Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe
Abstract
1. Introduction
2. Explaining Negative Temperature Dependencies in Carbon Growth from Steady-State Kinetic Studies: Linearity of the Weight vs. Time Register Observed
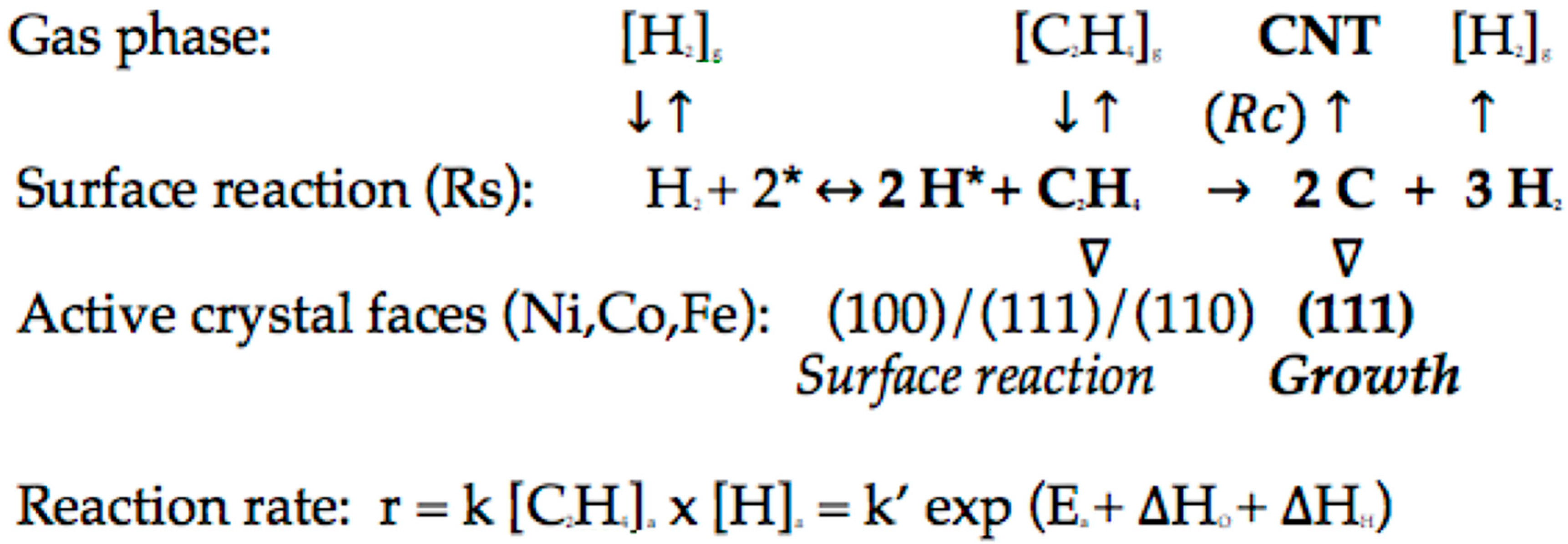
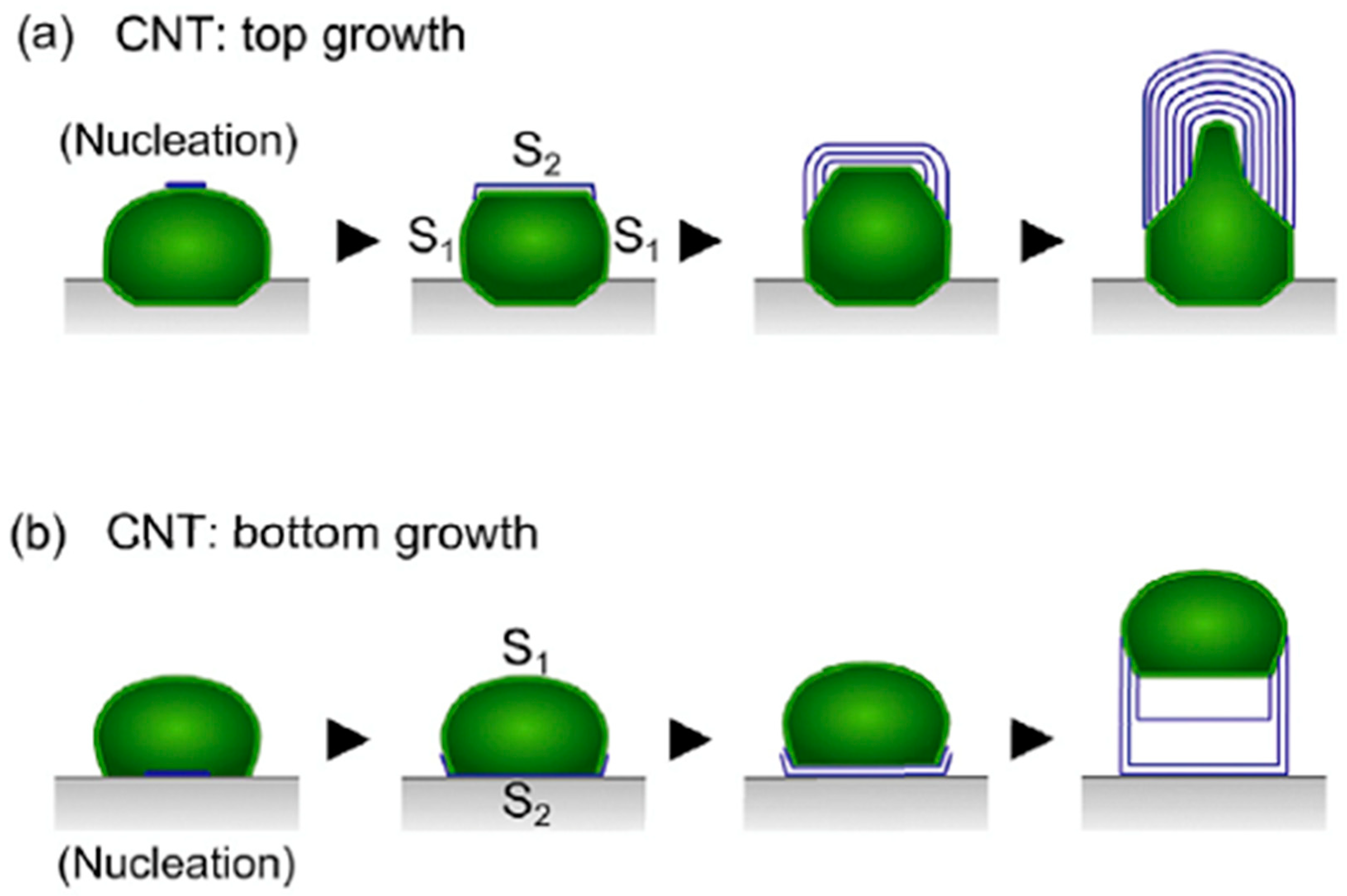
3. H Spillover Effect: An Illusion?
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lobo, L.S. Catalytic carbon formation: Clarifying the alternative kinetic routes and defining a kinetic linearity for sustained growth concept. Reac. Kinet. Mech. Catal. 2016, 118, 393–414. [Google Scholar] [CrossRef]
- Lobo, L.S. Nucleation and growth of carbon nanotubes and nanofibers: Mechanism and catalytic geometry control. Carbon 2017, 114, 411–417. [Google Scholar] [CrossRef]
- Lobo, L.S.; Trimm, D.L. Complex temperature dependencies of rate of carbon deposition on nickel. Nat. Phys. Sci. 1971, 234, 15–16. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Merkulov, I.A.; Rouleau, C.M.; Eres, G.; Geohegan, D.B. Revealing the surface and bulk regimes of isothermal graphene nucleation and growth on Ni with in situ kinetic measurements and modeling. Carbon 2014, 79, 256–264. [Google Scholar] [CrossRef]
- Bernardo, C.A.; Lobo, L.S. Kinetics of Carbon Formation from acetylene and 1-Butene on Cobalt. In Studies in Surface Science and Catalysis; Delmon, B., Froment, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; pp. 409–420. [Google Scholar]
