Fluence Enhanced Optical Response of Ag Implanted Amorphous Carbon Thin Films
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Raman Spectroscopy
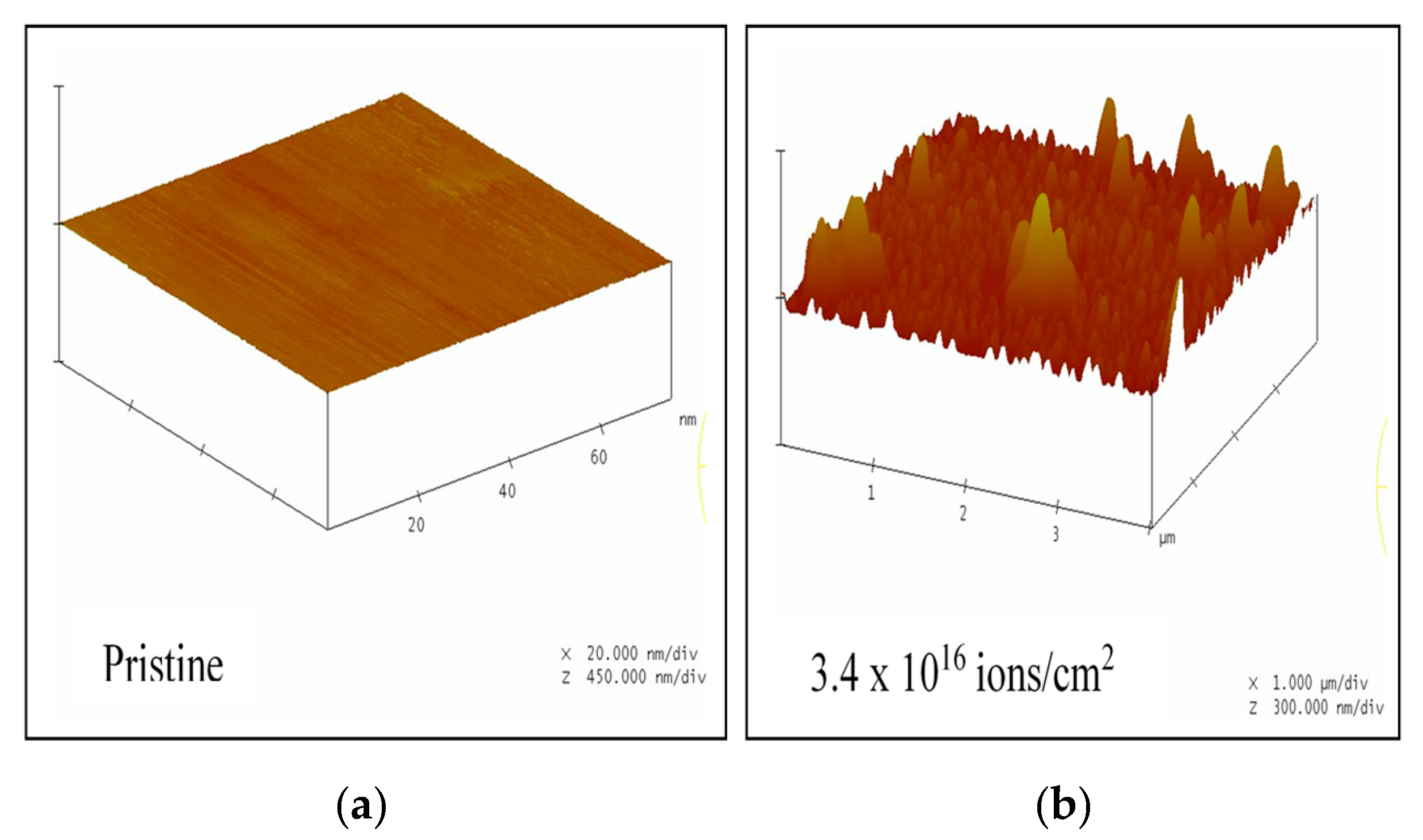
3.2. Atomic Force Microscopy
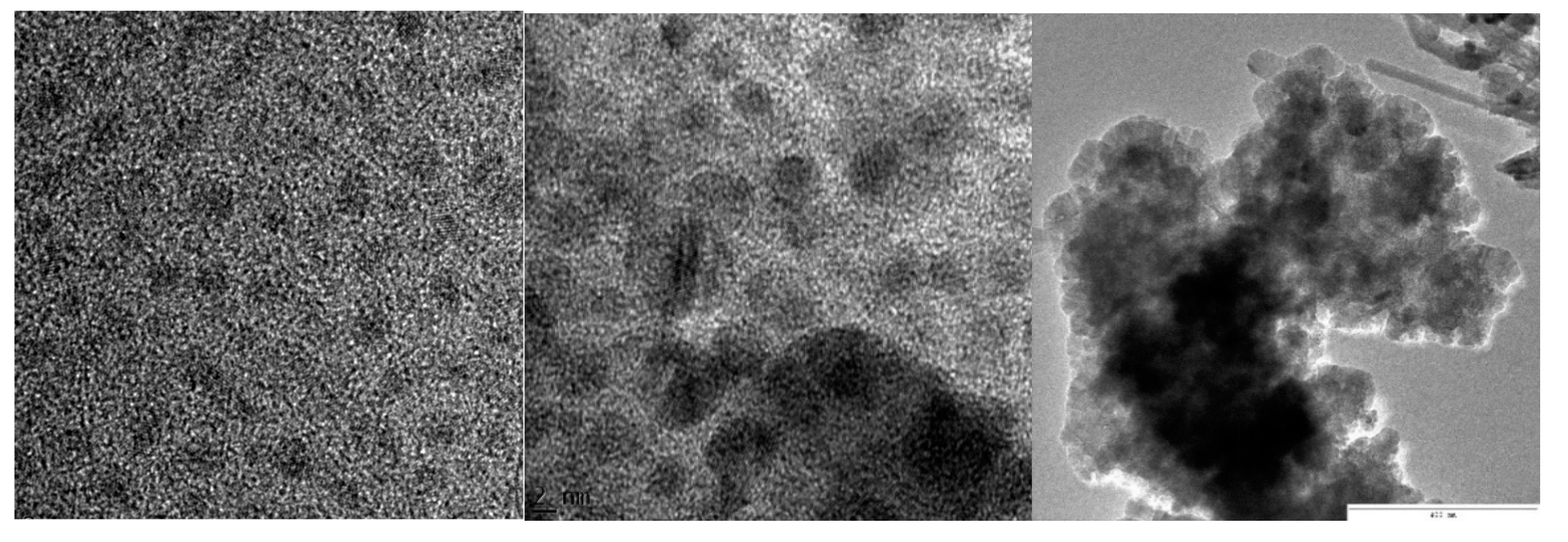
3.3. Transmission Electron Microscopy
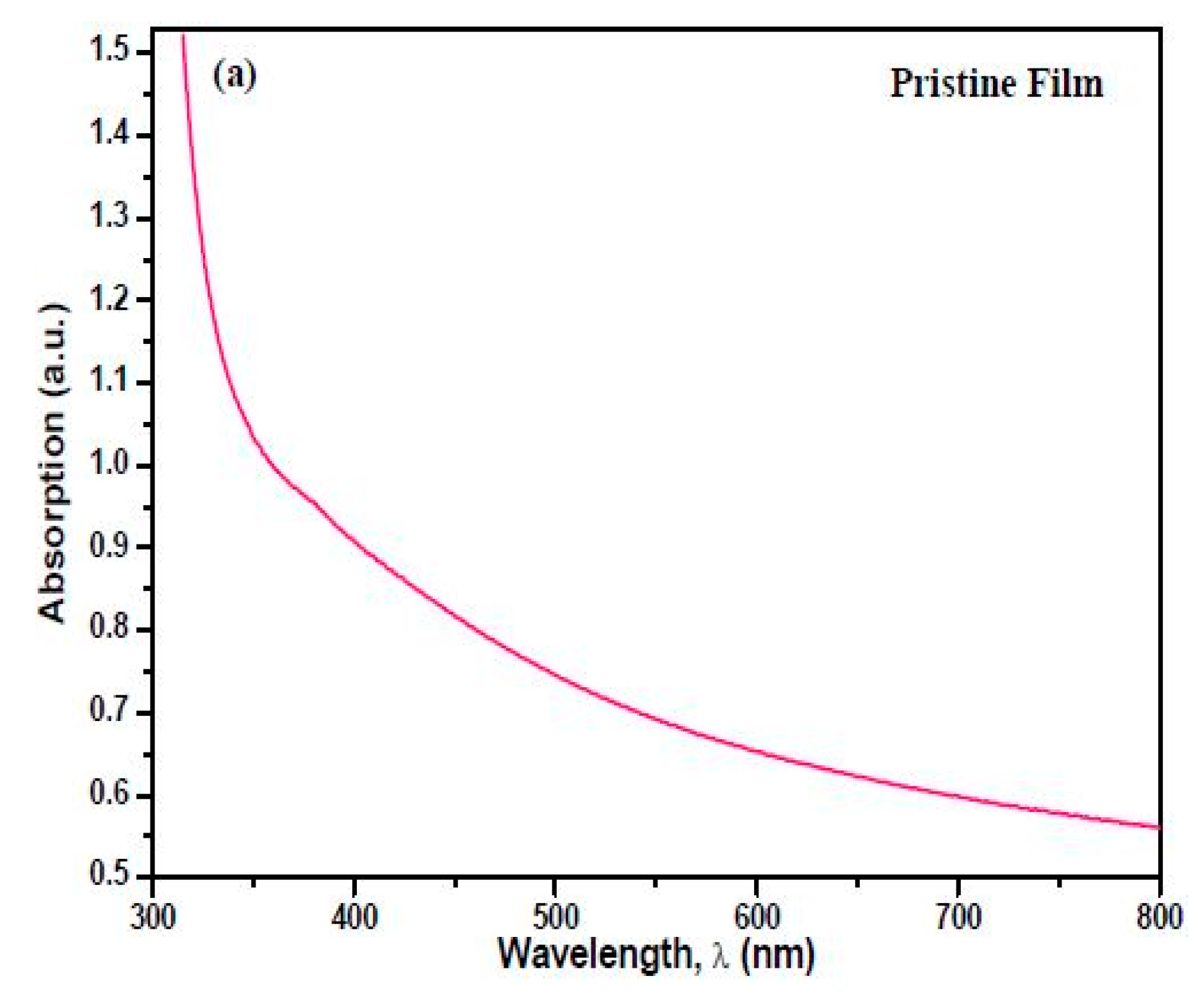
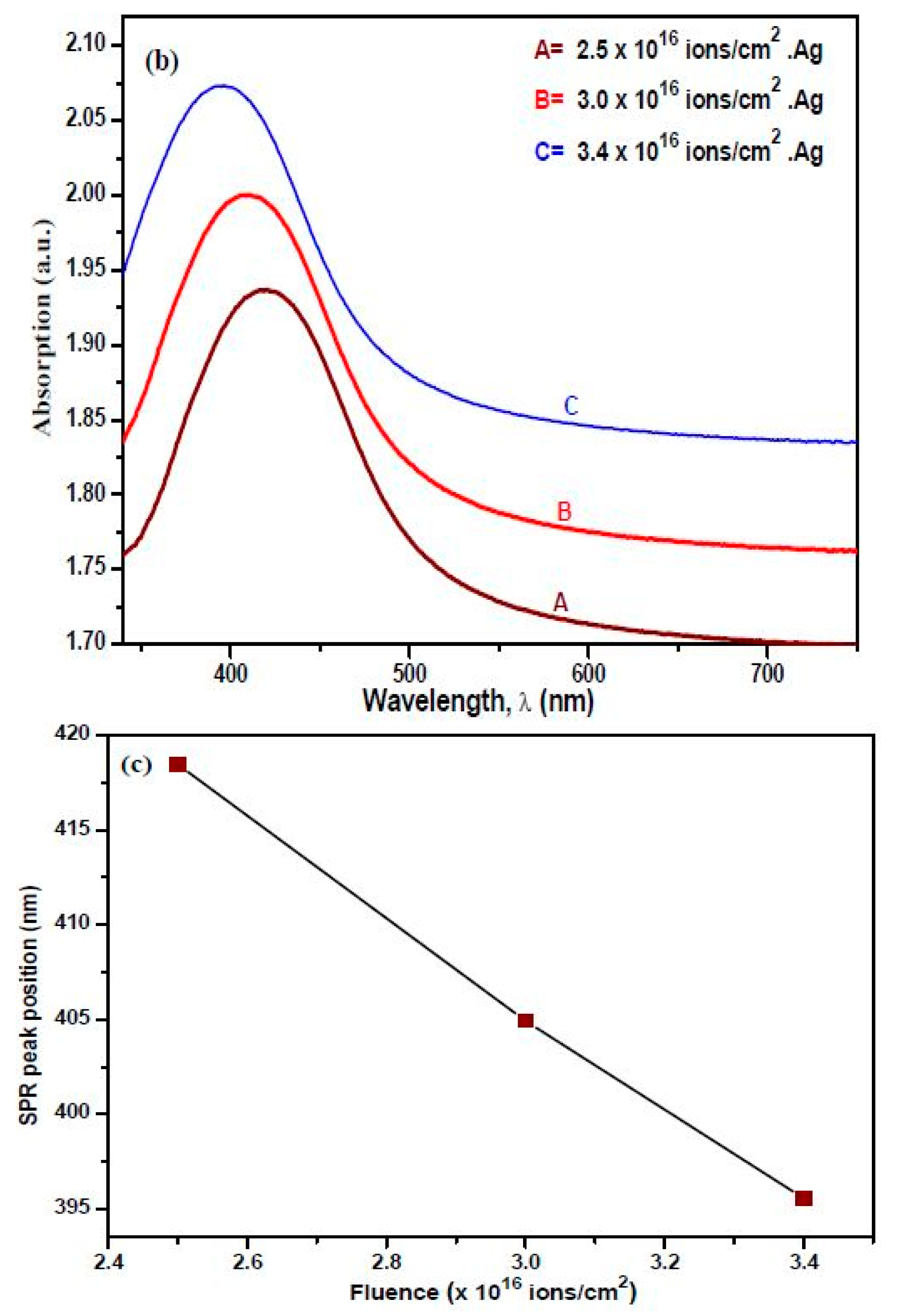
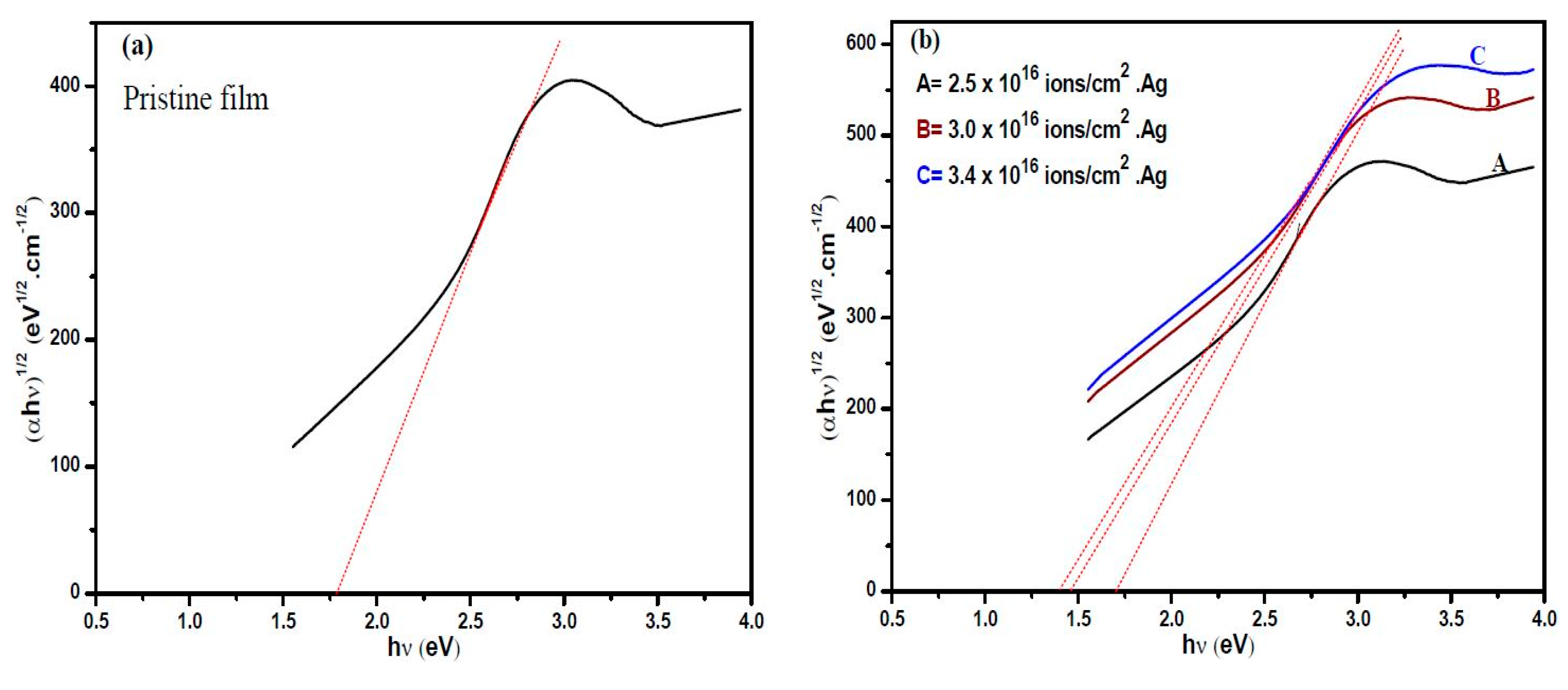
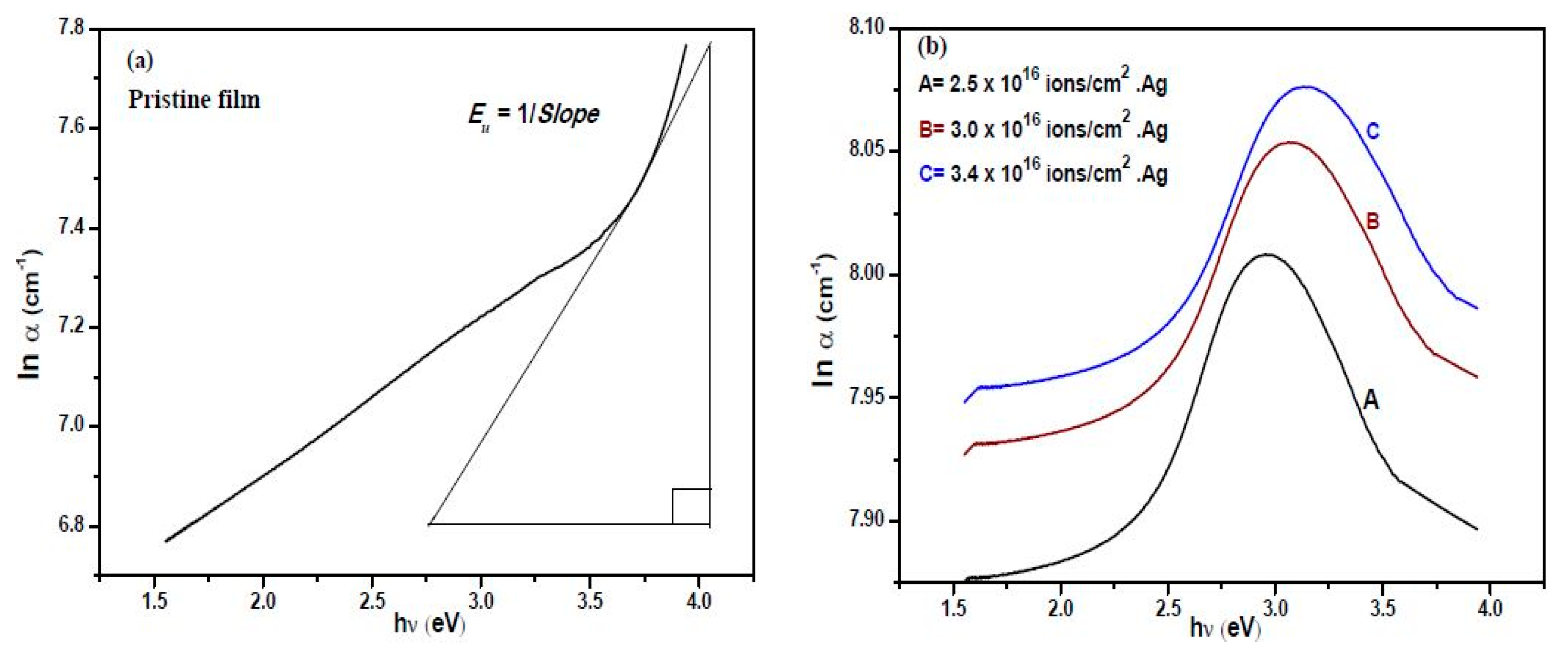
3.4. UV-Visible Absorption Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karthik, P.; Himaja, A.; Singh, S.P. Carbon-allotropes: Synthesis methods, applications and future perspectives. Carbon Lett. 2014, 15, 219–237. [Google Scholar] [CrossRef]
- Kroto, H. Space, Stars, C60, and Soot. Science 1986, 242, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, A. Carrier type modulation in current annealed graphene layers. Appl. Phys. Lett. 2014, 104, 083517. [Google Scholar] [CrossRef]
- Singhal, R.; Pivin, J.; Chandra, R.; Avasthi, D. Ion irradiation studies of silver/amorphous carbon nanocomposite thin film. Surf. Coat. Technol. 2013, 229, 50–54. [Google Scholar] [CrossRef]
- Stepanov, A.L. Synthesis of silver nanoparticles in dielectric matrix by ion implantation: A review. Rev. Adv. Mater. Sci. 2010, 26, 1–29. [Google Scholar]
- Zhou, L.; Zhang, C.; Yang, Y.; Li, B.; Zhang, L.; Fu, Y.; Zhang, H. Formation of Au nanoparticles in sapphire by using Ar ion implantation and thermal annealing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2009, 267, 58–62. [Google Scholar] [CrossRef]
