Tetrel Bonding Interactions Involving Carbon at Work: Recent Advances in Crystal Engineering and Catalysis
Abstract
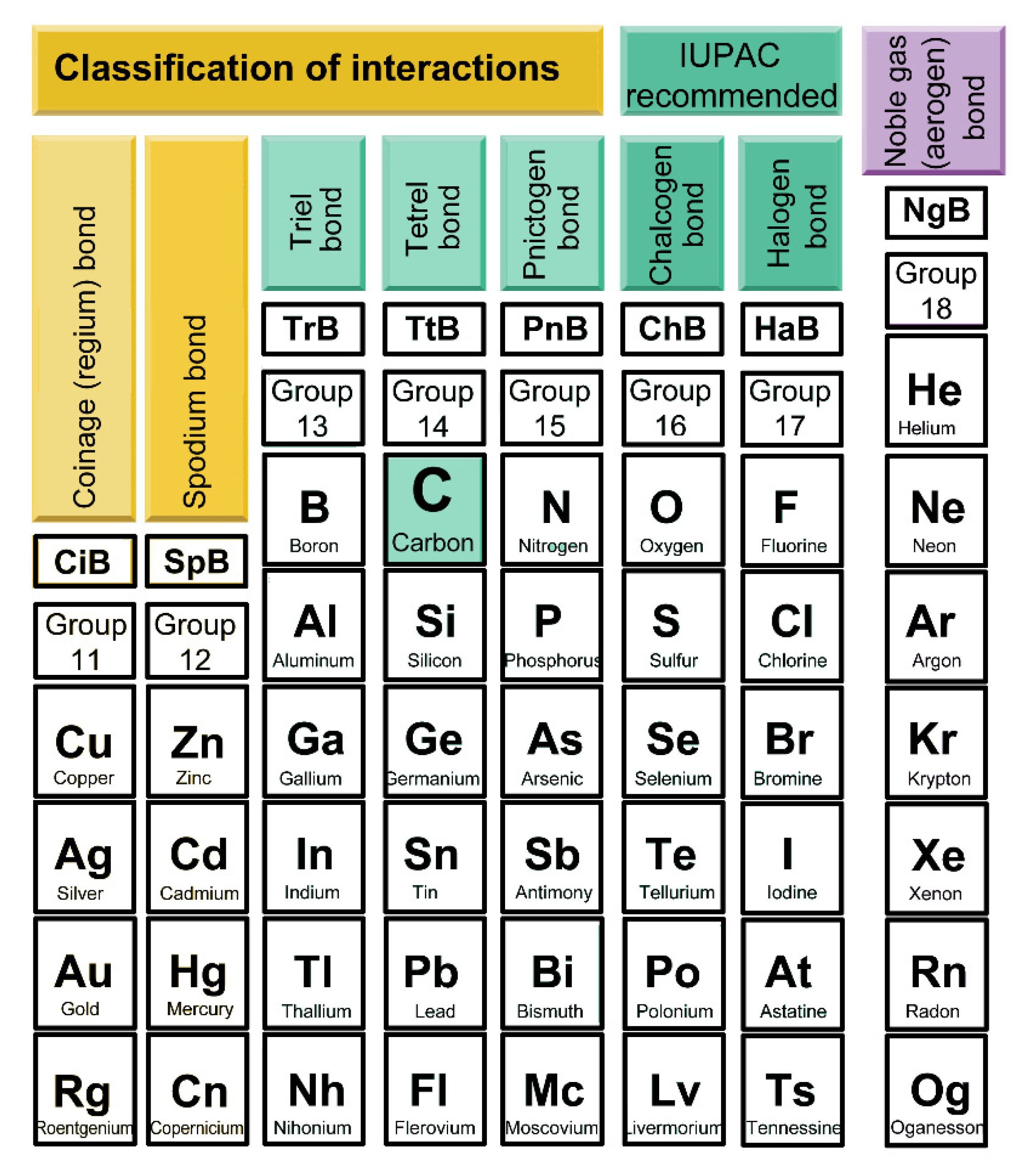
1. Introduction
2. Results and Discussion
2.1. Crystal Engineering
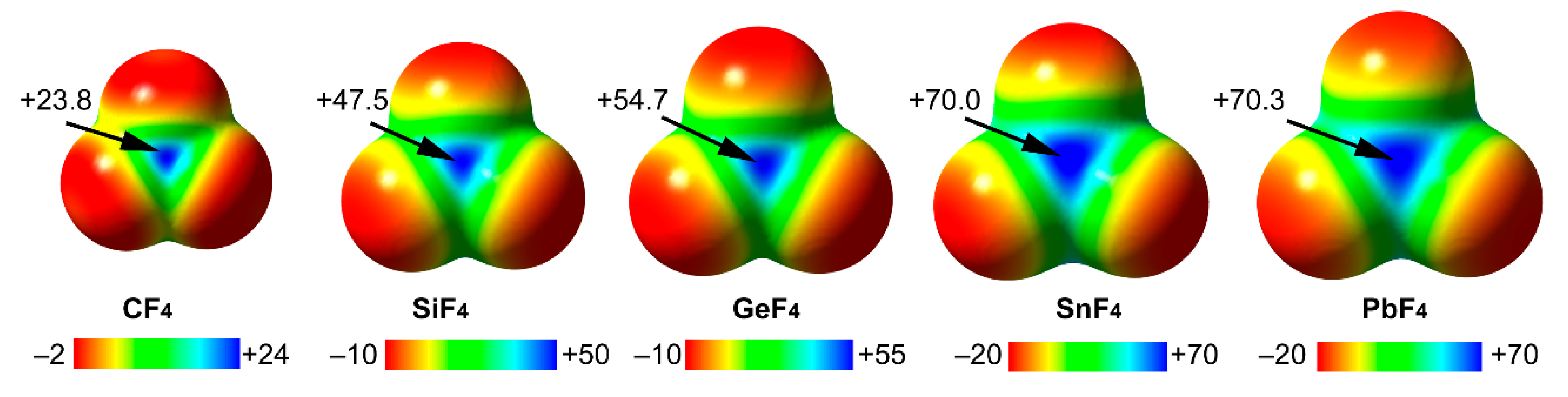
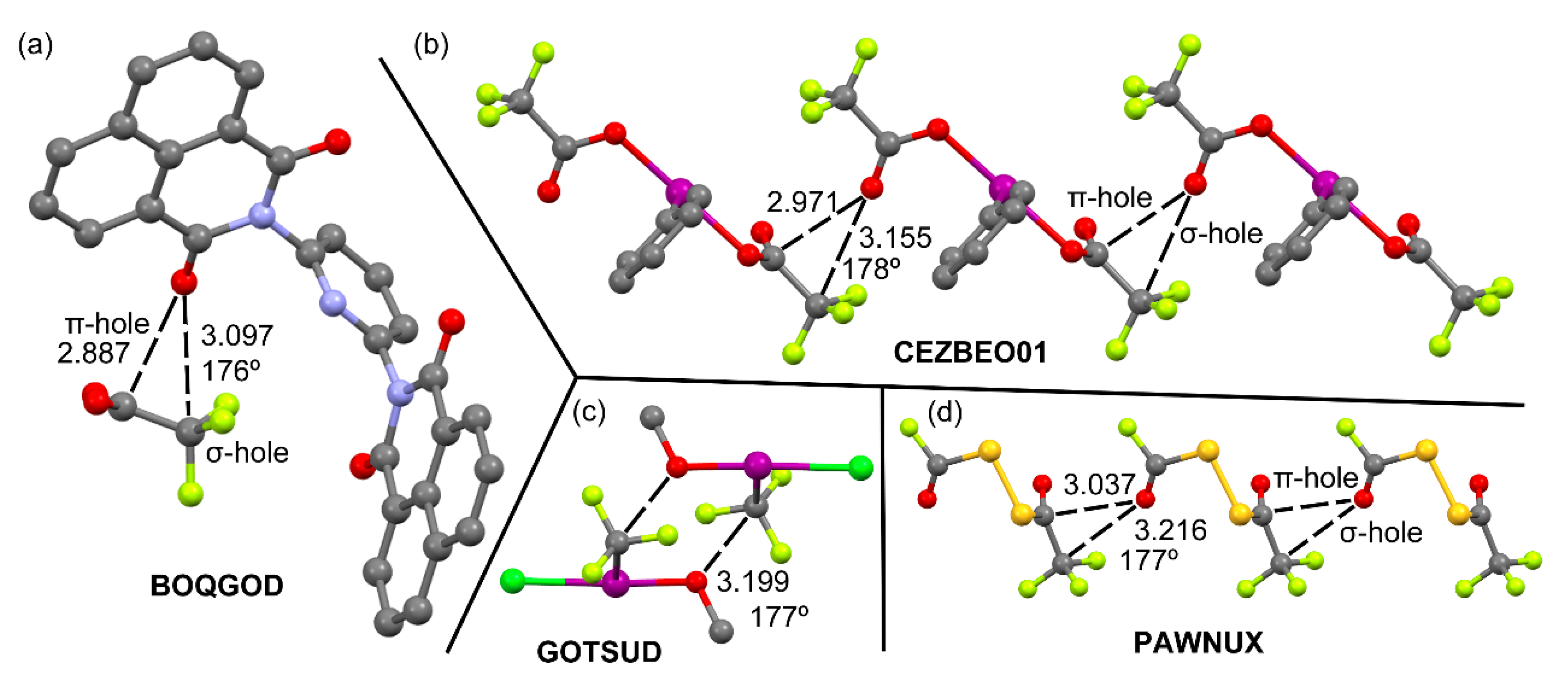
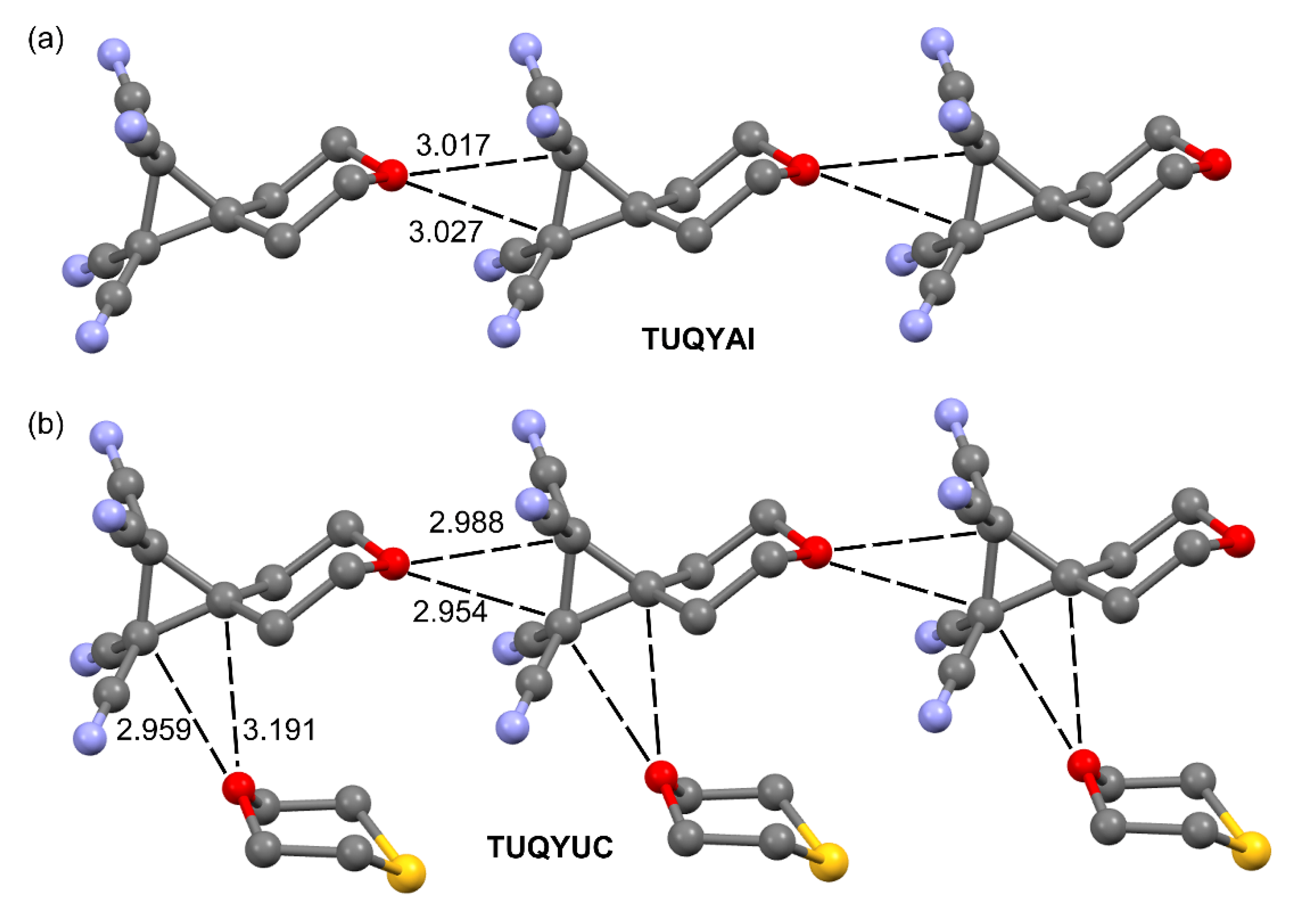
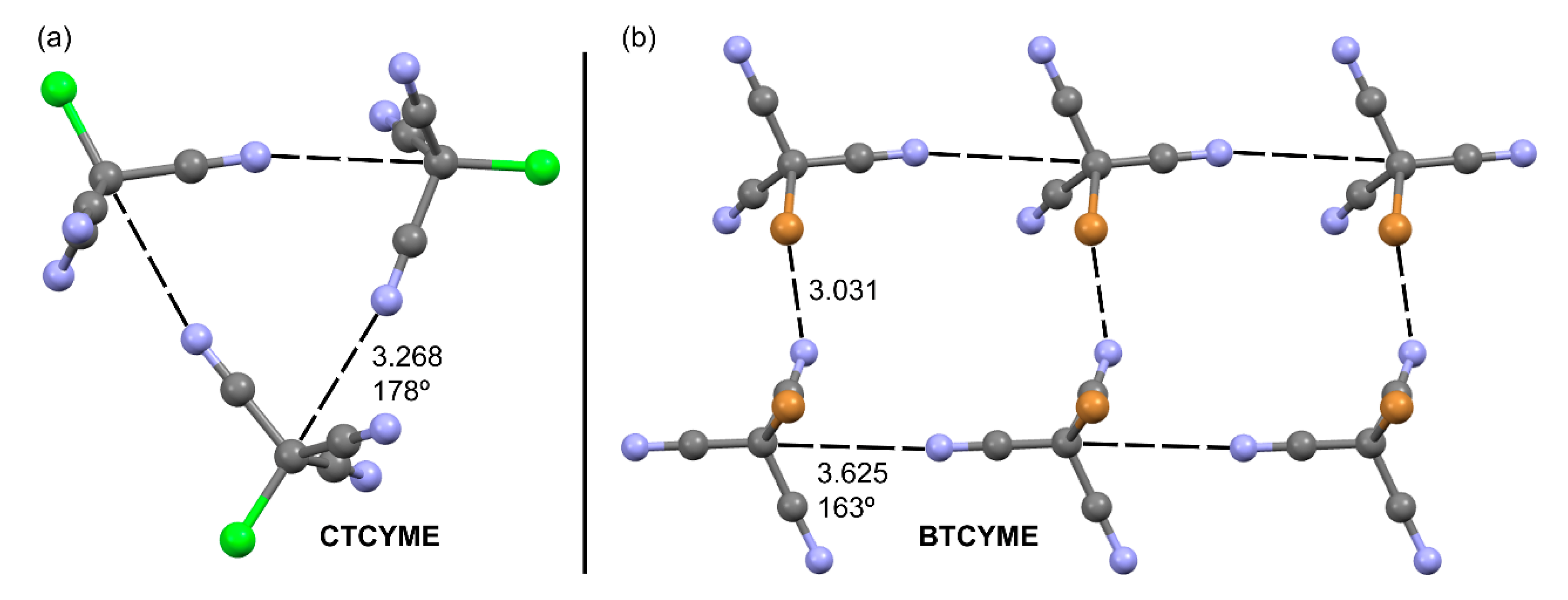
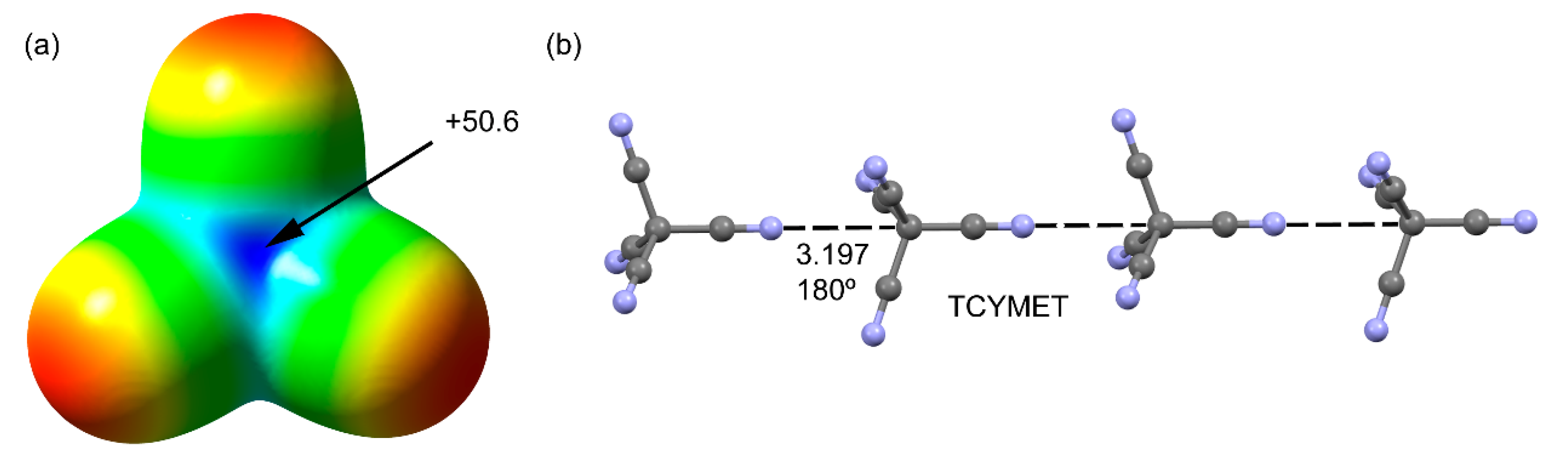
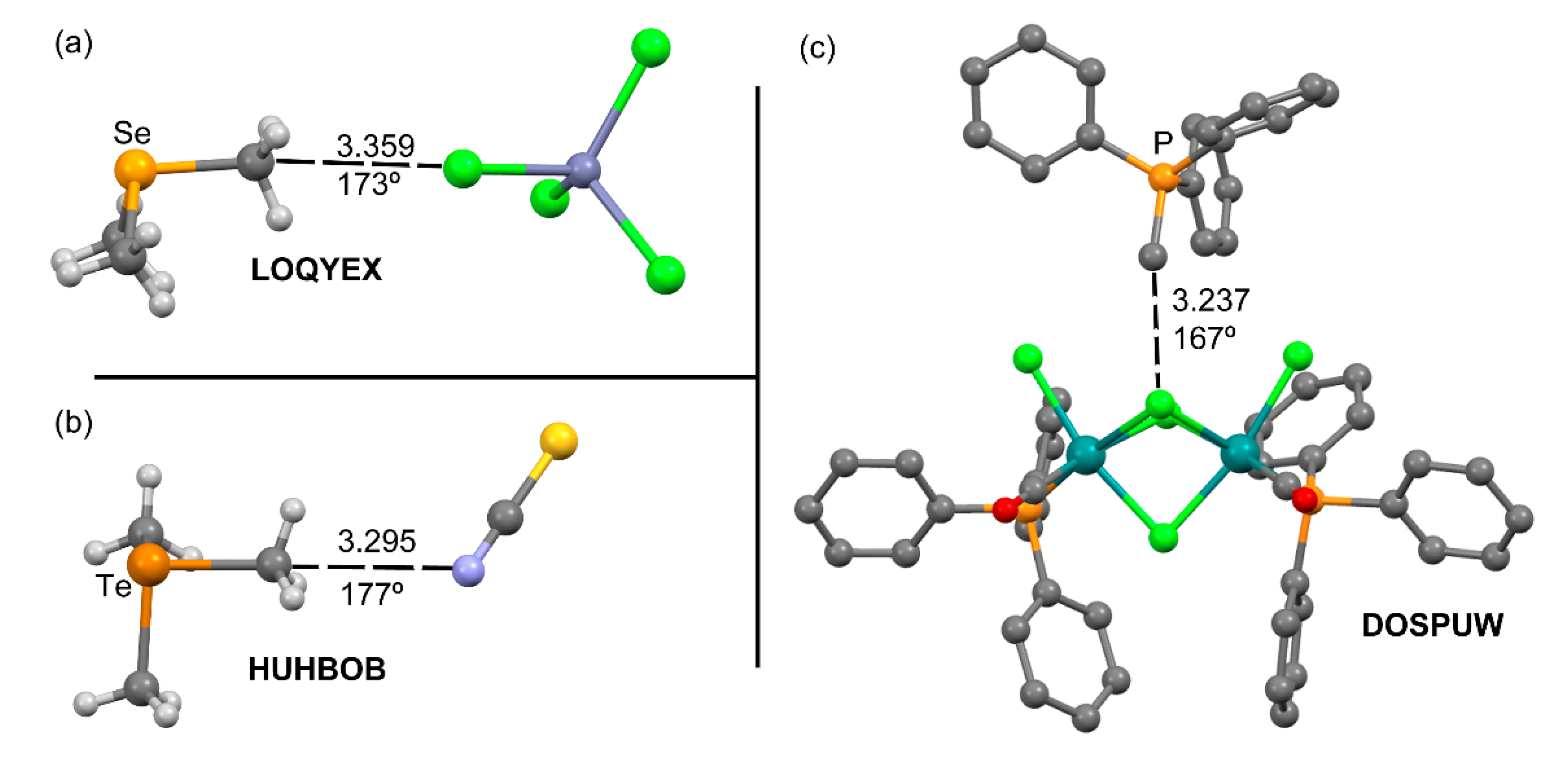
2.1.1. Neutral TtBs
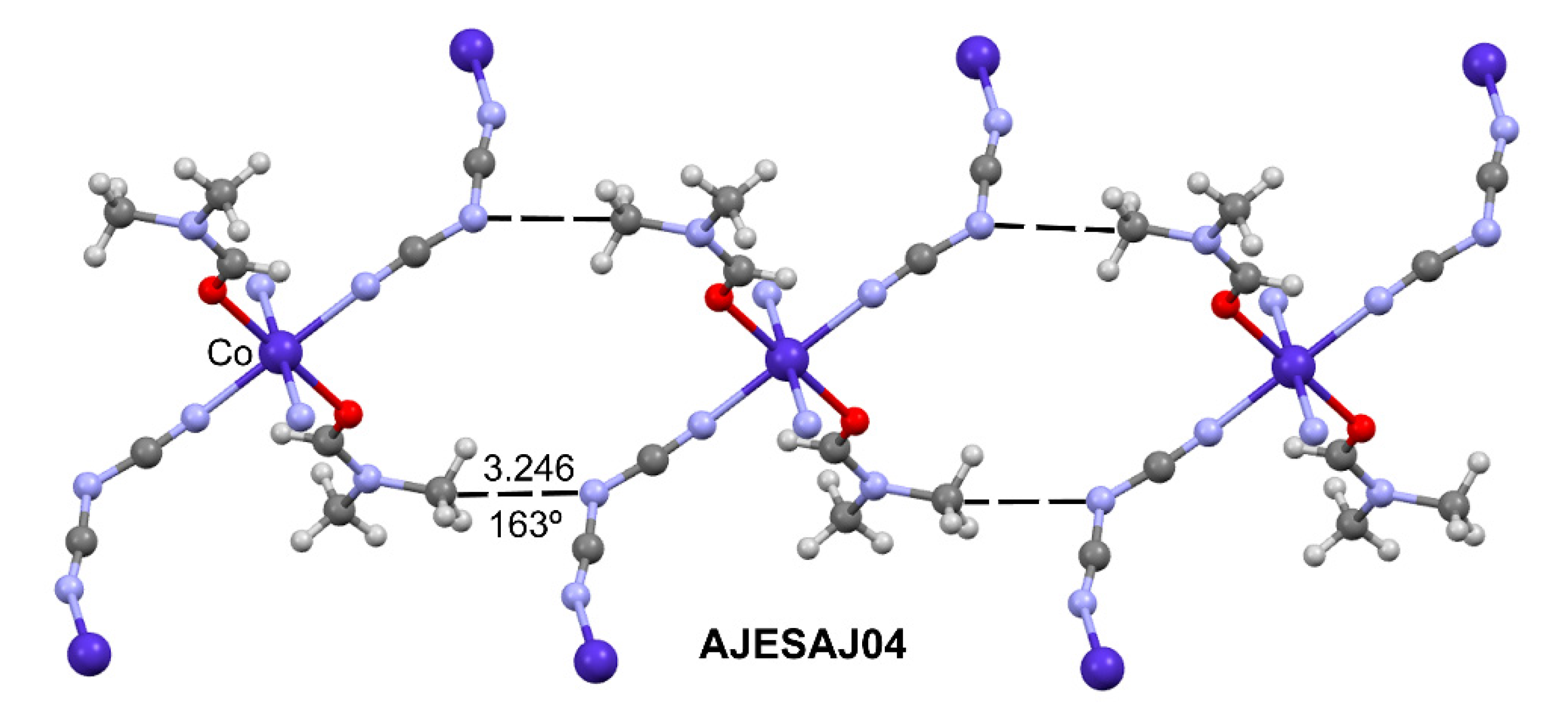
2.1.2. Charge assisted TtBs
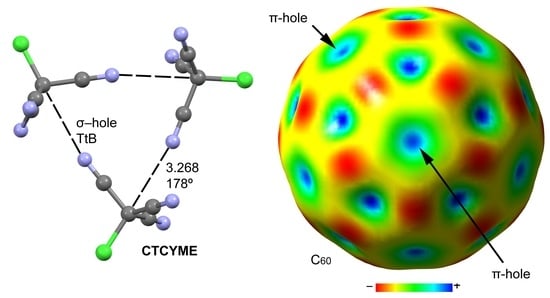
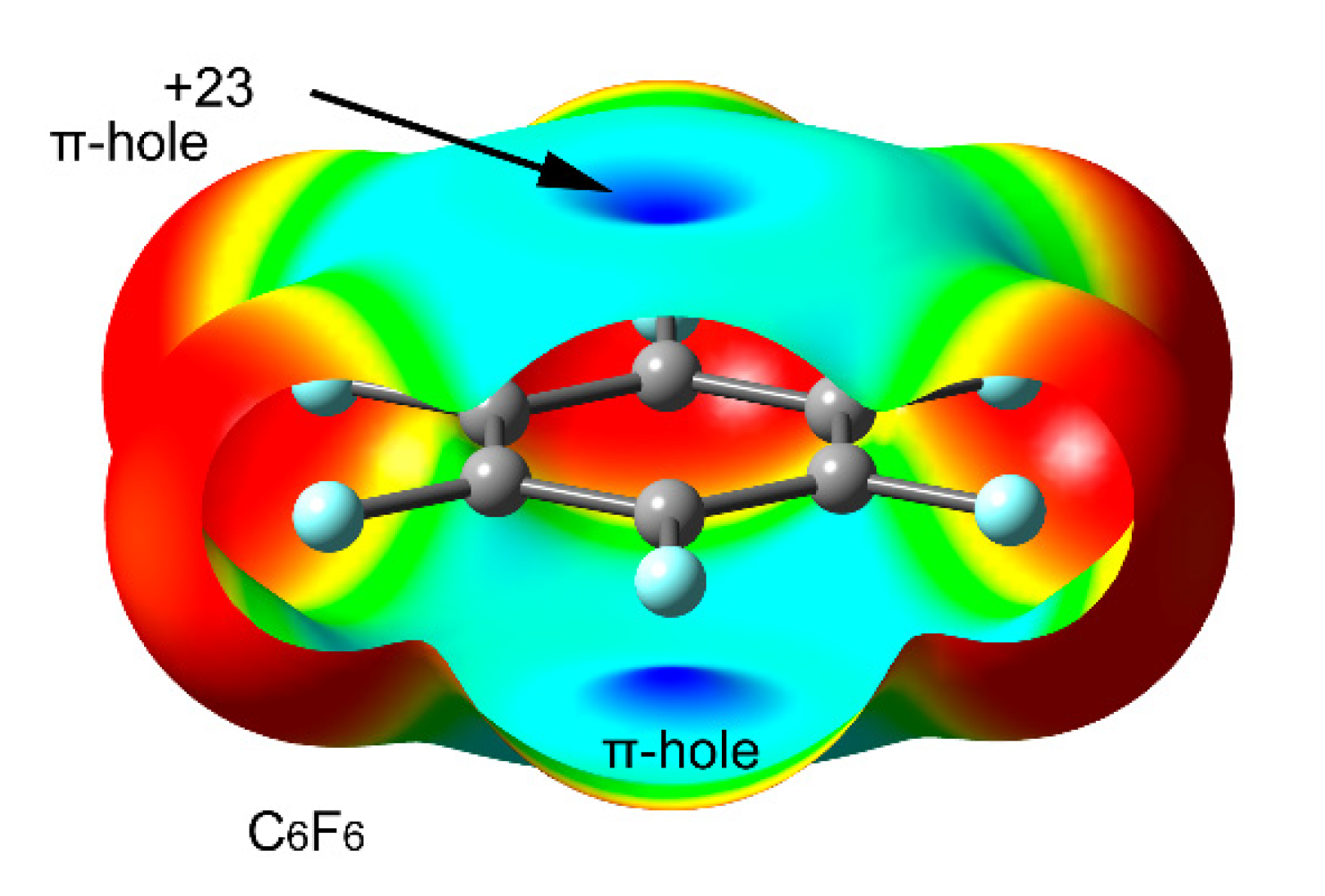
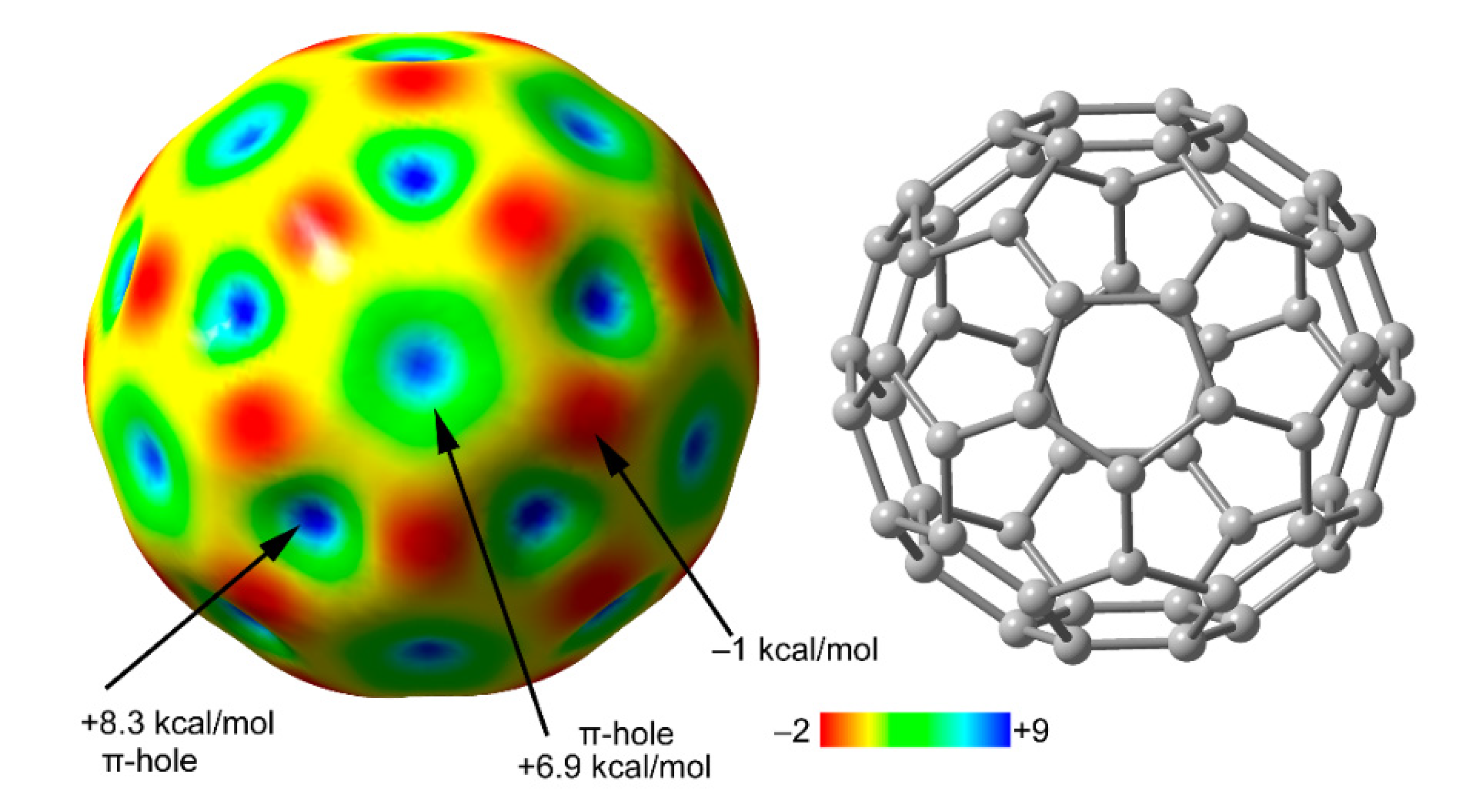
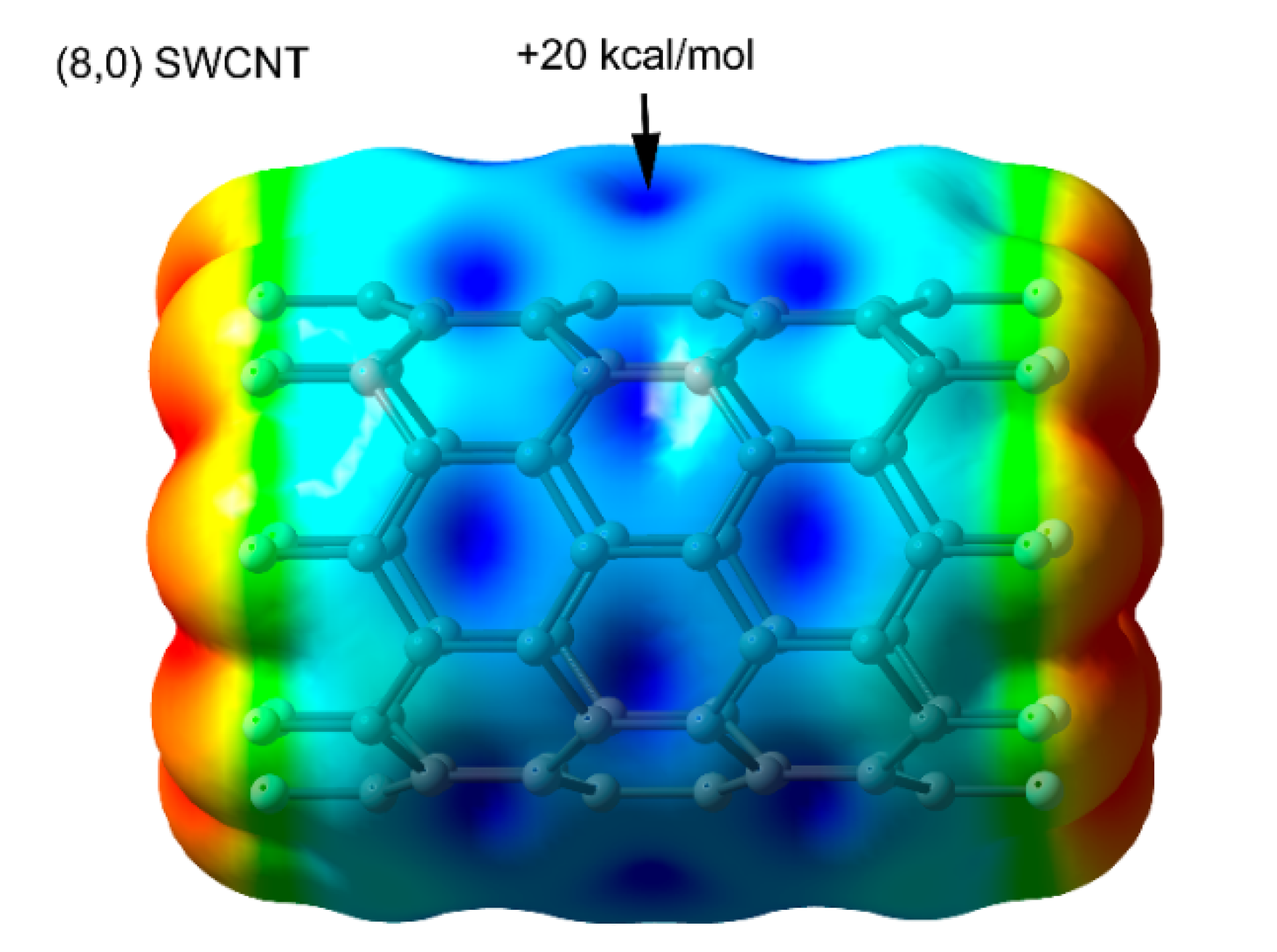
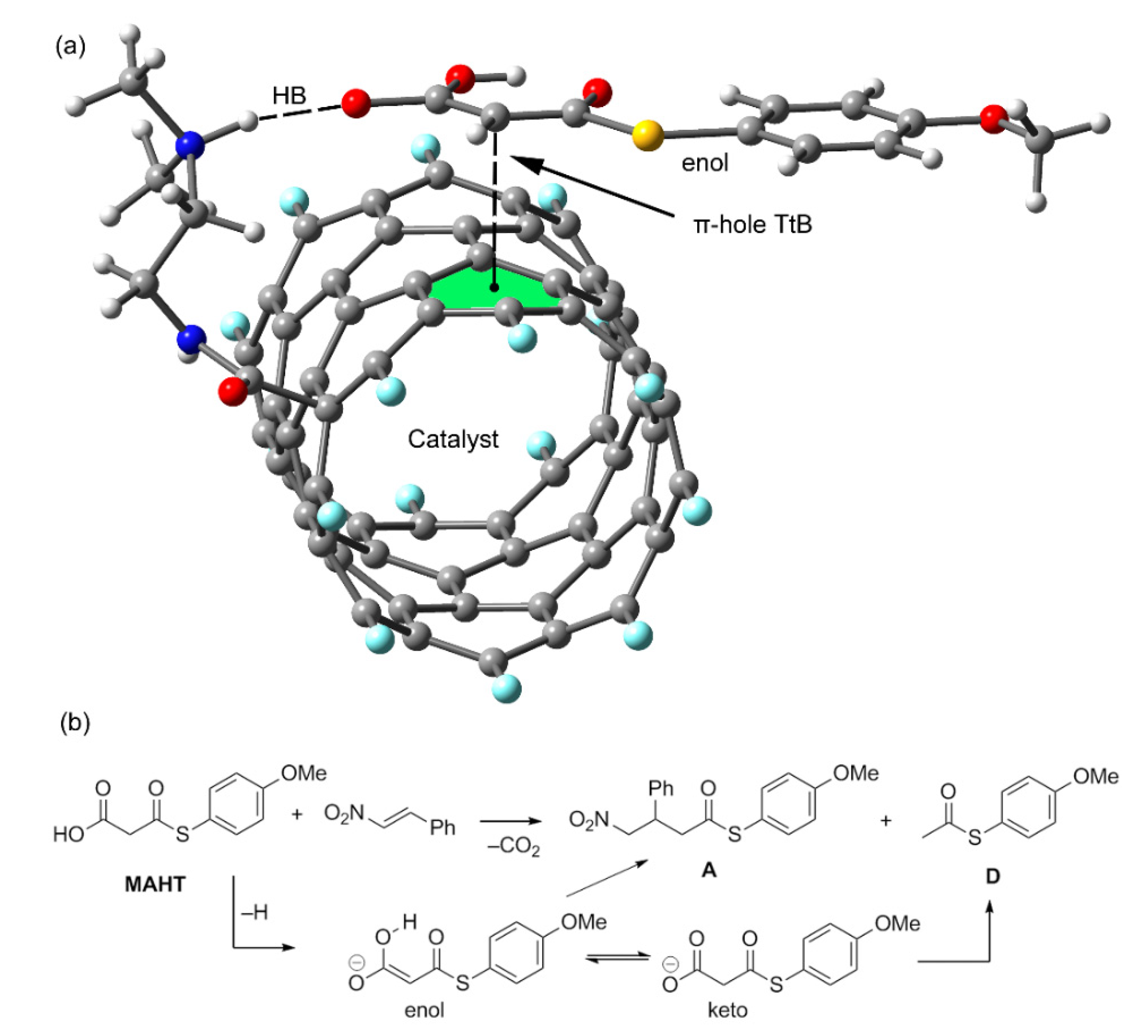
2.2. Anion–π (π-Hole) Catalysis with Carbon Allotropes
3. Conclusions
Funding
Conflicts of Interest
References
- Feringa, B.L. The Art of Building Small: From Molecular Switches to Motors (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef]
- Newberry, R.W.; Raines, R.T. The n→π* Interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Varughese, S. Non-covalent routes to tune the optical properties of molecular materials. J. Mater. Chem. C 2014, 2, 3499–3516. [Google Scholar] [CrossRef]
- Scheiner, S. Noncovalent Forces; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Yuan, C.; Ji, W.; Xing, R.; Li, J.; Gazit, E.; Yan, X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3, 567–588. [Google Scholar] [CrossRef]
- Spackman, P.R.; Yu, L.-J.; Morton, C.J.; Parker, M.W.; Bond, C.S.; Spackman, M.A.; Jayatilaka, D.; Thomas, S.P. Bridging Crystal Engineering and Drug Discovery by Utilizing Intermolecular Interactions and Molecular Shapes in Crystals. Angew. Chem. Int. Ed. 2019, 58, 16780–16784. [Google Scholar] [CrossRef]
- Daze, K.D.; Hof, F. The Cation−π Interaction at Protein–Protein Interaction Interfaces: Developing and Learning from Synthetic Mimics of Proteins That Bind Methylated Lysines. Acc. Chem. Res. 2013, 46, 937–945. [Google Scholar] [CrossRef]
