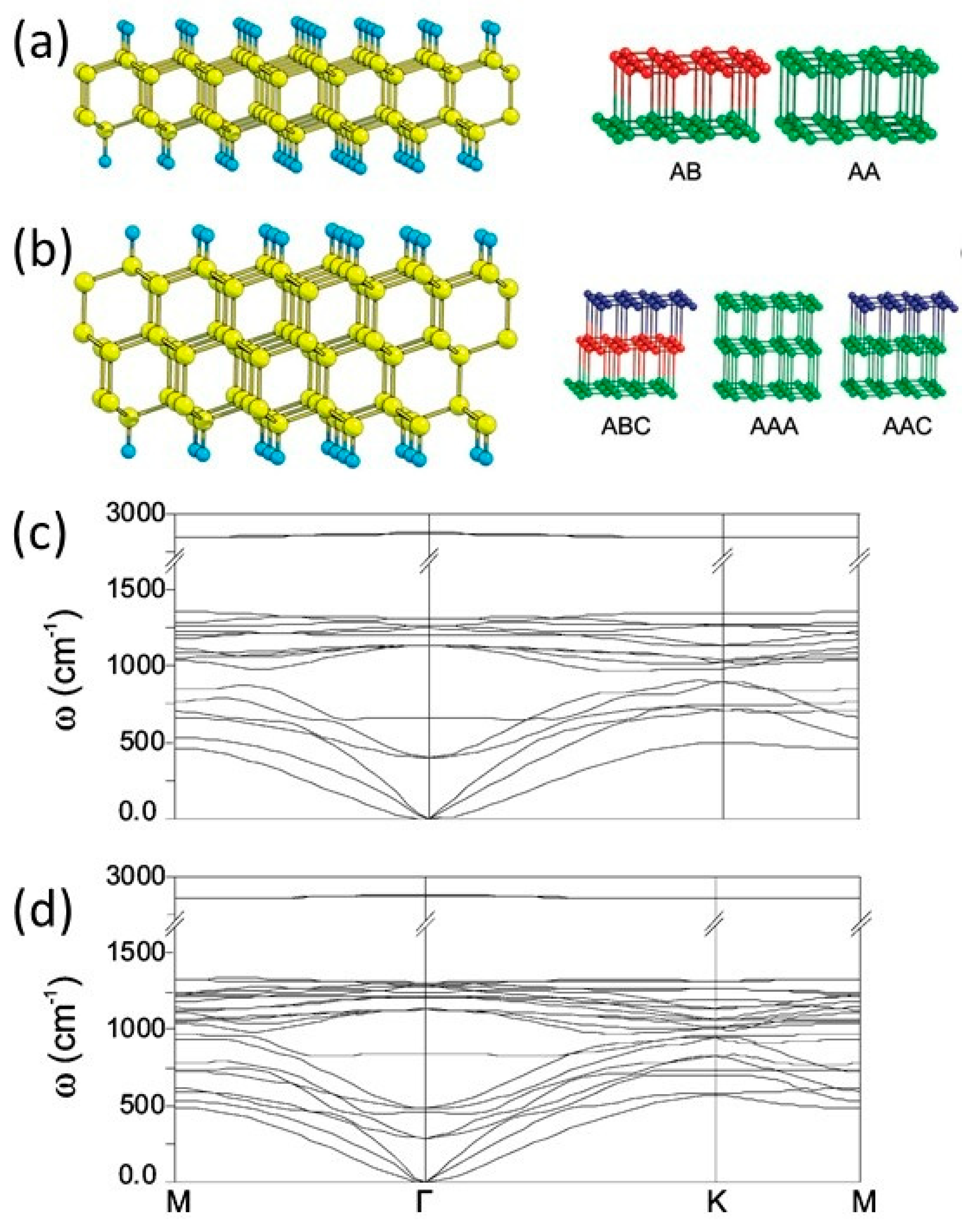
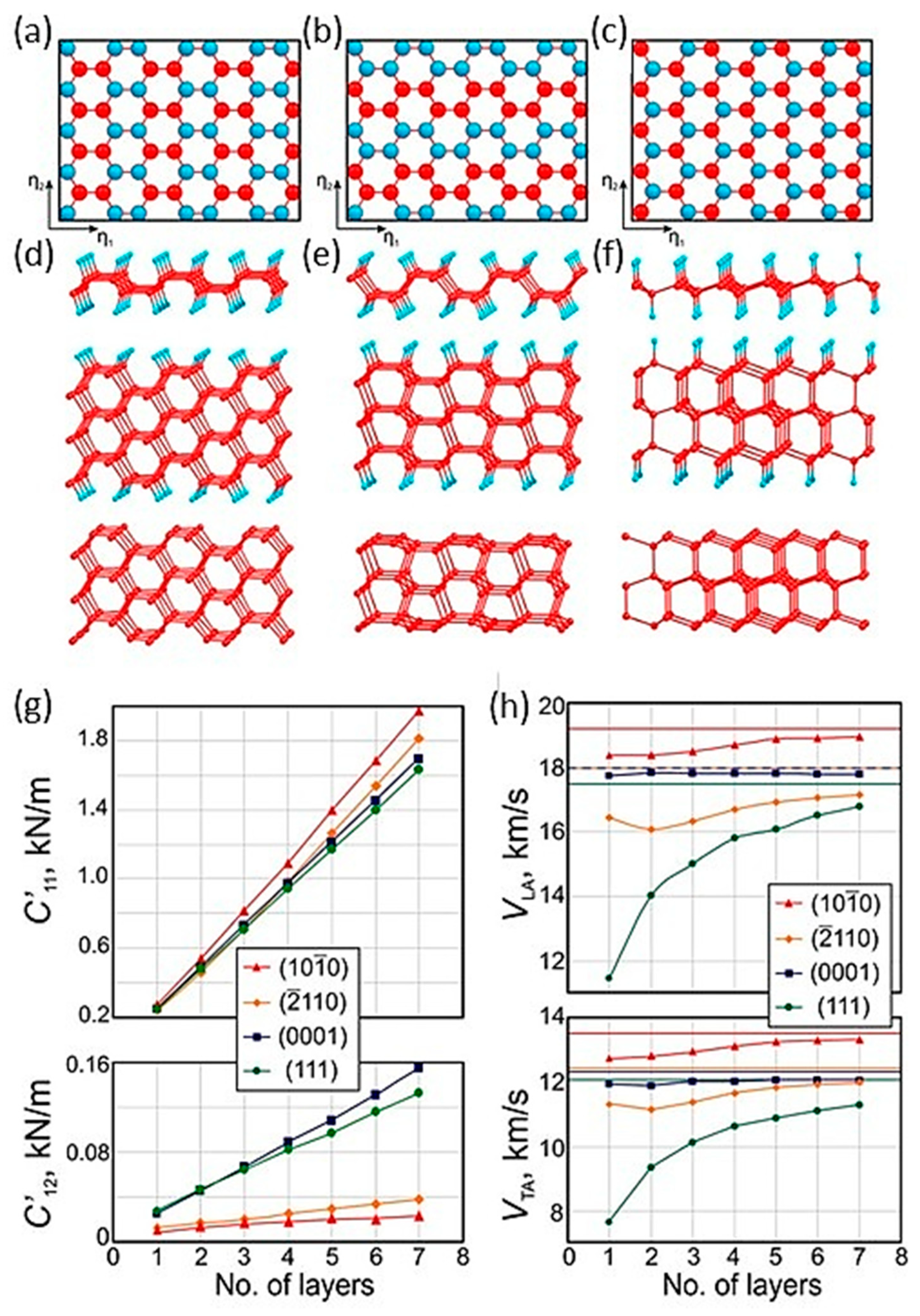
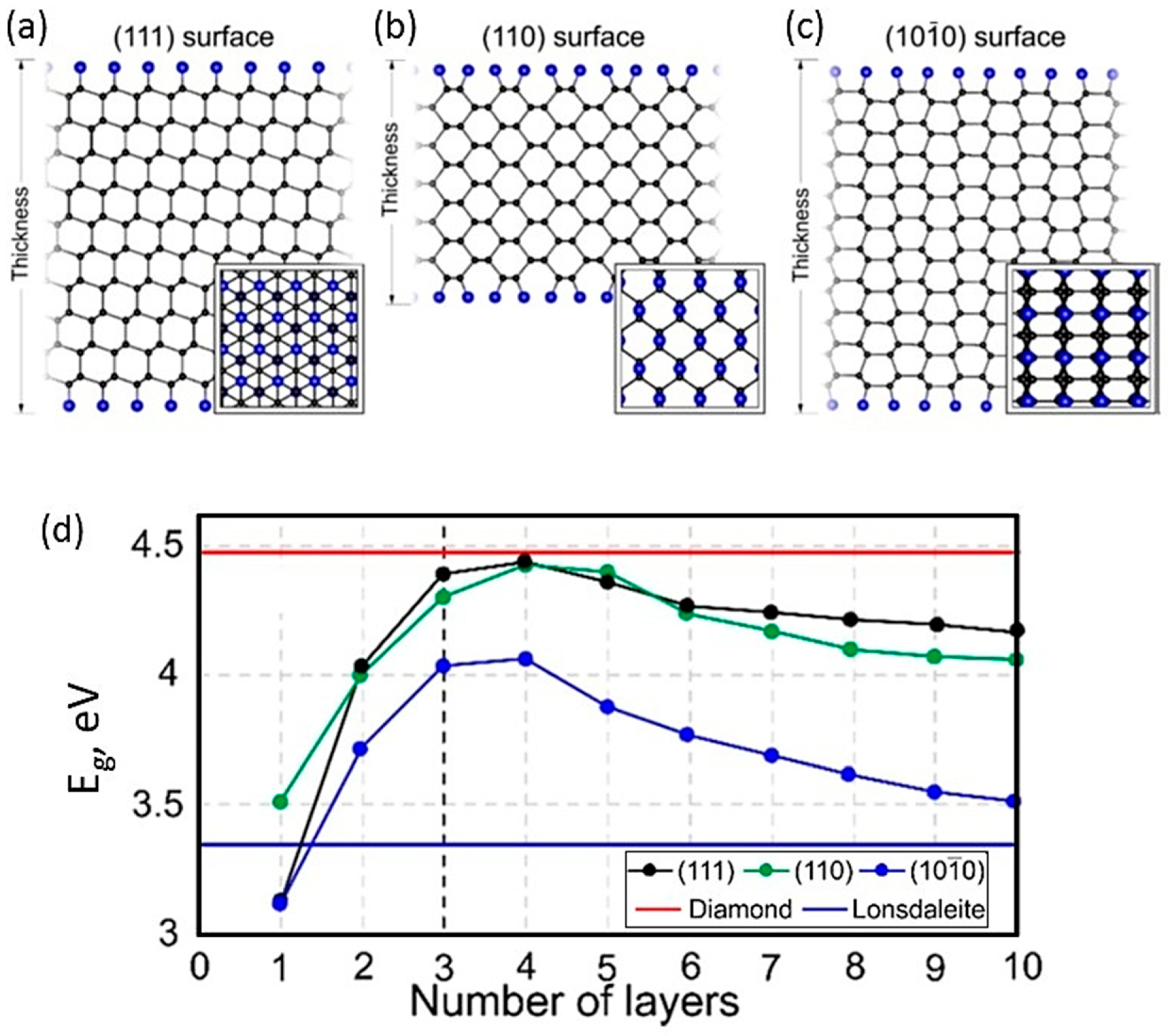
1.3. Multilayer Diamanes—Theoretical Studies
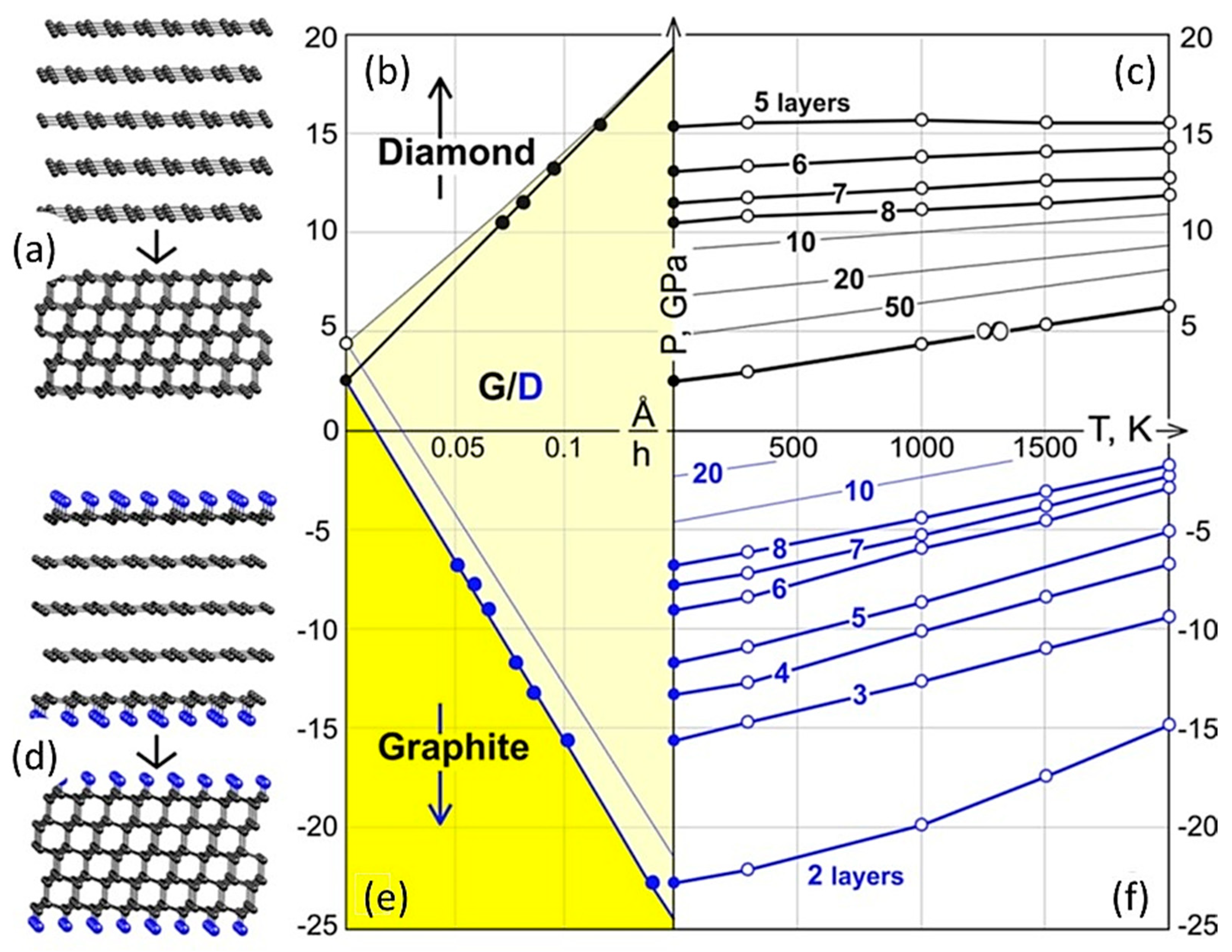
1.4. Phase (P-T) Diagram of Quasi-Two-Dimensional Carbon Formations
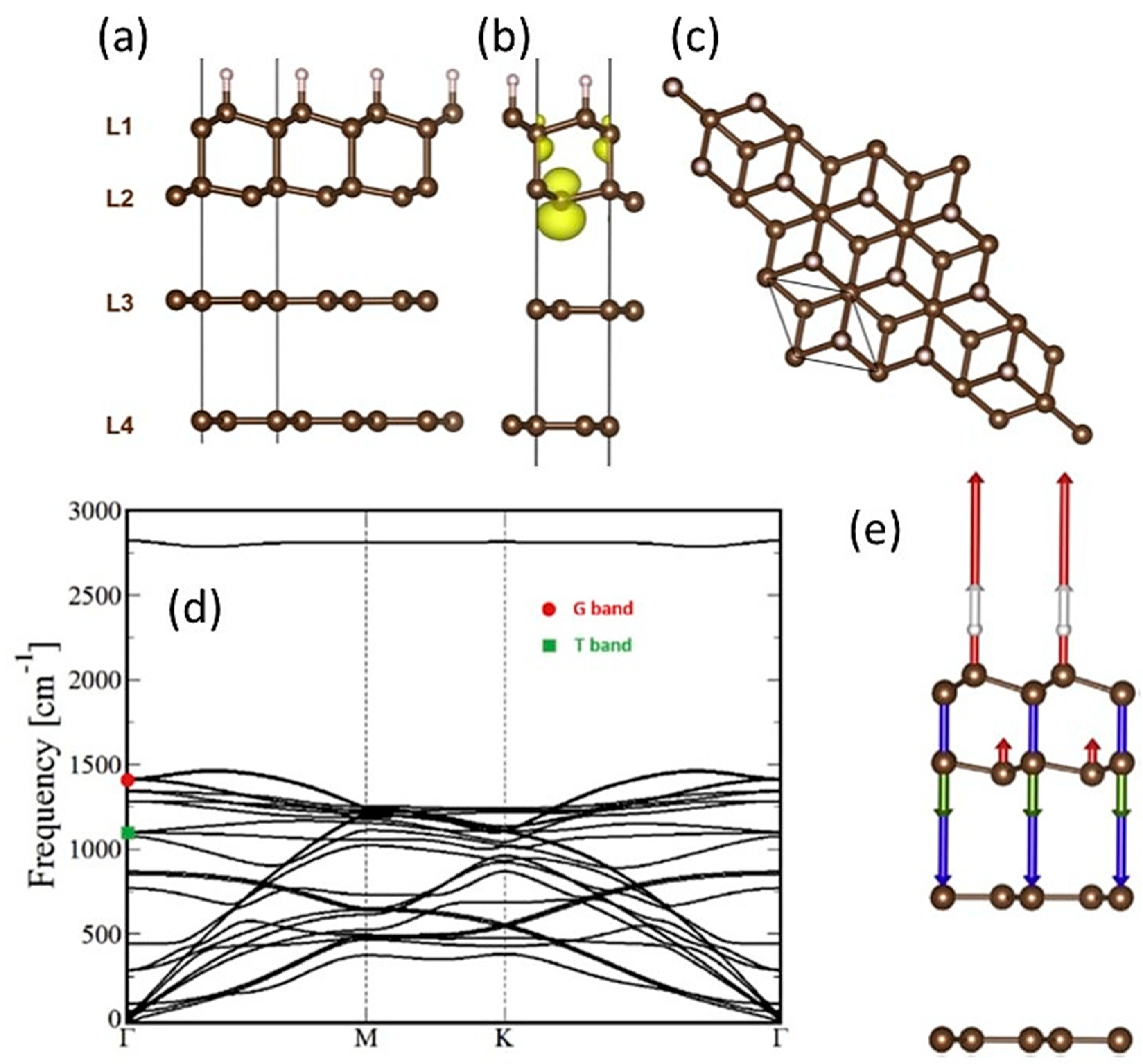
1.5. Diamanes with Lonsdaleite Structure—Theoretical Studies
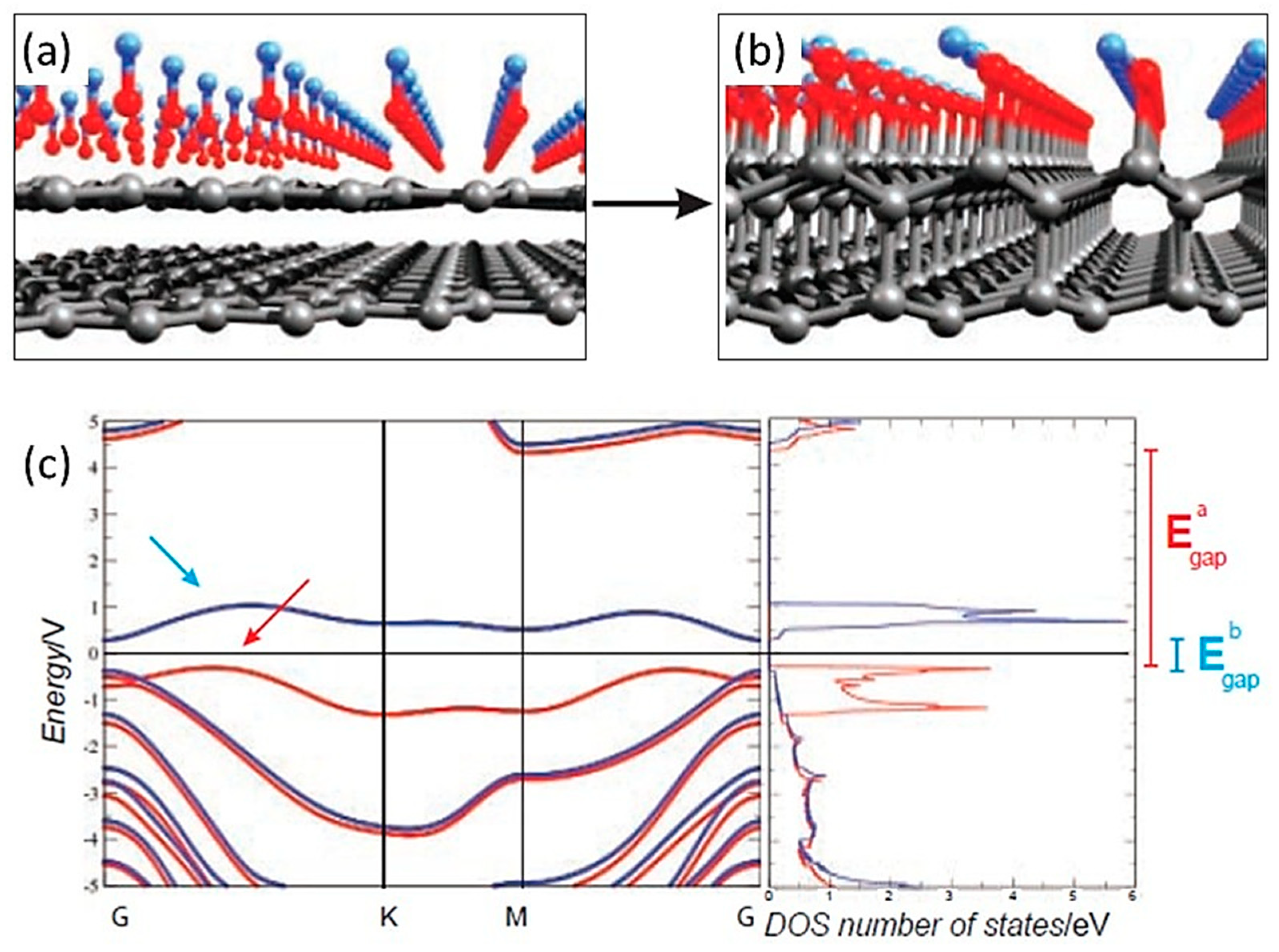
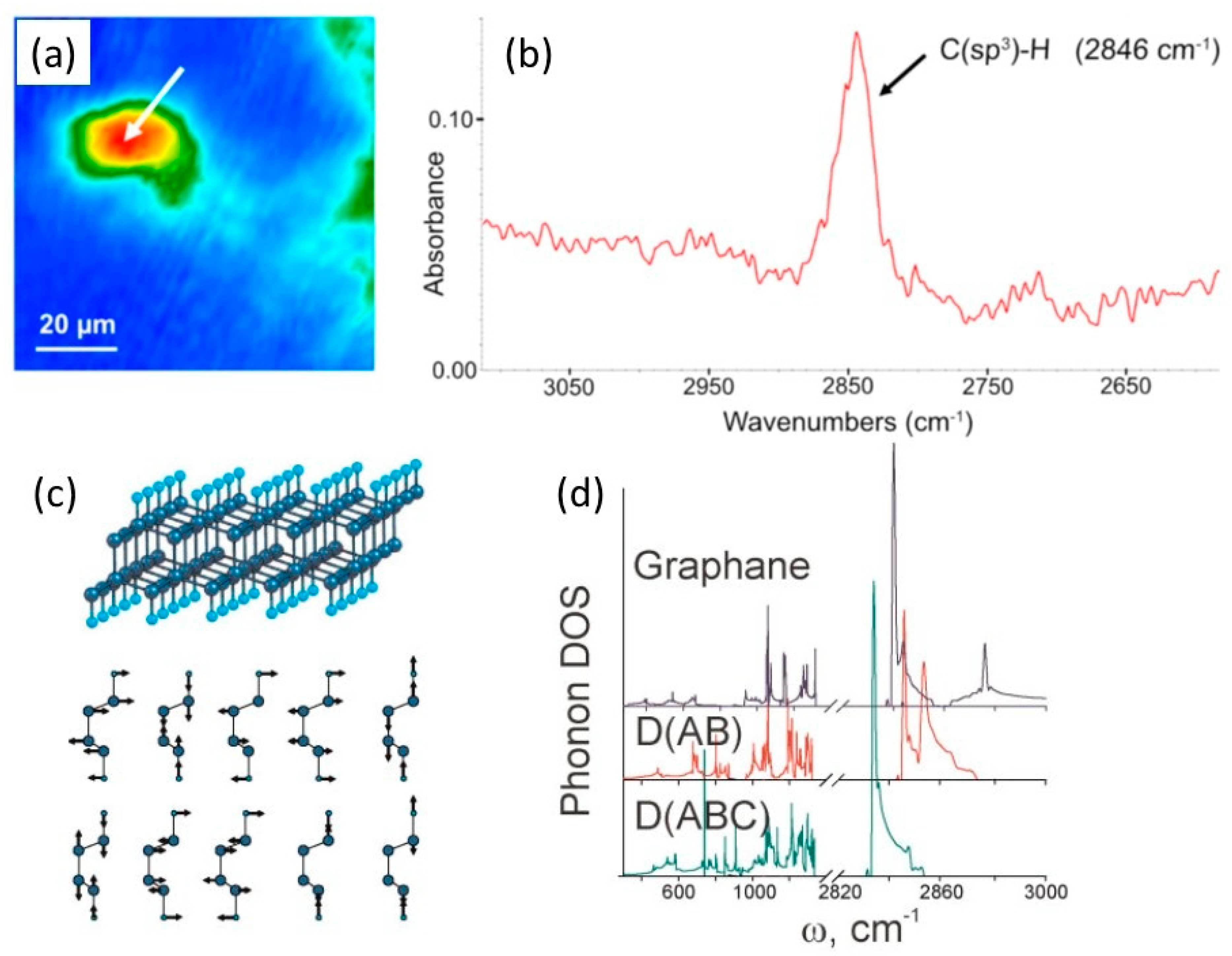
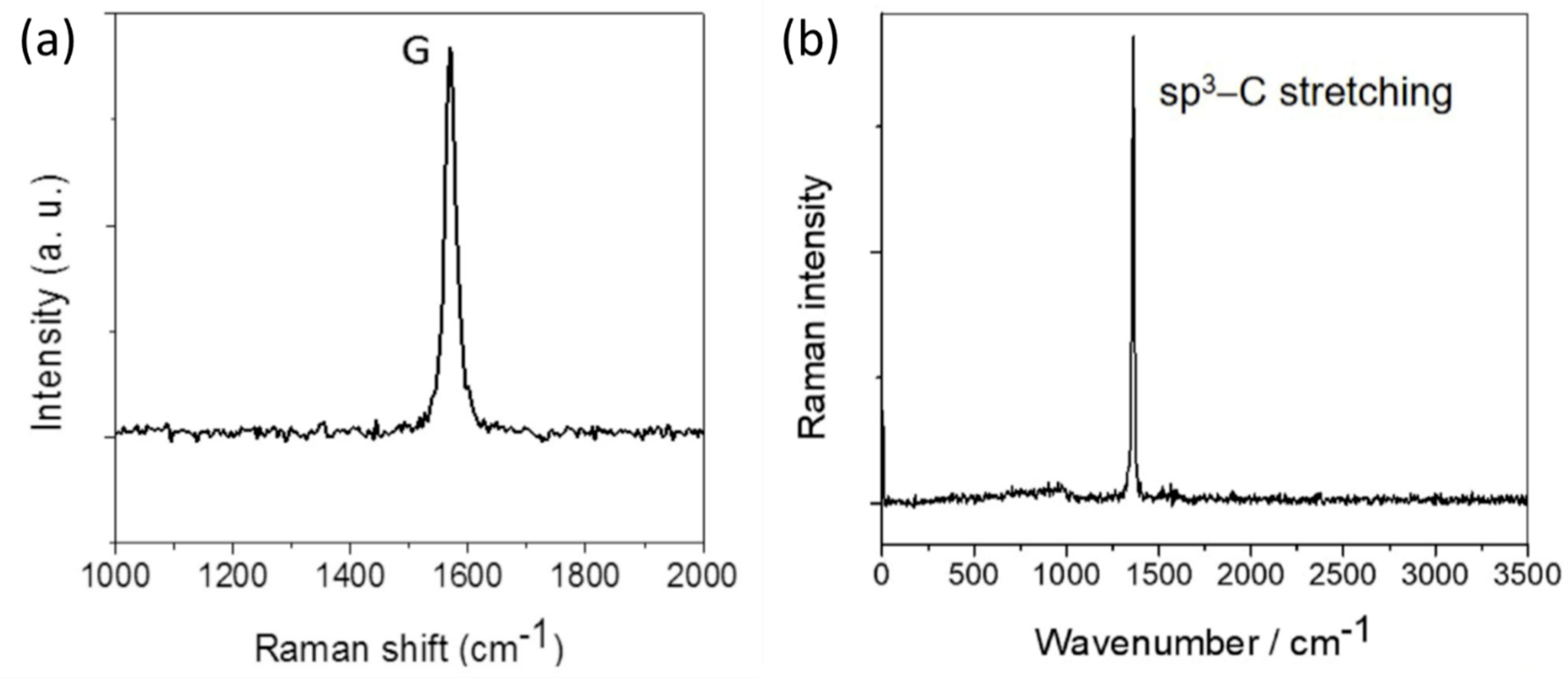
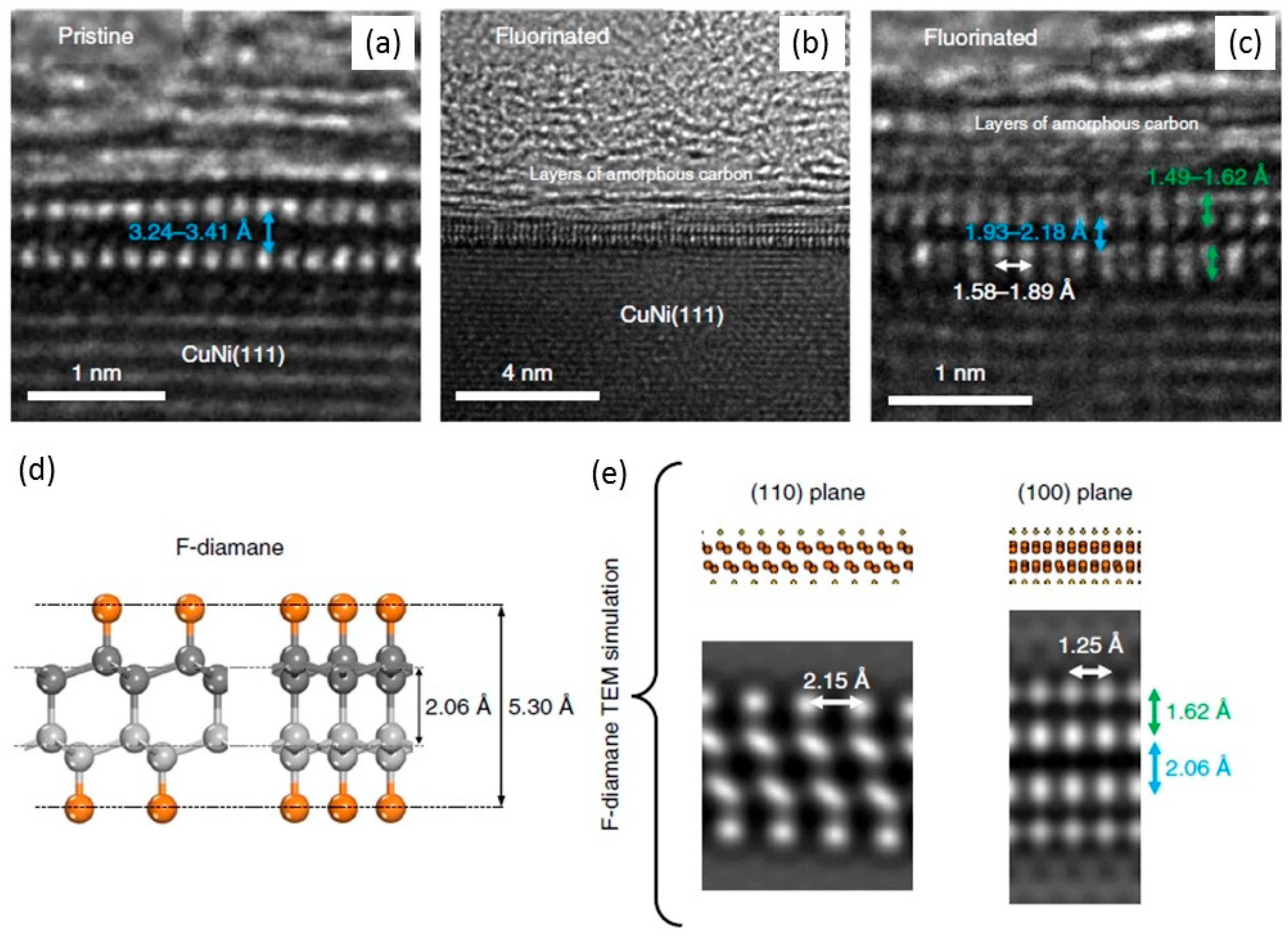
1.6. Experimental Studies of Diamanes
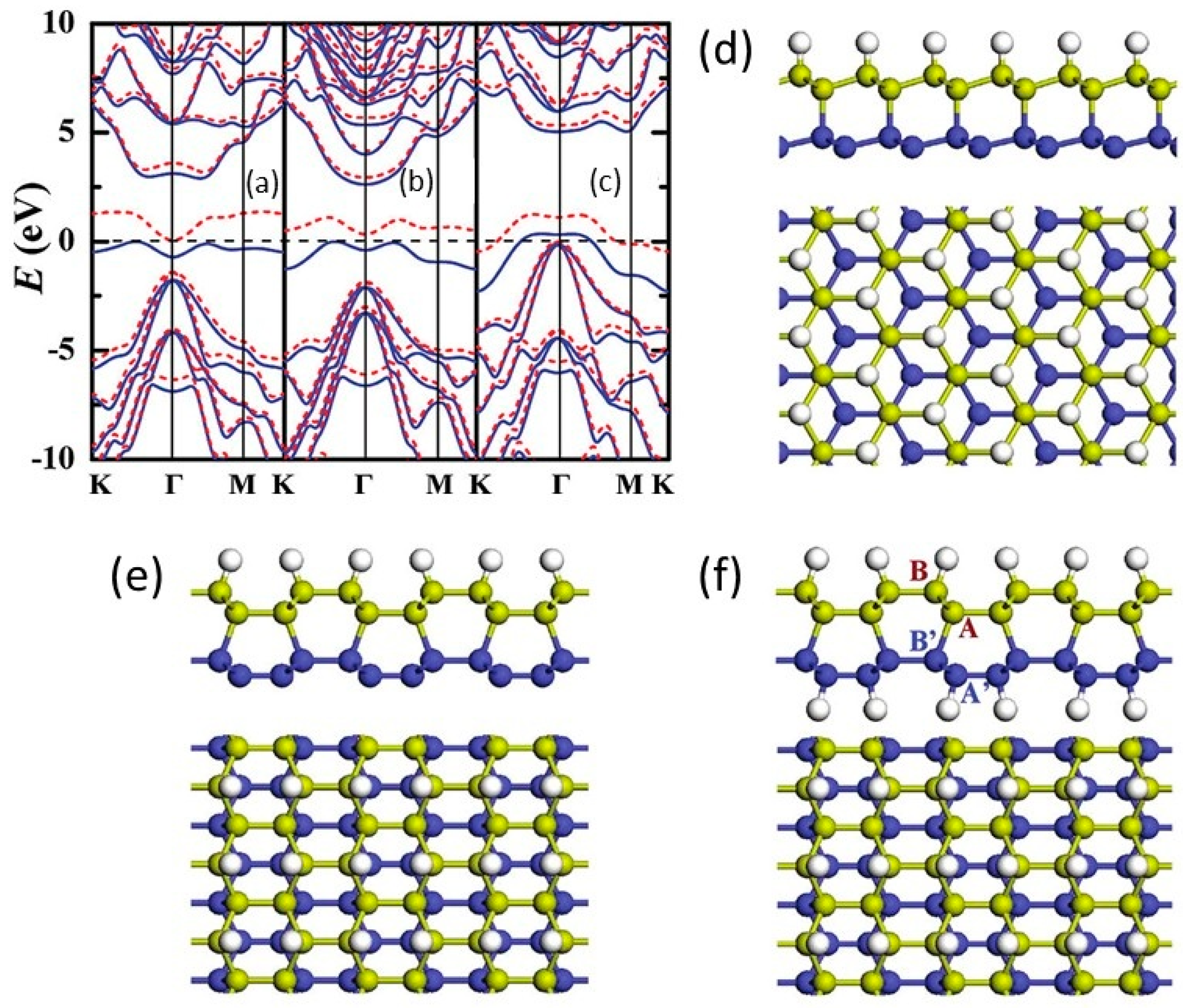
1.7. Fluorinated Diamanes—Theoretical and Experimental Studies
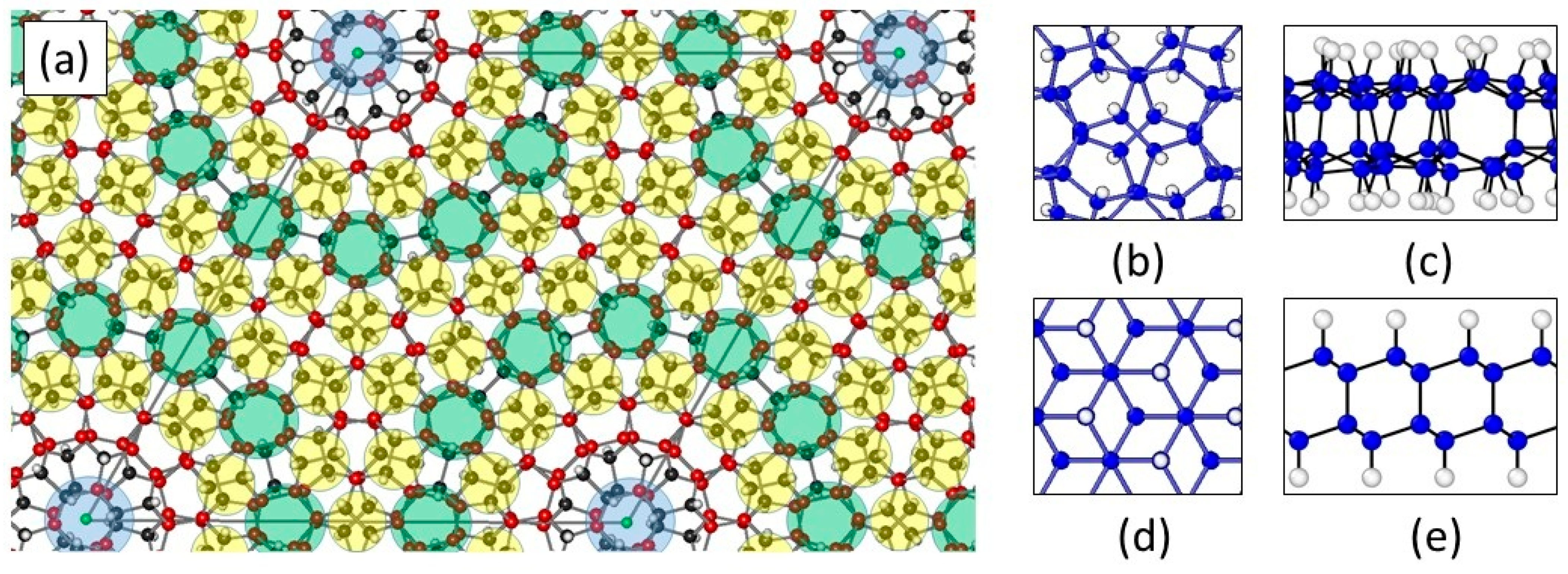
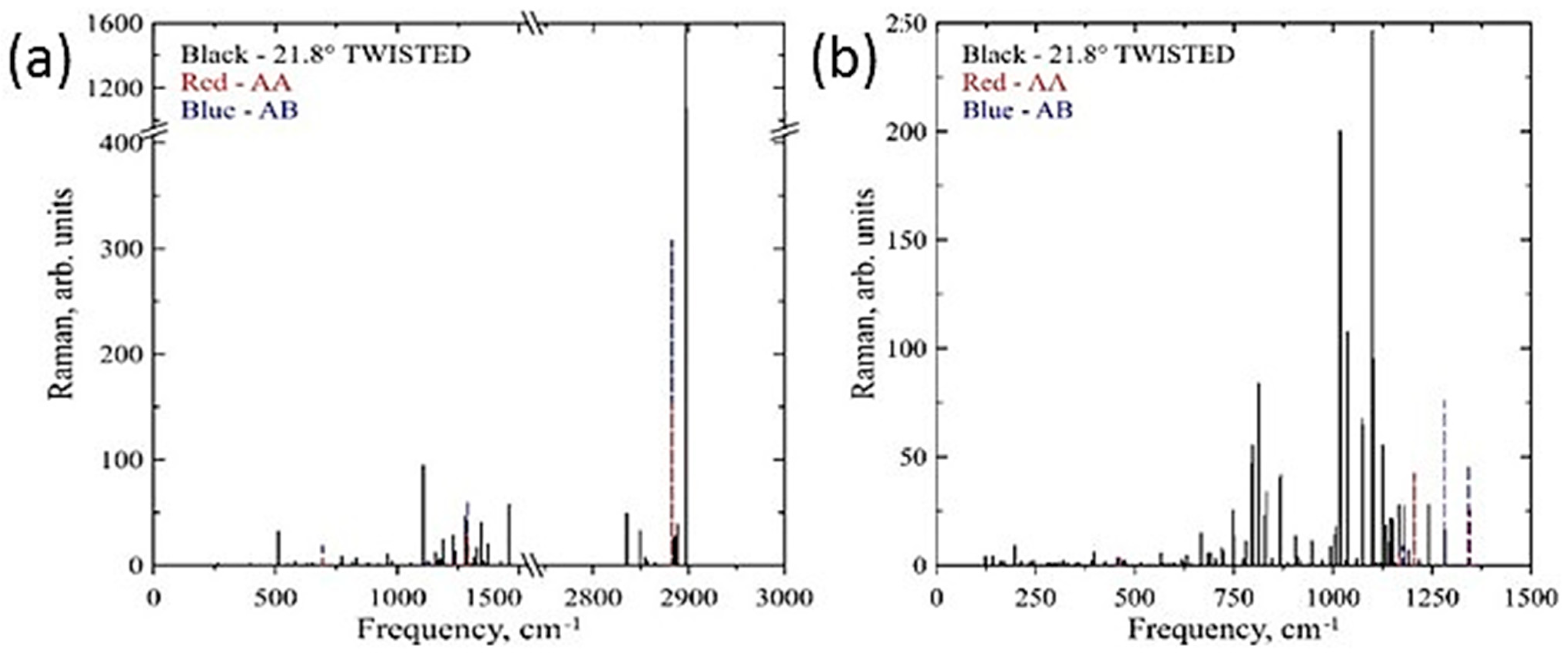
1.8. Moiré Diamanes—Theoretical Studies
2. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S. Electric Field Ef fect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Andreeva, D.V.; Ren, W.; Shan, G. Graphene and Other Two-Dimensional Materials. Front. Phys. 2019, 14, 13301. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Sorokin, P.B.; Kvashnin, A.G.; Kvashnin, D.G. Diamond-like C2H Nanolayer, Diamane: Simulation of the Structure and Properties. JETP Lett. 2009, 90, 134–138. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Sivek, J.; Leenaerts, O.; Partoens, B.; Peeters, F.M. First-Principles Investigation of Bilayer Fluorographene. J. Phys. Chem. C 2012, 116, 19240–19245. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Avramov, P.V.; Kvashnin, D.G.; Chernozatonskii, L.A.; Sorokin, P.B. Features of Electronic, Mechanical, and Electromechanical Properties of Fluorinated Diamond Films of Nanometer Thickness. J. Phys. Chem. C 2017, 121, 28484–28489. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Matos, M.J.S.; Paschoal, A.R.; Freire, P.T.C.; Andrade, N.F.; Aguiar, A.L.; Kong, J.; Neves, B.R.A.; de Oliveira, A.B.; Mazzoni, M.S.C.; et al. Raman Evidence for Pressure-Induced Formation of Diamondene. Nat. Commun. 2017, 8, 96. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, T. Suppressed Thermal Conductivity in Fluorinated Diamane: Optical Phonon Dominant Thermal Transport. Appl. Phys. Lett. 2019, 115, 151904. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Ding, F. Giant Thermal Conductivity in Diamane and the Influence of Horizontal Reflection Symmetry on Phonon Scattering. Nanoscale 2019, 11, 4248–4257. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, L. Sensitively Tuning the Thermal Conductivity of Diamane via Engineering the Mass of Functional Groups. Nanotechnology 2020, 31, 435409. [Google Scholar] [CrossRef]
- Raeisi, M.; Mortazavi, B.; Podryabinkin, E.V.; Shojaei, F.; Zhuang, X.; Shapeev, A.V. High Thermal Conductivity in Semiconducting Janus and Non-Janus Diamanes. Carbon 2020, 167, 51–61. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhan, H.; Nie, Y.; Xu, X.; Qi, D.; Gu, Y. Single Layer Diamond—A New Ultrathin 2D Carbon Nanostructure for Mechanical Resonator. Carbon 2020, 161, 809–815. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Yin, Y.; Li, S.; Ding, G.; Zhou, H.; Zhang, G. The Important Role of Strain on Phonon Hydrodynamics in Diamond-like Bi-Layer Graphene. Nanotechnology 2020, 31, 335711. [Google Scholar] [CrossRef] [PubMed]
- Erohin, S.V.; Ruan, Q.; Sorokin, P.B.; Yakobson, B.I. Nano-Thermodynamics of Chemically Induced Graphene–Diamond Transformation. Small 2020, 16, 2004782. [Google Scholar] [CrossRef]
- Pimenta Martins, L.G.; Silva, D.L.; Smith, J.S.; Lu, A.-Y.; Su, C.; Hempel, M.; Occhialini, C.; Ji, X.; Pablo, R.; Alencar, R.S.; et al. Hard, Transparent, Sp3-Containing 2D Phase Formed from Few-Layer Graphene under Compression. Carbon 2021, 173, 744–757. [Google Scholar] [CrossRef]