- Choi, H.W.; Dauskardt, R.H.; Lee, S.-C.; Lee, K.-R.; Oh, K.H. Characteristic of silver doped DLC films on surface properties and protein adsorption. Diam. Relat. Mater. 2008, 17, 252–257. [Google Scholar] [CrossRef]
- Mak, K.F.; Ju, L.; Wang, F.; Heinz, T.F. Optical spectroscopy of graphene: From the far infrared to the ultraviolet. Solid State Commun. 2012, 152, 1341–1349. [Google Scholar] [CrossRef]
- Laidani, N.; Bartali, R.; Gottardi, G.; Anderle, M.; Cheyssac, P. Optical absorption parameters of amorphous carbon films from Forouhi-Bloomer and Tauc-Lorentz models: A comparative study. J. Phys. Condens. Matter 2009, 20, 015216. [Google Scholar] [CrossRef]
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Optical Properties of Silver Nanoparticles for Surface Plasmon Resonance (SPR)-Based Biosensor Applications. J. Mod. Phys. 2015, 6, 1071–1076. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Iwaki, M. Progress in ion implantation technology for metal surface treatments and other related topics. In Proceedings of the 1998 International Conference on Ion Implantation Technology. Proceedings (Cat. No. 98EX144), Kyoto, Japan, 22–26 June 1998; Volume 2, pp. 824–826. [Google Scholar]
- Amir, O.; Kalish, R. Properties of nitrogen-doped amorphous hydrogenated carbon films. J. Appl. Phys. 1991, 70, 4958–4962. [Google Scholar] [CrossRef]
