Sensitive Voltammetric Detection of Chloroquine Drug by Applying a Boron-Doped Diamond Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
3. Results and Discussion
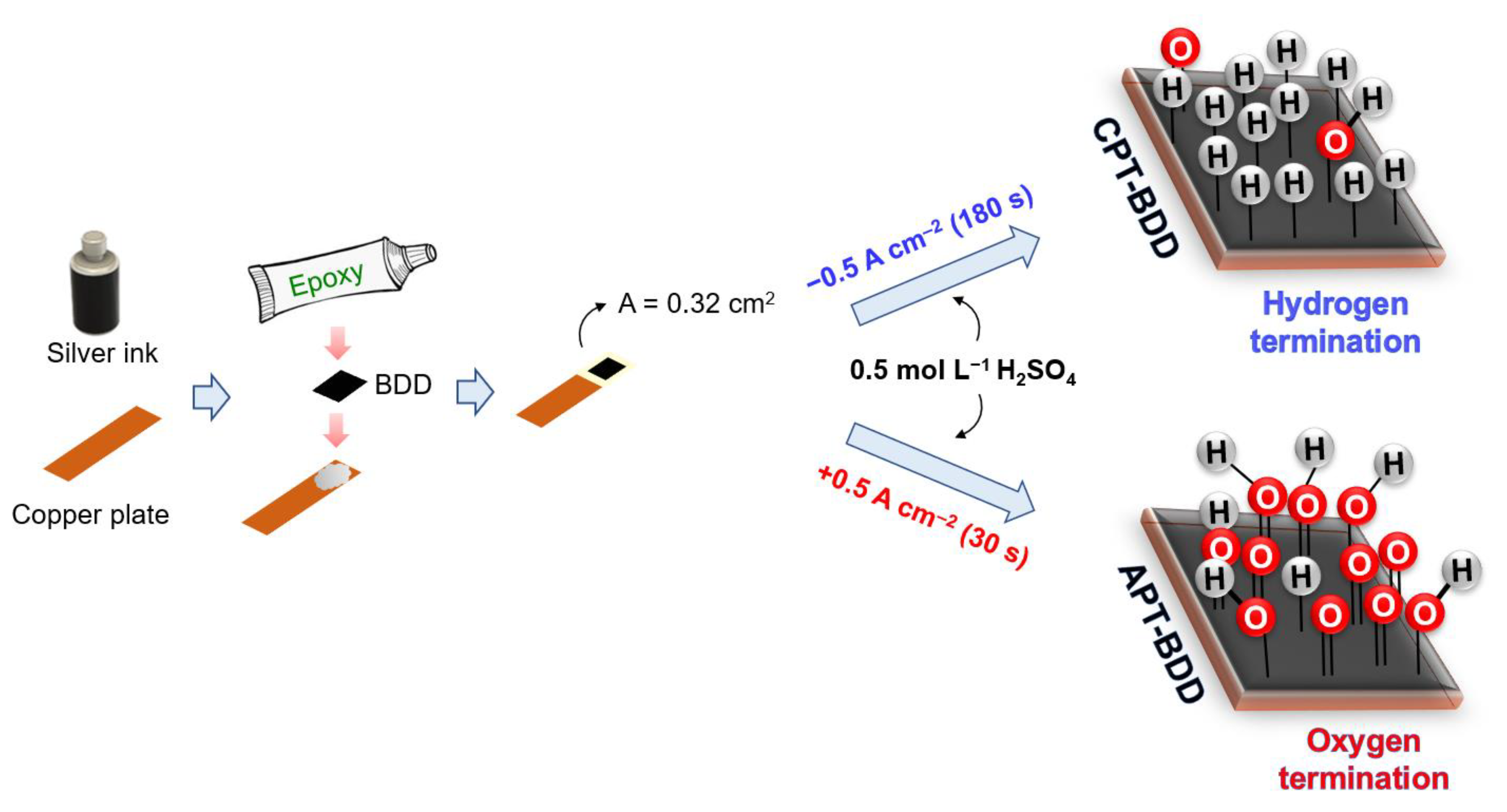
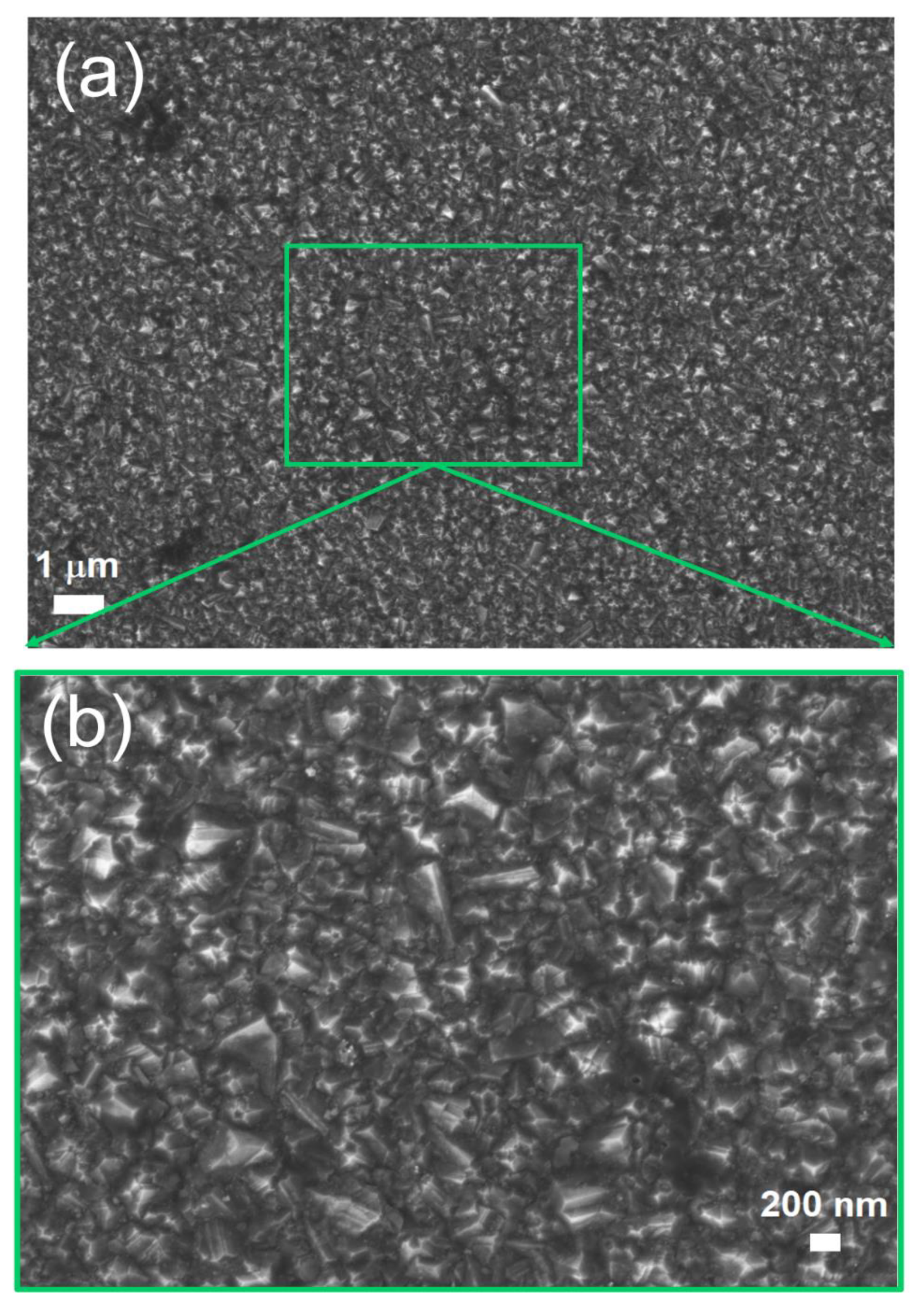
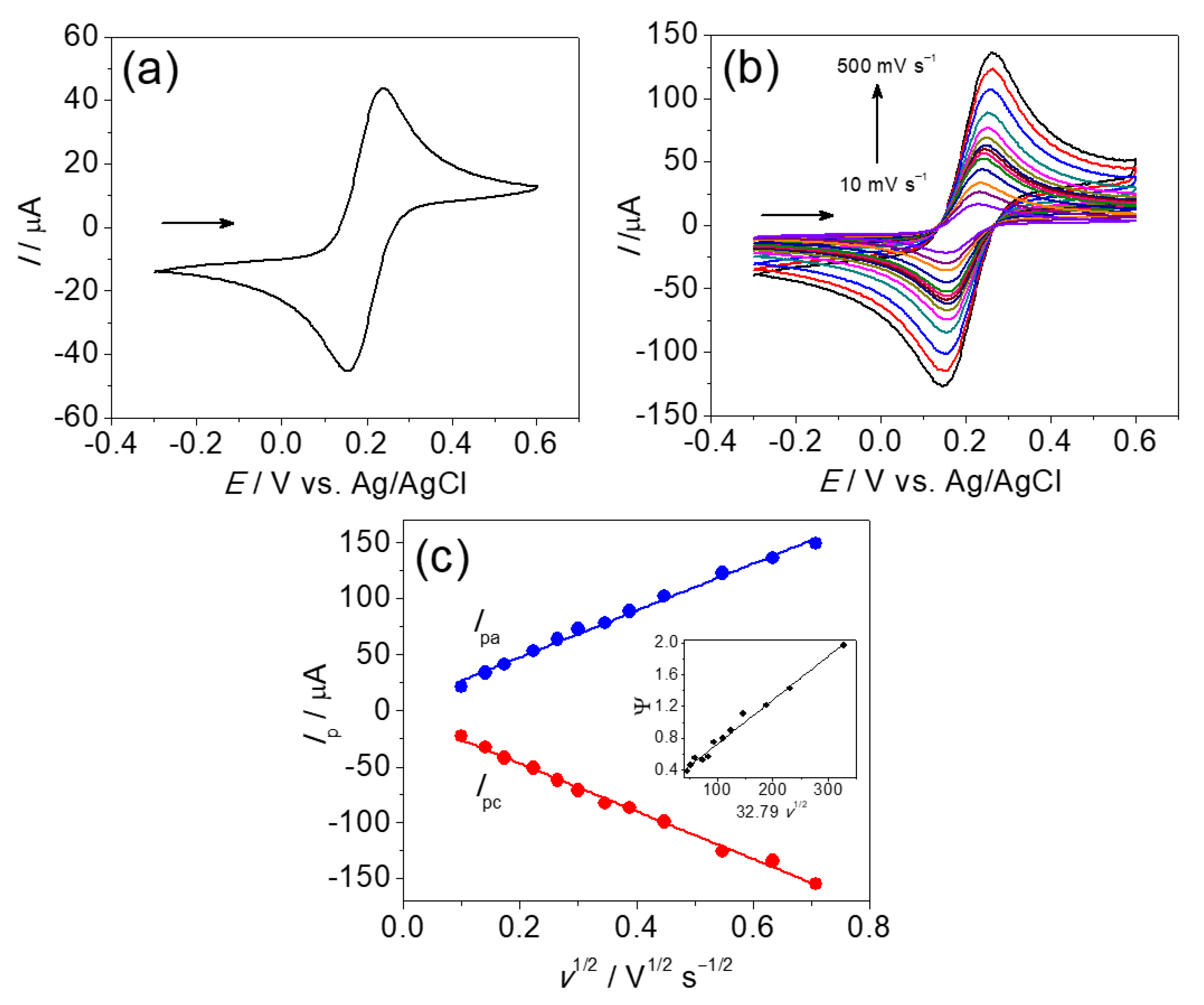
3.1. Working Electrode Characterization
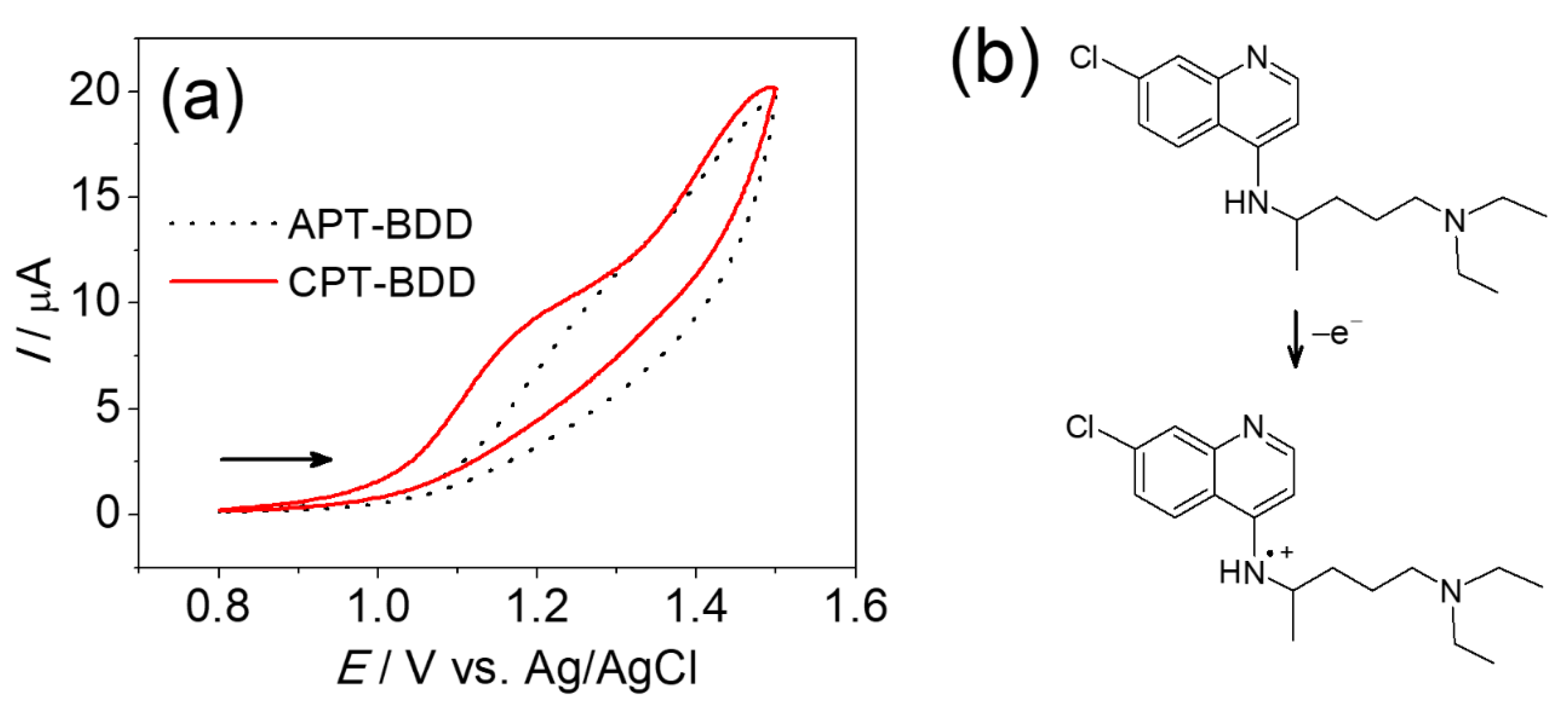
3.2. Chloroquine Electrochemistry
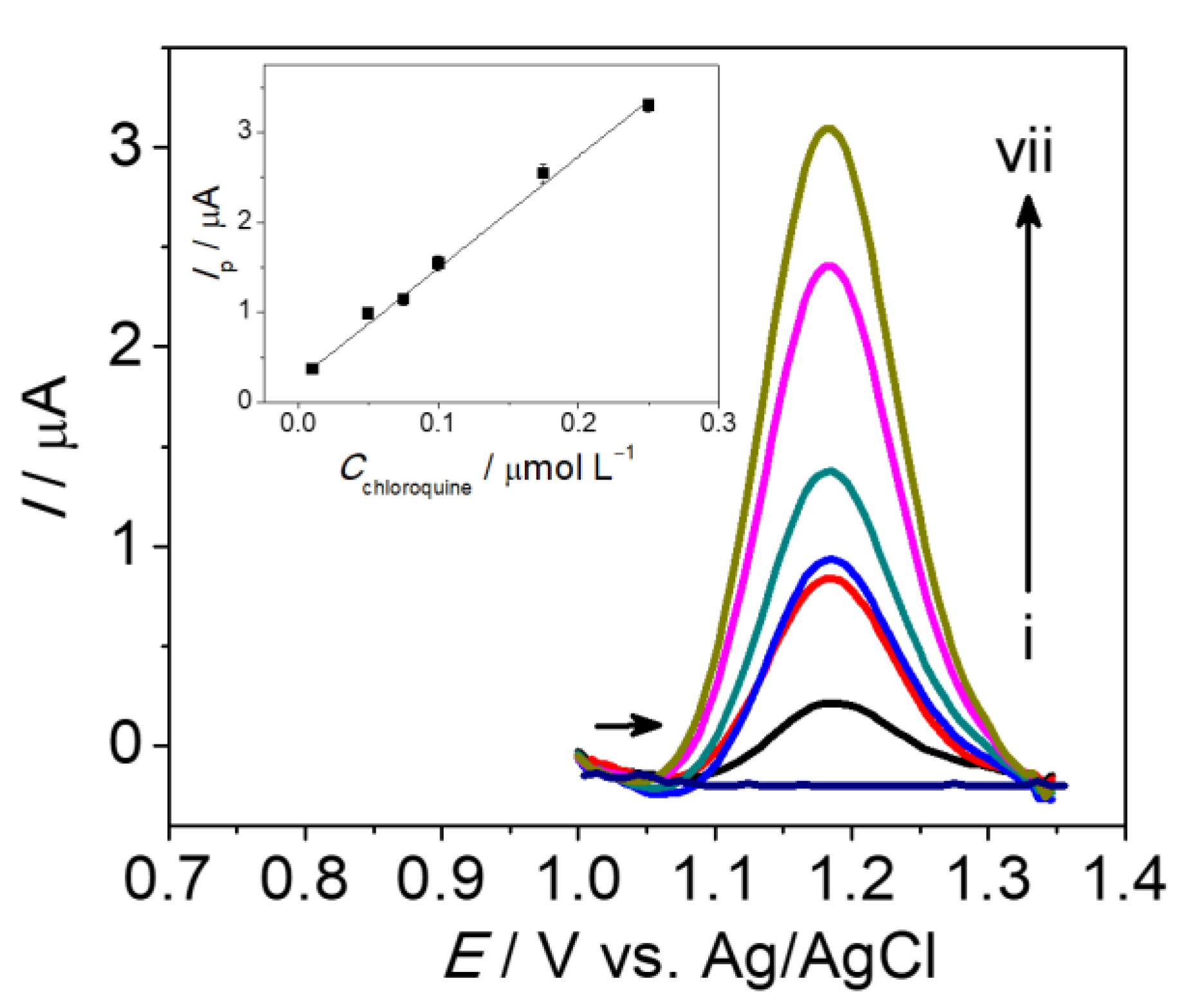
3.3. Square-Wave Voltammetry (SWV) Detection of Chloroquine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, A.; Tosounidou, S.; Gordon, C. Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: A brand-related issue? J. Rheumatol. 2017, 44, 398. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Retinopathy: What Is It? Harvard Health Publishing: Boston, MA, USA, 2017.
- Touret, F.; de Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- de Araujo Andreotti, I.A.; Orzari, L.O.; Camargo, J.R.; Faria, R.C.; Marcolino-Junior, L.H.; Bergamini, M.F.; Gatti, A.; Janegitz, B.C. Disposable and flexible electrochemical sensor made by recyclable material and low cost conductive ink. J. Electroanal. Chem. 2019, 840, 109–116. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Figueiredo, L.C.S.; Janegitz, B.C.; Santiago, A.; Pereira, E.R.; Fatibello-Filho, O. Factorial design and response surface: Voltammetric method optimization for the determination of ag(i) employing a carbon nanotubes paste electrode. Quim. Nova 2011, 34, 825–830. [Google Scholar]
- Kalinke, C.; Neumsteir, N.V.; de Oliveira Aparecido, G.; de Barros Ferraz, T.V.; Dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.S.; Fatibello, O. Nanostructured carbon black for simultaneous sensing in biological fluids. Sens. Actuator B-Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Camargo, E.R.; Baccarin, M.; Raymundo-Pereira, P.A.; Campos, A.M.; Oliveira, G.G.; Fatibello, O.; Oliveira, O.N.; Janegitz, B.C. Electrochemical biosensor made with tyrosinase immobilized in a matrix of nanodiamonds and potato starch for detecting phenolic compounds. Anal. Chim. Acta 2018, 1034, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Pereira, G.F.; Fatibello-Filho, O.