- Gupta, S.; Yang, J.-H.; Yakobson, B.I. Two-Level Quantum Systems in Two-Dimensional Materials for Single Photon Emission. Nano Lett. 2019, 19, 408–414. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, Q.; Cheng, S.; Gao, N.; Li, H. Electronic Structures of Two-Dimensional Hydrogenated Bilayer Diamond Films with Si Dopant and Si-V Center. Results Phys. 2019, 13, 102240. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.; Gao, N.; Zhang, M.; Mu, J.; Gao, L.; Li, H. First-Principles Calculations for Li, P Dopants and Vacancy Defect in Ultra-Thin Hydrogenated Diamond Nanofilms: Structural, Electronic and Optical Properties. Diam. Relat. Mater. 2019, 99, 107526. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Sorokin, P.B.; Kuzubov, A.A.; Sorokin, B.P.; Kvashnin, A.G.; Kvashnin, D.G.; Avramov, P.V.; Yakobson, B.I. Influence of Size Effect on the Electronic and Elastic Properties of Diamond Films with Nanometer Thickness. J. Phys. Chem. C 2011, 115, 132–136. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shojaei, F.; Javvaji, B.; Azizi, M.; Zhan, H.; Rabczuk, T.; Zhuang, X. First-Principles Investigation of Mechanical, Electronic and Optical Properties of H-, F- and Cl-Diamane. Appl. Surf. Sci. 2020, 528, 147035. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Hydrogenation of Bilayer Graphene and the Formation of Bilayer Graphane from First Principles. Phys. Rev. B 2009, 80. [Google Scholar] [CrossRef]
- Samarakoon, D.K.; Wang, X.-Q. Tunable Band Gap in Hydrogenated Bilayer Graphene. ACS Nano 2010, 4, 4126–4130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Q.; Sun, Q.; Chen, X.S.; Kawazoe, Y.; Jena, P. Ferromagnetism in Semihydrogenated Graphene Sheet. Nano Lett. 2009, 9, 3867–3870. [Google Scholar] [CrossRef]