- Amir, O.; Kalish, R. Doping of amorphous-hydrogenated carbon films by ion implantation. Diam. Relat. Mater. 1992, 1, 364–368. [Google Scholar] [CrossRef]
- Freire, F.; Achete, C.; Mariotto, G. Nitrogen implantation into amorphous carbon films: XPS, AES and Raman analyses. Nucl. Inst. Methods Phys. Res. Beam. Interact. Mater. Atoms 1995, 99, 606–609. [Google Scholar] [CrossRef]
- Jun, Z.; Hui, Z.; Zhi-Hua, W.; Rui-Peng, S. Structure and mechanical properties of tungsten-containing hydrogenated diamond like carbon coatings for space applications. Phys. Procedia 2011, 18, 245–250. [Google Scholar] [CrossRef]
- Dimigen, H.; Hubsch, H.; Memming, R. Tribological and electrical properties of metal-containing hydrogenated carbon films. Appl. Phys. Lett. 1987, 50, 1056–1058. [Google Scholar] [CrossRef]
- Gerhards, I.; Ronning, C.; Vetter, U.; Hofsäss, H.; Gibhardt, H.; Eckold, G.; Li, Q.; Lee, S.; Huang, Y.; Seibt, M. Ion beam synthesis of amorphous carbon thin films containing metallic nanoclusters. Surf. Coat. Technol. 2002, 158, 114–119. [Google Scholar] [CrossRef]
- Constantinou, M.; Nikolaou, P.; Koutsokeras, L.; Avgeropoulos, A.; Moschovas, D.; Varotsis, C.; Patsalas, P.; Kelires, P.; Constantinides, G. Metal (Ag/Ti)-Containing Hydrogenated Amorphous Carbon Nanocomposite Films with Enhanced Nanoscratch Resistance: Hybrid PECVD/PVD System and Microstructural Characteristics. Nanomaterials 2018, 8, 209. [Google Scholar] [CrossRef]