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Electroanalytical sensing of indigo carmine dye in water samples using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2016, 769, 28–34. [Google Scholar] [CrossRef]
- Janegitz, B.C.; Baccarin, M.; Raymundo-Pereira, P.A.; dos Santos, F.A.; Oliveira, G.G.; Machado, S.A.S.; Lanza, M.R.V.; Fatibello, O.; Zucolotto, V. The use of dihexadecylphosphate in sensing and biosensing. Sens. Actuator B Chem. 2015, 220, 805–813. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 14. [Google Scholar] [CrossRef]
- Janegitz, B.C.; Silva, T.A.; Wong, A.; Ribovski, L.; Vicentini, F.C.; Taboada Sotomayor, M.D.P.; Fatibello-Filho, O. The application of graphene for in vitro and in vivo electrochemical biosensing. Biosens. Bioelectron. 2017, 89, 224–233. [Google Scholar] [CrossRef]
- Wong, A.; Silva, T.; Caetano, F.; Bergamini, M.; Marcolino-Junior, L.; Fatibello-Filho, O.; Janegitz, B. An Overview of Pesticide Monitoring at Environmental Samples Using Carbon Nanotubes-Based Electrochemical Sensors. C—J. Carbon Res. 2017, 3, 8. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Riboviski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta 2020. [Google Scholar] [CrossRef]
- Luong, J.H.; Male, K.B.; Glennon, J.D. Boron-doped diamond electrode: Synthesis, characterization, functionalization and analytical applications. Analyst 2009, 134, 1965–1979. [Google Scholar] [CrossRef]
- Srikanth, V.V.; Sampath Kumar, P.; Kumar, V.B. A brief review on the in situ synthesis of boron-doped diamond thin films. Int. J. Electrochem. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Xu, J.; Granger, M.C.; Chen, Q.; Strojek, J.W.; Lister, T.E.; Swain, G.M. Peer Reviewed: Boron-Doped Diamond Thin-Film Electrodes. Anal. Chem. 1997, 69, 591A–597A. [Google Scholar] [CrossRef]