- Kharche, N.; Nayak, S.K. Quasiparticle Bandgap Engineering of Graphene and Graphone on Hexagonal Boron Nitride Substrate. Nano Lett. 2011, 11, 5274–5278. [Google Scholar] [CrossRef]
- Barboza, A.P.M.; Guimaraes, M.H.D.; Massote, D.V.P.; Campos, L.C.; Neto, N.M.B.; Cancado, L.G.; Lacerda, R.G.; Chacham, H.; Mazzoni, M.S.C.; Neves, B.R.A. Room-Temperature Compression-Induced Diamondization of Few-Layer Graphene. Adv. Mater. 2011, 23, 3014–3017. [Google Scholar] [CrossRef]
- Watanabe, N. Two Types of Graphite Fluorides, (CF)n and (C2F)n, and Discharge Characteristics and Mechanisms of Electrodes of (CF)n and (C2F)n in Lithium Batteries. Solid State Ion. 1980, 1, 87–110. [Google Scholar] [CrossRef]
- Touhara, H.; Kadono, K.; Fujii, Y.; Watanabe, N. On the Structure of Graphite Fluoride. Z. Für Anorg. Und Allg. Chem. 1987, 544, 7–20. [Google Scholar] [CrossRef]
- Novoselov, K.S. Nobel Lecture: Graphene: Materials in the Flatland. Rev. Mod. Phys. 2011, 83, 837–849. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Abild-Pedersen, F.; Ogasawara, H.; Nilsson, A.; Kaya, S. Interlayer Carbon Bond Formation Induced by Hydrogen Adsorption in Few-Layer Supported Graphene. Phys. Rev. Lett. 2013, 111. [Google Scholar] [CrossRef]
- Piazza, F.; Monthioux, M.; Puech, P.; Gerber, I.C. Towards a Better Understanding of the Structure of Diamanoïds and Diamanoïd/Graphene Hybrids. Carbon 2020, 156, 234–241. [Google Scholar] [CrossRef]
- McSkimin, H.J.; Andreatch, P. Elastic Moduli of Diamond as a Function of Pressure and Temperature. J. Appl. Phys. 1972, 43, 2944–2948. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Shao, J.-L.; Zheng, Z.; Zhan, H. Mechanical Properties of a Single-Layer Diamane under Tension and Bending. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, H.; Chen, Q.; Wang, S.; Wang, J.; Ding, F. Formation and Electronic Properties of Hydrogenated Few Layer Graphene. Nanotechnology 2011, 22, 185202. [Google Scholar] [CrossRef] [PubMed]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase Diagram of Quasi-Two-Dimensional Carbon, From Graphene to Diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Sorokin, P.B. Lonsdaleite Films with Nanometer Thickness. J. Phys. Chem. Lett. 2014, 5, 541–548. [Google Scholar] [CrossRef]
- Piazza, F.; Gough, K.; Monthioux, M.; Puech, P.; Gerber, I.; Wiens, R.; Paredes, G.; Ozoria, C. Low Temperature, Pressureless Sp2 to Sp3 Transformation of Ultrathin, Crystalline Carbon Films. Carbon 2019, 145, 10–22. [Google Scholar] [CrossRef]
- Piazza, F.; Cruz, K.; Monthioux, M.; Puech, P.; Gerber, I. Raman Evidence for the Successful Synthesis of Diamane. Carbon 2020, 169, 129–133. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Mavrin, B.N.; Sorokin, P.B. Determination of Ultrathin Diamond Films by Raman Spectroscopy. Phys. Status Solidi B 2012, 249, 1550–1554. [Google Scholar] [CrossRef]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically Induced Transformation of Chemical Vapour Deposition Grown Bilayer Graphene into Fluorinated Single-Layer Diamond. Nat. Nanotechnol. 2020, 15, 59–66. [Google Scholar] [CrossRef]
- Chen, X.; Dubois, M.; Radescu, S.; Rawal, A.; Zhao, C. Liquid-Phase Exfoliation of F-Diamane-Like Nanosheets. Carbon 2020. [Google Scholar] [CrossRef]
- Son, J.; Ryu, H.; Kwon, J.; Huang, S.; Yu, J.; Xu, J.; Watanabe, K.; Taniguchi, T.; Ji, E.; Lee, S.; et al. Tailoring Single- and Double-Sided Fluorination of Bilayer Graphene via Substrate Interactions. Nano Lett. 2020. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, Z.; Liu, Z. High Elastic Moduli, Controllable Bandgap and Extraordinary Carrier Mobility in Single-Layer Diamond. J. Mater. Chem. C 2020, 8, 13819–13826. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A.; Kvashnin, D.G. Ultrawide-Bandgap Moiré Diamanes Based on Bigraphenes with the Twist Angles Θ ∼30°. Appl. Phys. Lett. 2020, 117, 253104. [Google Scholar] [CrossRef]
- Campanera, J.M.; Savini, G.; Suarez-Martinez, I.; Heggie, M.I. Density Functional Calculations on the Intricacies of Moiré Patterns on Graphite. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef]
- Artyukhov, V.I.; Chernozatonskii, L.A. Structure and Layer Interaction in Carbon Monofluoride and Graphane: A Comparative Computational Study. J. Phys. Chem. A 2010, 114, 5389–5396. [Google Scholar] [CrossRef]
- Li, H.; Duan, T.; Haldar, S.; Sanyal, B.; Eriksson, O.; Jafri, H.; Hajjar-Garreau, S.; Simon, L.; Leifer, K. Direct Writing of Lateral Fluorographene Nanopatterns with Tunable Bandgaps and Its Application in New Generation of Moiré Superlattice. Appl. Phys. Rev. 2020, 7, 011403. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Katin, K.P.; Demin, V.A.; Maslov, M.M. Moiré Diamanes Based on the Hydrogenated or Fluorinated Twisted Bigraphene: The Features of Atomic and Electronic Structures, Raman and Infrared Spectra. Appl. Surf. Sci. 2021, 537, 148011. [Google Scholar] [CrossRef]
- Wang, J.; Mu, X.; Wang, L.; Sun, M. Properties and Applications of New Superlattice: Twisted Bilayer Graphene. Mater. Today Phys. 2019, 9, 100099. [Google Scholar] [CrossRef]
- Muniz, A.R.; Maroudas, D. Opening and Tuning of Band Gap by the Formation of Diamond Superlattices in Twisted Bilayer Graphene. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Muniz, A.R.; Maroudas, D. Formation of Fullerene Superlattices by Interlayer Bonding in Twisted Bilayer Graphene. J. Appl. Phys. 2012, 111, 043513. [Google Scholar] [CrossRef]