- Grischke, H.; Bewilogua, M.; Dimigen, K. Preparation, properties and structure of metal containing amorphous hydrogenated carbon films. Mater. Manufacur. Process. 2007, 8, 407–417. [Google Scholar] [CrossRef]
- Szunerits, S.; Castel, X.; Boukherroub, R. Surface Plasmon Resonance Investigation of Silver and Gold Films Coated with Thin Indium Tin Oxide Layers: Influence on Stability and Sensitivity. J. Phys. Chem. C 2008, 112, 15813–15817. [Google Scholar] [CrossRef]
- Raza, S.; Yan, W.; Stenger, N.; Wubs, M.; Mortensen, N.A. Blueshift of the surface plasmon resonance in silver nanoparticles: Substrate effects. Opt. Express 2013, 21, 27344–27355. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Agarwal, D.C.; Mishra, Y.K.; Singh, F.; Pivin, J.C.; Chandra, R.; Avasthi, D.K. Electronic excitation induced tuning of surface plasmon resonance of Ag nanoparticles in fullerene C70 matrix. J. Phys. D Appl. Phys. 2009, 42, 155103. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, Y.; He, L.; Xie, B.; Song, F.; Han, M.; Wang, G. Systemically tuning the surface plasmon resonance of high-density silver nanoparticle films. Eur. Phys. J. D 2013, 67, 87. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Xiang, X.; Zu, X.T.; Zhu, S.; Wei, Q.M.; Zhang, C.F.; Sun, K.; Wang, L.M. ZnO nanoparticles embedded in sapphire fabricated by ion implantation and annealing. Nanotechnology 2006, 17, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bhaumik, S.; Panda, J.K.; Ojha, S.; Dhar, A.; Kabiraj, D.; Roy, A. Strain buildup in GaAs due to 100 MeV Ag ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 316, 192–197. [Google Scholar] [CrossRef]