- Haque, A.; Sumaiya, S. An overview on the formation and processing of nitrogen-vacancy photonic centers in diamond by ion implantation. J. Manuf. Mater. Process. 2017, 1, 6. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Brocenschi, R.F.; Medeiros, R.A.; Fatibello-Filho, O.; Rocha-Filho, R.C. Analytical Applications of Electrochemically Pretreated Boron-Doped Diamond Electrodes. ChemElectroChem 2020, 7, 1291–1311. [Google Scholar] [CrossRef]
- Teófilo, R.F.; Ceragioli, H.J.; Peterlevitz, A.C.; Da Silva, L.M.; Damos, F.S.; Ferreira, M.M.C.; Baranauskas, V.; Kubota, L.T. Improvement of the electrochemical properties of “as-grown” boron-doped polycrystalline diamond electrodes deposited on tungsten wires using ethanol. J. Solid State Electrochem. 2007, 11, 1449–1457. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Silva, T.A.; Zanin, H.; May, P.W.; Corat, E.J.; Fatibello-Filho, O. Promising electrochemical performance of high-surface-area boron-doped diamond/carbon nanotube electroanalytical sensors. J. Solid State Electrochem. 2016, 20, 2403–2409. [Google Scholar] [CrossRef]
- Marken, F.; Compton, R.G.; Goeting, C.H.; Foord, J.S.; Bull, S.D.; Davies, S.G. Fast electrochemical triple-interface processes at boron-doped diamond electrodes. J. Solid State Electrochem. 2001, 5, 88–93. [Google Scholar] [CrossRef]
- Haque, A.; Sachan, R.; Narayan, J. Synthesis of diamond nanostructures from carbon nanotube and formation of diamond-CNT hybrid structures. Carbon 2019, 150, 388–395. [Google Scholar] [CrossRef]
- Deroco, P.B.; Vicentini, F.C.; Oliveira, G.G.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-wave voltammetric determination of hydroxychloroquine in pharmaceutical and synthetic urine samples using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2014, 719, 19–23. [Google Scholar] [CrossRef]