- Muniz, A.R.; Maroudas, D. Superlattices of Fluorinated Interlayer-Bonded Domains in Twisted Bilayer Graphene. J. Phys. Chem. C 2013, 117, 7315–7325. [Google Scholar] [CrossRef]
- Machado, A.S.; Maroudas, D.; Muniz, A.R. Tunable Mechanical Properties of Diamond Superlattices Generated by Interlayer Bonding in Twisted Bilayer Graphene. Appl. Phys. Lett. 2013, 103, 013113. [Google Scholar] [CrossRef]
- Muniz, A.R.; Machado, A.S.; Maroudas, D. Mechanical Behavior of Interlayer-Bonded Nanostructures Obtained from Bilayer Graphene. Carbon 2015, 81, 663–677. [Google Scholar] [CrossRef]
- Chen, M.; Muniz, A.R.; Maroudas, D. Formation and Mechanical Behavior of Nanocomposite Superstructures from Interlayer Bonding in Twisted Bilayer Graphene. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Cellini, F.; Lavini, F.; Cao, T.; de Heer, W.; Berger, C.; Bongiorno, A.; Riedo, E. Epitaxial Two-Layer Graphene under Pressure: Diamene Stiffer than Diamond. FlatChem 2018, 10, 8–13. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, L.; Chen, Y.; Yin, K.; Wang, C.; Tzeng, Y.-K.; Lin, Y.; Dong, H.; Liu, Z.; Tse, J.S.; et al. Synthesis of Atomically Thin Hexagonal Diamond with Compression. Nano Lett. 2020, 20, 5916–5921. [Google Scholar] [CrossRef]
- Paul, S.; Momeni, K.; Levitas, V.I. Shear-Induced Diamondization of Multilayer Graphene Structures: A Computational Study. Carbon 2020, 167, 140–147. [Google Scholar] [CrossRef]

| Structure | EB (eV) | Eg (eV) | L (Å) |
|---|---|---|---|
| Fully hydrogenated chair | −12.00 | 3.24 | 1.54 |
| Fully hydrogenated boat | −11.93 | 2.92 | 1.54 |
| Semi-hydrogenated chair | −10.55 | 0.54 | 1.65 |
| Semi-hydrogenated boat I | −10.79 | 2.35 | 1.63 |
| Semi-hydrogenated boat II | −10.85 | 0.50 | 3.26 |
| ωopt Groups | |||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VTA | VLA |