- Singhal, R.; Agarwal, D.; Mishra, Y.; Mohapatra, S.; Avasthi, D.; Chawla, A.; Chandra, R.; Pivin, J. Swift heavy ion induced modifications of optical and microstructural properties of silver-fullerene C60 nanocomposite. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2009, 267, 1349–1352. [Google Scholar] [CrossRef]
- Yaremchuk, I.; Tamulevičiene, A.; Tamulevičius, T.; Šlapikas, K.; Balevičius, Z.; Tamulevičius, S. Modeling of the plasmonic properties of DLC-Ag nanocomposite films. Phys. Status Solidi Appl. Mater. Sci. 2014, 211, 329–335. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.; Biersack, J. SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S. Observation of an anomolously sharp feature in the 2nd order Raman spectrum of graphite. Solid State Commun. 1977, 23, 417–420. [Google Scholar] [CrossRef]
- Wang, Y.D.; Alsmeyer, C.; McCreery, R.L. Raman-Spectroscopy of Carbon Materials—Structural Basis of Observed Spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Dennison, J.R.; Holtz, M.; Swain, G. Raman Spectroscopy of Carbon Materials. Spectroscopy. 1996, 11, 38–45. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.R.C.; Kumar, K.J.; Subrahmanyam, A.; Champion, P.M.; Ziegler, L.D. Silver oxide (AgO) thin films for Surface Enhanced Raman Scattering (SERS) studies. AIP Conf. Proc. 2010, 1267, 1005–1006. [Google Scholar]