- Tuikka, M.; Hirva, P.; Rissanen, K.; Korppi-Tommola, J.; Haukka, M. Halogen bonding—A key step in charge recombination of the dye-sensitized solar cell. Chem. Commun. 2011, 47, 4499–4501. [Google Scholar] [CrossRef]
- Song, P.; Wang, H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mat. 2020, 32, 1901244. [Google Scholar] [CrossRef]
- Molcanov, K.; Milasinovic, V.; Kojic-Prodic, B. Contribution of Different Crystal Packing Forces in π-Stacking: From Noncovalent to Covalent Multicentric Bonding. Cryst. Growth Des. 2019, 19, 5967–5980. [Google Scholar] [CrossRef]
- Frontera, A.; Gamez, P.; Mascal, M.; Mooinbroek, T.J.; Reedijk, J. Putting Anion–π Interactions Into Perspective. Angew. Chem. Int. Ed. 2011, 50, 9564–9583. [Google Scholar] [CrossRef]
- Yamada, S. Cation−π Interactions in Organic Synthesis. Chem. Rev. 2018, 118, 11353–11432. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Frontera, A. Noble Gas Bonding Interactions Involving Xenon Oxides and Fluorides. Molecules 2020, 25, 3419. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Covalent and Non-covalent Noble Gas Bonding Interactions in XeFn Derivatives (n = 2–6): A Combined Theoretical and ICSD Analysis. Front. Chem. 2020, 8, 395. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. σ/π-Hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2020, 404, 213112. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Naming Interactions from the Electrophilic Site. Cryst. Growth Des. 2014, 14, 2697–2702. [Google Scholar] [CrossRef]
- Terraneo, G.; Resnati, G. Bonding Matters. Cryst. Growth Des. 2017, 17, 1439–1440. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzà, A. Regium–π bonds: An Unexplored Link between Noble Metal Nanoparticles and Aromatic Surfaces. Chem. Eur. J. 2018, 24, 7228–7234. [Google Scholar] [CrossRef] [PubMed]
- Legon, A. Tetrel, pnictogen and chalcogen bonds identified in the gas phase before they had names: A systematic look at non-covalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 14884–14896. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Michalczyk, M.; Zierkiewicz, W.; Scheiner, S. Triel-Bonded Complexes between TrR3 (Tr=B, Al, Ga; R=H, F, Cl, Br, CH3) and Pyrazine. Chem. Phys. Chem. 2018, 19, 3122–3133. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Daolio, A.; Baggioli, A.; Famulari, A.; Leroy, C.; Bryce, D.L.; Resnati, G. 4,4′-Dipyridyl Dioxide·SbF3 Cocrystal: Pnictogen Bond Prevails over Halogen and Hydrogen Bonds in Driving Self-Assembly. Cryst. Growth Des. 2020, 20, 916–922. [Google Scholar] [CrossRef]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef]
- Mikuriya, M.; Okawa, H.; Kidam, S. Binuclear metal complexes. XXXII [1]. Further studies of binuclear copper(II) complexes with N-(2-alkylthioethyl)-3-aminopropanol: Synthesis, Spectral and magnetic properties and the crystal structure. Inorg. Chem. Acta 1980, 42, 233–239. [Google Scholar] [CrossRef]
- Healy, P.C.; Kildea, J.D.; White, A.H. Lewis-Base Adducts of Group 11 Metal(I) Compounds. XLIV. Synthesis and Conformational Systematics of 1:1 Copper(I) Halide Nitrogen Base Adducts as [(N-base)CuX2Cu(N-base)] Dimers (X = Cl, Br, I) and [(N-Base)CuX] Monomers (X = Cl, Br). Aust. J. Chem. 1989, 42, 137–148. [Google Scholar] [CrossRef]
- Jones, P.G.; Ahrens, B. Gold(I) complexes with amine ligands. 3 Competition between auriophilic and hydrogen bonding interactions in dimeric species. New J. Chem. 1998, 22, 1041–1042. [Google Scholar] [CrossRef]
- Wang, R.; Yang, S.; Li, Q. Coinage-Metal Bond between [1.1.1]Propellane and M2/MCl/MCH3 (M = Cu, Ag, and Au): Cooperativity and Substituents. Molecules 2019, 24, 2601. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. σ-Holes and Si···N intramolecular interactions. J. Mol. Mod. 2019, 25, 101. [Google Scholar] [CrossRef]
- Lu, J.; Scheiner, S. Effects of Halogen, Chalcogen, Pnicogen, and Tetrel Bonds on IR and NMR Spectra. Molecules 2019, 24, 2822. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. S⋅⋅⋅Sn Tetrel Bonds in the Activation of Peroxisome Proliferator-Activated Receptors (PPARs) by Organotin Molecules. Chem. Eur. J. 2018, 24, 16582–16587. [Google Scholar] [CrossRef]
- Escudero-Adán, E.C.; Bauzà, A.; Frontera, A.; Ballester, P. Nature of Noncovalent Carbon-Bonding Interactions Derived from Experimental Charge-Density Analysis. Chem. Phys. Chem. 2015, 16, 2530–2533. [Google Scholar] [CrossRef]