- Guedes, T.d.J.; Alecrim, M.F.; Oliveira, F.M.; Lima, A.B.; Barbosa, S.L.; dos Santos, W.T.P. Determination of prazosin in pharmaceutical samples by flow injection analysis with multiple-pulse amperometric detection using boron-doped diamond electrode. J. Solid State Electrochem. 2016, 20, 2445–2451. [Google Scholar] [CrossRef]
- Silva, T.A.; Pereira, G.F.; Fatibello-Filho, O.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Square-wave voltammetric determination of rosuvastatin calcium in pharmaceutical and biological fluid samples using a cathodically pretreated boron-doped diamond electrode. Diam. Relat. Mater. 2015, 58, 103–109. [Google Scholar] [CrossRef]
- Santos, A.M.; Vicentini, F.C.; Deroco, P.B.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-Wave Voltammetric Determination of Paracetamol and Codeine in Pharmaceutical and Human Body Fluid Samples Using a Cathodically Pretreated Boron-Doped Diamond Electrode. J. Braz. Chem. Soc. 2015, 26, 2159–2168. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lourencao, B.C.; Rocha-Filho, R.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of synthetic colorants in food using a cathodically pretreated boron-doped diamond electrode. Talanta 2012, 97, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Brocenschi, R.F.; Silva, T.A.; Lourencao, B.C.; Fatibello-Filho, O.; Rocha-Filho, R.C. Use of a boron-doped diamond electrode to assess the electrochemical response of the naphthol isomers and to attain their truly simultaneous electroanalytical determination. Electrochim. Acta 2017, 243, 374–381. [Google Scholar] [CrossRef]
- Souza, G.A.; Arantes, L.C.; Guedes, T.J.; de Oliveira, A.C.; Marinho, P.A.; Muñoz, R.A.A.; dos Santos, W.T.P. Voltammetric signatures of 2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamines on boron-doped diamond electrodes: Detection in blotting paper samples. Electrochem. Commun. 2017, 82, 121–124. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Medeiros, R.A.; Thomasi, S.S.; Ferreira, A.G.; Rocha-Filho, R.C.; Fatibello-Filho, O. Amperometric flow-injection determination of the anthelmintic drugs ivermectin and levamisole using electrochemically pretreated boron-doped diamond electrodes. Sens. Actuators B 2016, 222, 181–189. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Medeiros, R.A.; Fatibello-Filho, O. Simultaneous determination of antihypertensive drugs by flow injection analysis using multiple pulse amperometric detection with a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2015, 754, 154–159. [Google Scholar] [CrossRef]
- Deroco, P.B.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Selective and simultaneous determination of indigo carmine and allura red in candy samples at the nano-concentration range by flow injection analysis with multiple pulse amperometric detection. Food Chem. 2018, 247, 66–72. [Google Scholar] [CrossRef]
- Silva, W.P.; Silva, L.A.J.; França, C.H.; Sousa, R.M.F.; Muñoz, R.A.A.; Richter, E.M. Square-wave Voltammetric Determination of Propyphenazone, Paracetamol, and Caffeine: Comparative Study between Batch Injection Analysis and Conventional Electrochemical Systems. Electroanalysis 2017, 29, 1860–1866. [Google Scholar] [CrossRef]
- Freitas, J.M.; Oliveira, T.d.C.; Gimenes, D.T.; Munoz, R.A.A.; Richter, E.M. Simultaneous determination of three species with a single-injection step using batch injection analysis with multiple pulse amperometric detection. Talanta 2016, 146, 670–675. [Google Scholar] [CrossRef]
- Oliveira, T.d.C.; Freitas, J.M.; Abarza Munoz, R.A.; Richter, E.M. A batch injection analysis system with square-wave voltammetric detection for fast and simultaneous determination of naphazoline and zinc. Talanta 2016, 152, 308–313. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Pedrosa, V.A.; Codognoto, L.; Machado, S.A.S.; Rocha-Filho, R.C.; Avaca, L.A. Enhanced electrochemical response of boron-doped diamond electrodes brought on by a cathodic surface pre-treatment. Electrochim. Acta 2004, 49, 4021–4026. [Google Scholar] [CrossRef]
- Haque, A.; Pant, P.; Narayan, J. Large-area diamond thin film on Q-carbon coated crystalline sapphire by HFCVD. J. Cryst. Growth 2018, 504, 17–25. [Google Scholar] [CrossRef]