- Débarre, A.; Jaffiol, R.; Julien, C.; Tchénio, P.; Mostafavi, M. Raman scattering from single Ag aggregates in presence of EDTA. Chem. Phys. Lett. 2004, 386, 244–247. [Google Scholar] [CrossRef]
- Sharma, P.; Singhal, R.; Vishnoi, R.; Agarwal, D.C.; Banerjee, M.K.; Chand, S.; Kanjilal, D.; Avasthi, D.K. Effect of Ag Ion Implantation on SPR of Cu-C60 Nanocomposite Thin Film. Plasmonics 2017, 13, 669–679. [Google Scholar] [CrossRef]
- Maisels, A.; Fissan, H.; Stappert, S.; Kruis, F.E.; Nanda, K.K. Higher Surface Energy of Free Nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar]
- Lu, H.M.; Jiang, Q. Size-Dependent Surface Energies of Nanocrystals. J. Phys. Chem. B 2004, 108, 5617–5619. [Google Scholar] [CrossRef]
- Medasani, B.; Park, Y.H.; Vasiliev, I. Theoretical study of the surface energy, stress, and lattice contraction of silver nanoparticles. Phys. Rev. B 2007, 75, 235436. [Google Scholar] [CrossRef]
- Vollath, D.; Fischer, F.D.; Holec, D. Surface energy of nanoparticles—Influence of particle size and structure. Beilstein J. Nanotechnol. 2018, 9, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Noguez, C. Surface plasmons on metal nanoparticles: The influence of shape and physical environment. J. Phys. Chem. C 2007, 111, 3606–3619. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.; Avalos-Borja, M.; Castillón-Barraza, F.; Posada-Amarillas, A. Classical theoretical characterization of the surface plasmon absorption band for silver spherical nanoparticles suspended in water and ethylene glycol. Phys. E Low-Dimens. Syst. NanoStruct. 