- Bauzà, A.; Frontera, A.; Mooibroek, T.J. 1,1,2,2-Tetracyanocyclopropane (TCCP) as supramolecular synthon. Phys. Chem. Chem. Phys. 2016, 18, 1693–1698. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Mod. 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D.; Shefter, E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973, 95, 5065–5067. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D. From crystal statics to chemical dynamics. Acc. Chem. Res. 1983, 16, 153–161. [Google Scholar] [CrossRef]
- Persch, E.; Dumele, O.; Diederich, F. Molecular Recognition in Chemical and Biological Systems. Angew. Chem. Int. Ed. 2015, 54, 3290–3327. [Google Scholar] [CrossRef] [PubMed]
- Paulini, R.; Müller, K.; Diederich, F. Orthogonal Multipolar Interactions in Structural Chemistry and Biology. Angew. Chem. Int. Ed. 2005, 44, 1788–1805. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.M.; Raithby, P.R. Carbonyl-Carbonyl Interactions can be Competitive with Hydrogen Bonds. Acta Cryst. 1998, B54, 320–329. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 48, 2686–2695. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooinbroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. Chem. Phys. Chem. 2015, 16, 2496–2517. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. The Chalcogen Bond in Crystalline Solids: A World Parallel to Halogen Bond. Acc. Chem. Res. 2019, 52, 1313–1324. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. Fluorinated elements of Group 15 as pnictogen bond donor sites. J. Fluor. Chem. 2017, 203, 62–74. [Google Scholar] [CrossRef]
- Scilabra, P.; Kumar, V.; Ursini, M.; Resnati, G. Close contacts involving germanium and tin in crystal structures: Experimental evidence of tetrel bonds. J. Mol. Mod. 2018, 24, 37. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Sahoo, D.K.; Bhaumik, S.; Jena, S.; Chandrakar, A.; Biswal, H.S. Noncovalent Carbon-Bonding Interactions in Proteins. Angew. Chem. Int. Ed. 2018, 57, 16496–16500. [Google Scholar] [CrossRef] [PubMed]