- Haque, A.; Gupta, S.; Narayan, J. Characteristics of Diamond Deposition on Al2O3, Diamond-like Carbon, and Q-Carbon. ACS Appl. Electron. Mater. 2020, 2, 1323–1334. [Google Scholar] [CrossRef]
- Salazar-Banda, G.R.; de Carvalho, A.E.; Andrade, L.S.; Rocha-Filho, R.C.; Avaca, L.A. On the activation and physical degradation of boron-doped diamond surfaces brought on by cathodic pretreatments. J. Appl. Electrochem. 2010, 40, 1817–1827. [Google Scholar] [CrossRef]
- Granger, M.C.; Witek, M.; Xu, J.; Wang, J.; Hupert, M.; Hanks, A.; Koppang, M.D.; Butler, J.E.; Lucazeau, G.; Mermoux, M.; et al. Standard Electrochemical Behavior of High-Quality, Boron-Doped Polycrystalline Diamond Thin-Film Electrodes. Anal. Chem. 2000, 72, 3793–3804. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.M.; Ramesham, R. The electrochemical activity of boron-doped polycrystalline diamond thin film electrodes. Anal. Chem. 1993, 65, 345–351. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons Inc.: New York, NY, USA, 2001; Volume 2. [Google Scholar]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351. [Google Scholar] [CrossRef]
- Silva, T.A.; Zanin, H.; May, P.W.; Corat, E.J.; Fatibello-Filho, O. Electrochemical Performance of Porous Diamond-like Carbon Electrodes for Sensing Hormones, Neurotransmitters, and Endocrine Disruptors. ACS Appl. Mater. Interfaces 2014, 6, 21086–21092. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Simioni, N.B.; Silva, T.A.; Oliveira, G.G.; Fatibello-Filho, O. A nanodiamond-based electrochemical sensor for the determination of pyrazinamide antibiotic. Sens. Actuators B 2017, 250, 315–323. [Google Scholar] [CrossRef]
- Wong, A.; Silva, T.A.; Fatibello-Filho, O. Graphite Oxide and Gold Nanoparticles as Alternative Materials in the Design of a Highly Sensitive Electrochemical Sensor for the Simultaneous Determination of Biological Species. Electroanalysis 2017, 29, 2491–2497. [Google Scholar] [CrossRef]