2005, 27, 104–112. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C. Influence of Morphology on the Optical Properties of Metal Nanoparticles. J. Comput. Theor. Nanosci. 2007, 4, 1249–1252. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structures of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Moon, M.-W.; Lee, K.-R. Enhancement of electron field emission property with silver incorporation into diamond like carbon matrix. Appl. Phys. Lett. 2008, 92, 193502. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Moon, M.-W.; Lee, K.-R. Effect of silver doping on optical property of diamond like carbon films. Thin Solid Films 2009, 517, 4035–4038. [Google Scholar] [CrossRef]
- Robertson, J. Electronic Structure and Bonding of a-C:H. Mater. Sci. Forum 1990, 52, 125–150. [Google Scholar] [CrossRef]
- Robertson, J.; O’Reilly, E. Electronic and atomic structure of amorphous carbon. Phys. Rev. B 1987, 35, 2946–2957. [Google Scholar] [CrossRef]
- Robertson, J. Gap states in diamond-like amorphous carbon. Philos. Mag. B Phys. Condens. Matter Stat. Mech. Electron. Opt. Magn. Prop. 1997, 76, 335–350. [Google Scholar] [CrossRef]
- Studenyak, I.; Kranj, M.; Kurik, M. Urbach Rule in Solid State Physics. Int. J. Opt. Appl. 2014, 4, 76–83. [Google Scholar]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Patsalas, P. Optical properties of amorphous carbons and their applications and perspectives in photonics. Thin Solid Films 2011, 519, 3990–3996. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