- Mooibroek, T.J. Intermolecular Non-Covalent Carbon-Bonding Interactions with Methyl Groups: A CSD, PDB and DFT Study. Molecules 2019, 24, 3370. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. The fluorine atom as a halogen bond donor, viz. a positive site. CrystEngComm 2011, 13, 6593–6596. [Google Scholar] [CrossRef]
- Ruedenberg, K. The physical nature of the chemical bond. Rev. Mod. Phys. 1962, 34, 326–352. [Google Scholar] [CrossRef]
- Feinberg, M.J.; Ruedenberg, K. Paradoxical Role of the Kinetic-Energy Operator in the Formation of the Covalent Bond. J. Chem. Phys. 1971, 54, 1495–1511. [Google Scholar] [CrossRef]
- Feinberg, M.J.; Ruedenberg, K. Heteropolar One-Electron Bond. J. Chem. Phys. 1971, 55, 5804–5818. [Google Scholar] [CrossRef]
- Feinberg, M.J.; Ruedenberg, K.; Mehler, E.L. The Origin of Binding and Antibinding in the Hydrogen Molecule-lon. Adv. Quantum Chem. 1970, 5, 27–98. [Google Scholar] [CrossRef]
- Sethio, D.; Oliveira, V.; Kraka, E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules 2018, 23, 2763. [Google Scholar] [CrossRef]
- Yannacone, S.; Freindorf, M.; Tao, Y.; Zou, W.; Kraka, E. Local Vibrational Mode Analysis of π–Hole Interactions between Aryl Donors and Small Molecule Acceptors. Crystals 2020, 10, 556. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, Y.; Zou, W.; Nanayakkara, S.; Yannacone, S.; Kraka, E. In Situ Assessment of Intrinsic Strength of X-I⋯OA-Type Halogen Bonds in Molecular Crystals with Periodic Local Vibrational Mode Theory. Molecules 2020, 25, 1589. [Google Scholar] [CrossRef]
- Grabowki, S. Tetrel bond–σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Akiba, K.-Y.; Moriyama, Y.; Mizozoe, M.; Inohara, H.; Nishii, T.; Yamamoto, Y.; Minoura, M.; Hashizume, D.; Iwasaki, F.; Takagi, N.; et al. Synthesis and Characterization of Stable Hypervalent Carbon Compounds (10-C-5) Bearing a 2,6-Bis(p-substituted phenyloxymethyl)benzene Ligand. J. Am. Chem. Soc. 2005, 127, 5893–5901. [Google Scholar] [CrossRef]
- Akiba, K.-Y.; Yamashita, M.; Yamamoto, Y.; Nagase, S. Synthesis and Isolation of Stable Hypervalent Carbon Compound (10-C-5) Bearing a 1,8-Dimethoxyanthracene Ligand. J. Am. Chem. Soc. 1999, 121, 10644–10645. [Google Scholar] [CrossRef]