- Srivastava, M.; Tiwari, P.; Mall, V.K.; Srivastava, S.K.; Prakash, R. Voltammetric determination of the antimalarial drug chloroquine using a glassy carbon electrode modified with reduced graphene oxide on WS2 quantum dots. Microchim. Acta 2019, 186, 10. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.F.; Deroco, P.B.; Silva, T.A.; Ferreira, H.S.; Fatibello-Filho, O.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Study of electrooxidation and enhanced voltammetric determination of β-blocker pindolol using a boron-doped diamond electrode. Diam. Relat. Mater. 2018, 82, 109–114. [Google Scholar] [CrossRef]
- Santos, A.M.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Flow injection analysis system with electrochemical detection for the simultaneous determination of nanomolar levels of acetaminophen and codeine. Arab. J. Chem. 2020, 13, 335–345. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Baccarin, M.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Differential pulse voltammetric determination of albendazole in pharmaceutical tablets using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2013, 707, 15–19. [Google Scholar] [CrossRef]
- Rodriguesa, G.N.; Silvaa, W.P.; Rochaa, D.P.; Richtera, E.M.; Munoza, R.A.; Batistaa, A.D. Electrochemical determination of 2-naphthylamine in perfume samples using boron-doped diamond electrode. Quim. Nova 2020, 43, 286–290. [Google Scholar]
- Oliveira, T.D.C.; Freitas, J.M.; Muñoz, R.A.A.; Richter, E.M. Development of a Novel Versatile Method for Determination of two Antihistamines in Association with Naphazoline Using Cathodically Pretreated Boron-doped Diamond Electrode. Electroanalysis 2018, 30, 868–876. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 7th ed.; Pearson Education: New York, NY, USA, 2018; p. 312. [Google Scholar]