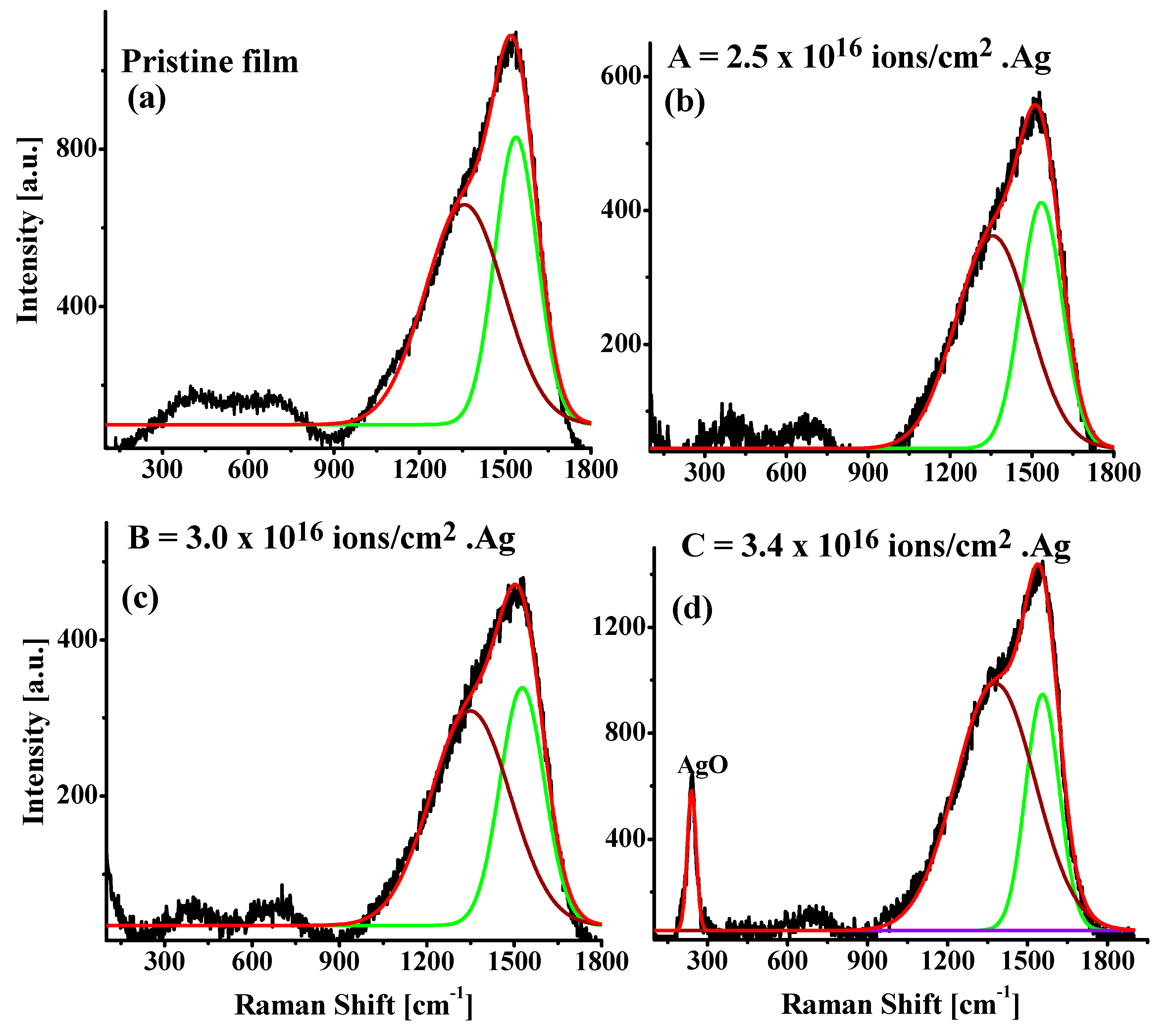

| Pristine Film | A | B | C | |
|---|---|---|---|---|
| G-Peak position (cm−1) | 1541 | 1535 | 1544 | 1557 |
| D-Peak position (cm−1) | 1360 | 1355 | 1364 | 1380 |
| G-Peak intensity, (cm−1) | 732 | 368 | 493 | 895 |
| D-Peak intensity, (cm−1) | 559 | 318 | 478 | 930 |
| Intensity ratio, | 0.76 | 0.86 | 0.97 | 1.04 |
| FWHM (G) (±4.0) | 148.0 | 151.1 | 155.1 | 125.9 |
| Cluster size, (nm) | 5.8 | 5.1 | 4.5 | 4.2 |
| Optical Parameters | Pristine Film | A | B | C |
|---|---|---|---|---|
| Optical band gap energy, (eV) (±0.03) | 1.79 | 1.71 | 1.48 | 1.41 |
| Urbach energy, (eV) (±0.2) | 0.01 | 9.7 | 10.9 | 12.0 |
| SPR peak position, (nm) | - | 418 | 401 | 395 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, S.R.; Ismaila, A. Fluence Enhanced Optical Response of Ag Implanted Amorphous Carbon Thin Films. C 2019, 5, 45. https://doi.org/10.3390/c5030045
Naidoo SR, Ismaila A. Fluence Enhanced Optical Response of Ag Implanted Amorphous Carbon Thin Films. C. 2019; 5(3):45. https://doi.org/10.3390/c5030045
Chicago/Turabian StyleNaidoo, Shunmugam Ramsamy, and Abdulsalam Ismaila. 2019. "Fluence Enhanced Optical Response of Ag Implanted Amorphous Carbon Thin Films" C 5, no. 3: 45. https://doi.org/10.3390/c5030045
APA StyleNaidoo, S. R., & Ismaila, A. (2019). Fluence Enhanced Optical Response of Ag Implanted Amorphous Carbon Thin Films. C, 5(3), 45. https://doi.org/10.3390/c5030045