- Karim, A.; Schulz, N.; Andersson, H.; Nekoueishahraki, B.; Carlsson, A.-C.C.; Sarabi, D.; Valkonen, A.; Rissanen, K.; Gräfenstein, J.; Keller, S.; et al. Carbon’s Three-Center, Four-Electron Tetrel Bond, Treated Experimentally. J. Am. Chem. Soc. 2018, 140, 17571–17579. [Google Scholar] [CrossRef] [PubMed]
- Kovalevsky, A.Y.; Shishkin, O.V.; Ponomarev, I.I. N,N’-(Pyridine-2,6-diyl)bis(naphthalenedicarboximide) trifluoroacetic acid monohydrate. Acta Cryst. 1999, C55, 1914–1915. [Google Scholar] [CrossRef]
- Alcock, N.W.; Harrison, W.D.; Howes, C. Secondary bonding. Part 13. Aryl-tellurium(IV) and -iodine(III) acetates and trifluoroacetates. The crystal and molecular structures of bis-(p-methoxyphenyl)tellurium diacetate, µ-oxo-bis[diphenyltrifluoroacetoxytellurium] hydrate, and [bis(trifluoroacetoxy)iodo]benzene. J. Chem. Soc. Dalton Trans. 1984, 8, 1709–1716. [Google Scholar] [CrossRef]
- Erben, F.; Vedova, C.O.D.; Willner, H.; Trautner, F.; Oberhammer, H.; Boese, R. Fluoroformyl Trifluoroacetyl Disulfide, FC(O)SSC(O)CF 3: Synthesis, Structure in Solid and Gaseous States, and Conformational Properties. Inorg. Chem. 2005, 44, 7070. [Google Scholar] [CrossRef]
- Minkwitz, R.; Berkei, M.; Ludwig, R. Synthesis and Characterization of Novel Iodine(III) Compounds; Crystal Structures of Methoxy(trifluoromethyl)iodine(III) Chloride [CF3I(Cl)OCH3] and Dimethoxy(trifluoromethyl)iodine(III) [CF3I(OCH3)2]. Eur. J. Inorg. Chem. 2000, 2387. [Google Scholar] [CrossRef]
- Heywood, V.L.; Alford, T.P.J.; Roeleveld, J.J.; Deprez, S.J.L.; Verhoofstad, A.M.; van der Vlugt, J.I.; Domingos, S.R.; Schnell, M.; Davis, A.P.; Mooibroek, T.J. Observations of tetrel bonding between sp3-carbon and THF. Chem. Sci. 2020, 11, 5289–5293. [Google Scholar] [CrossRef]
- Roeleveld, J.J.; Lekanne Deprez, S.J.; Verhoofstad, A.; Frontera, A.; van der Vlugt, J.I.; Mooibroek, T.J. Engineering Crystals Using sp3-C Centred Tetrel Bonding Interactions. Chem Eur. J. 2020, 26, 10126–10132. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Small Cycloalkane (CN)2C–C(CN)2 Structures Are Highly Directional Non-covalent Carbon-Bond Donors. Chem. Eur. J. 2014, 20, 10245–10248. [Google Scholar] [CrossRef] [PubMed]