- Radi, A. Accumulation and trace measurement of chloroquine drug at DNA-modified carbon paste electrode. Talanta 2005, 65, 271–275. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Akbarian, M. Voltammetric determination of some anti-malarial drugs using a carbon paste electrode modified with Cu(OH)2 nano-wire. Talanta 2009, 78, 1440–1445. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]

| Parameter | Evaluated Conditions | Optimum Condition |
|---|---|---|
| Supporting electrolyte | 0.1 mol L−1 H2SO4 0.1 mol L−1 Britton–Robson buffer 0.1 mol L−1 acetate buffer | 0.1 mol L−1 Britton–Robson buffer |
| pH (Britton–Robson buffer) | 2.0 to 8.0 | 6.0 |
| SWV frequency (f) | 10 to 150 Hz | 100 |
| SWV amplitude (A) | 5 to 60 mV | 50 |
| SWV potential increment (ΔE) | 1 to 10 mV | 5 |
| Electrode | Linear Range (µmol L−1) | LOD (µmol L−1) | Reference |
|---|---|---|---|
| rGO@WS2-QDs/ GC | 0.5–82.4 | 0.04 | [28] |
| dsDNA/CP | 0.1–10.0 | 0.03 | [33] |
| CuNW/CP | 0.13–13.3 | 0.02 | [34] |
| BDD | 0.01–0.25 | 0.002 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.G.; Azzi, D.C.; Silva, T.A.; Oliveira, P.R.d.; Fatibello-Filho, O.; Janegitz, B.C. Sensitive Voltammetric Detection of Chloroquine Drug by Applying a Boron-Doped Diamond Electrode. C 2020, 6, 75. https://doi.org/10.3390/c6040075
Oliveira GG, Azzi DC, Silva TA, Oliveira PRd, Fatibello-Filho O, Janegitz BC. Sensitive Voltammetric Detection of Chloroquine Drug by Applying a Boron-Doped Diamond Electrode. C. 2020; 6(4):75. https://doi.org/10.3390/c6040075
Chicago/Turabian StyleOliveira, Geiser Gabriel, Déborah Christine Azzi, Tiago Almeida Silva, Paulo Roberto de Oliveira, Orlando Fatibello-Filho, and Bruno Campos Janegitz. 2020. "Sensitive Voltammetric Detection of Chloroquine Drug by Applying a Boron-Doped Diamond Electrode" C 6, no. 4: 75. https://doi.org/10.3390/c6040075
APA StyleOliveira, G. G., Azzi, D. C., Silva, T. A., Oliveira, P. R. d., Fatibello-Filho, O., & Janegitz, B. C. (2020). Sensitive Voltammetric Detection of Chloroquine Drug by Applying a Boron-Doped Diamond Electrode. C, 6(4), 75. https://doi.org/10.3390/c6040075