| Graphane | - | - | - | 1123, 1123 | 1159, 1162, 1162, 1328, 1328, 2856, 2896 | 12.0 | 17.7 |
| D(AB) | 401, 401 | 664 | 1131, 1131, 1133, 1133 | 1201, 1249, 1260, 1260, 1313, 1313, 2865, 2875 | 12.1 | 17.8 | |
| D(ABC) | 291, 291 | 467, 483, 483 | 848 | 1132, 1132, 1133, 1133 | 1211, 1224, 1224, 1248, 1282, 1287, 1287, 1308, 1308, 2874, 2882 | 12.2 | 18.0 |
| Diamond (experiment) | 12.4 | 18.3 | |||||
| System | Unit Cell | Ef, eV/Atoms | Eg, eV |
|---|---|---|---|
| Graphane | C2H2 | −0.11 | 3.4 |
| F-graphane | C2F2 | −0.91 | 3.3 |
| DnAB | C4H2 | −0.03 | 3.1 |
| F-DnAB | C4F2 | −0.5 | 4.0 |
| Dn21.8 | C28H18 | 0.12 | 3.2 |
| F-Dn21.8 | C28F18 | −0.19 | 4.2 |
| Dn27.8 | C52H30 | 0.13 | 3.3 |
| F-Dn27.8 | C52F30 | −0.29 | 4.5 |
| Dn29.4 | C388H174 | 0.14 | 3.6 |
| F-Dn29.4 | C388F174 | −0.28 | 4.1 |
| Diamond | C8 | 0.12 | 4.2 |
| System | Frequinces, cm−1 | ||||
|---|---|---|---|---|---|
| Dn21.8 | 1343.9 | 1344.1 | 1459.9 | 2834.9 | |
| F-Dn21.8 | 797.8 | 1019.1 | 1035.5 | 1073.9 | 1101.9 |
| DnAB | 1272.0 | 1341.3 | 2859.4 | 2868.6 | |
| F-DnAB | 1212.0 | 1282.2 | 1342.1 | 1352.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernozatonskii, L.A.; Demin, V.A.; Kvashnin, D.G. Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies. C 2021, 7, 17. https://doi.org/10.3390/c7010017
Chernozatonskii LA, Demin VA, Kvashnin DG. Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies. C. 2021; 7(1):17. https://doi.org/10.3390/c7010017
Chicago/Turabian StyleChernozatonskii, Leonid A., Victor A. Demin, and Dmitry G. Kvashnin. 2021. "Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies" C 7, no. 1: 17. https://doi.org/10.3390/c7010017
APA StyleChernozatonskii, L. A., Demin, V. A., & Kvashnin, D. G. (2021). Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies. C, 7(1), 17. https://doi.org/10.3390/c7010017