- Daolio, A.; Scilabra, P.; Terraneo, G.; Resnati, G. C(sp3) atoms as tetrel bond donors: A crystallographic survey. Coord. Chem. Rev. 2020, 413, 213265. [Google Scholar] [CrossRef]
- Witt, J.R.; Britton, D.; Mahon, C. The crystal structures of BrC(CN)3, ClC(CN)3, and CH3C(CN)3. Acta Cryst. 1972, B28, 950. [Google Scholar] [CrossRef]
- Franconetti, A.; Quiñonero, D.; Frontera, A.; Resnati, G. Unexpected chalcogen bonds in tetravalent sulfur compounds. Unexpected chalcogen bonds in tetravalent sulfur compounds. Phys. Chem. Chem. Phys. 2019, 21, 11313–11319. [Google Scholar] [CrossRef]
- Britton, D. The crystal structure of tetracyanomethane, C(CN)4. Acta Cryst. 1974, B30, 1818–1821. [Google Scholar] [CrossRef]
- Taheriha, M.; Ghadermazi, M.; Amani, V. Dimeric and polymeric mercury(II) complexes of 1-methyl-1,2,3,4-tetrazole-5-thiol: Synthesis, crystal structure, spectroscopic characterization, and thermal analyses. J. Mol. Struct. 2016, 1107, 57–65. [Google Scholar] [CrossRef]
- Xu, X.; Ju, W.; Hou, W.; Zhu, D.; Xu, Y. Two octamolybdate-based complexes: Hydrothermal synthesis, structural characterization and properties. CrystEngComm 2014, 16, 82–88. [Google Scholar] [CrossRef]
- Lyakhov, A.S.; Voitekhovich, S.V.; Ivashkevich, L.S.; Gaponik, P.N. 5-Amino-1-methyl-4H-tetra zolium picrate. Acta Cryst. 2005, E61, o3645–o3647. [Google Scholar] [CrossRef]
- Pal, P.; Konar, S.; Lama, P.; Das, K.; Bauzá, A.; Frontera, A.; Mukhopadhyay, S. On the Importance of Noncovalent Carbon-Bonding Interactions in the Stabilization of a 1D Co(II) Polymeric Chain as a Precursor of a Novel 2D Coordination Polymer. J. Phys. Chem. B 2016, 120, 6803–6811. [Google Scholar] [CrossRef]
- Morgunov, R.B.; Mushenok, F.B.; Aldoshin, S.M.; Sanina, N.A.; Yureva, E.A.; Shilov, G.V.; Tkachev, V.V. Thermally-induced paramagnetism of spiropyrane iodides. New J. Chem. 2009, 33, 1374–1379. [Google Scholar] [CrossRef]
- Peter, A.; Fehr, S.M.; Dybbert, V.; Himmel, D.; Lindner, I.; Jacob, E.; Ouda, M.; Schaadt, A.; White, R.J.; Scherer, H.; et al. Towards a Sustainable Synthesis of Oxymethylene Dimethyl Ether by Homogeneous Catalysis and Uptake of Molecular Formaldehyde. Angew. Chem. Int. Ed. 2018, 57, 9461–9464. [Google Scholar] [CrossRef]
- Finze, M.; Reiss, G.J. Trimethyl sulfonium 1-amino-6-fluoro-2,3,4,5,7,8,9,10,11,12-decaiodo-1-carba-closo-dodeca borate. Acta Cryst. 2012, E68, o640. [Google Scholar] [CrossRef]
- Abramov, P.A.; Zakharchuk, N.F.; Virovets, A.V.; Mirzaeva, I.V.; Sokolov, M.N. Hydrogen selenide in M–Se and C–Se bond formation. [Cp*3Ir3Se2]2+ clusters: New synthesis, molecular and electronic structure and related studies. J. Organomet. Chem. 2014, 767, 65–71. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Lopez-Andarias, J.; Bauza, A.; Sakai, N.; Frontera, A.; Matile, S. Remote Control of Anion–π Catalysis on Fullerene-Centered Catalytic Triads. Angew. Chem. Int. Ed. 2018, 57, 10883–10887. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Andarias, J.; Frontera, A.; Matile, S. Anion-π Catalysis on Fullerenes. J. Am. Chem. Soc. 2017, 139, 13296–13299. [Google Scholar] [CrossRef]
- Bornhof, A.-B.; Vázquez-Nakagawa, M.; Rodríguez-Pérez, L.; Herranz, M.A.; Sakai, N.; Martín, N.; Matile, S.; López-Andarias, J. Anion–π Catalysis on Carbon Nanotubes. Angew. Chem. Int. Ed. 2019, 58, 16097–16100. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frontera, A. Tetrel Bonding Interactions Involving Carbon at Work: Recent Advances in Crystal Engineering and Catalysis. C 2020, 6, 60. https://doi.org/10.3390/c6040060
Frontera A. Tetrel Bonding Interactions Involving Carbon at Work: Recent Advances in Crystal Engineering and Catalysis. C. 2020; 6(4):60. https://doi.org/10.3390/c6040060
Chicago/Turabian StyleFrontera, Antonio. 2020. "Tetrel Bonding Interactions Involving Carbon at Work: Recent Advances in Crystal Engineering and Catalysis" C 6, no. 4: 60. https://doi.org/10.3390/c6040060
APA StyleFrontera, A. (2020). Tetrel Bonding Interactions Involving Carbon at Work: Recent Advances in Crystal Engineering and Catalysis. C, 6(4), 60. https://doi.org/10.3390/c6040060