- Figueiredo, J.L. Carbon Formation in Steam Reforming Catalysts. Ph.D. Thesis, Imperial College, London, UK, 1975. [Google Scholar]
- Lobo, L.S.; Franco, M.D. Kinetics of catalytic carbon formation on steel surfaces from light hydrocarbons. Catal. Today 1990, 7, 247–256. [Google Scholar] [CrossRef]
- Koziol, K.K.; Ducati, C.; Windle, A.H. Carbon nanotubes with catalyst controlled chiral angle. Chem. Mater. 2010, 22, 4904–4911. [Google Scholar] [CrossRef]
- Lobo, L.S. Carbon Formation from Hydrocarbons on Metals. Ph.D. Thesis, Imperial College, London, UK, 1971. Available online: http://hdl.handle.net/10044/1/16515 (accessed on 18 April 2019).
- Boudart, M.; Djéga-Mariadassou, G. Kinetics of Heterogeneous Catalytic Reactions; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Bernardo, C.A. Carbon Deposition and gasification in the context of nickel catalysts. Ph.D. Thesis, Imperial College, London, UK, 1977. [Google Scholar]
- Bernardo, C.A.; Lobo, L.S. Kinetics of carbon formation from acetylene on nickel. J. Catal. 1975, 37, 267–278. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. A molecular beam investigation of the catalytic oxidation of CO on Pd (111). J. Chem. Phys. 1978, 69, 1267. [Google Scholar] [CrossRef]
- Harris, P. Carbon Nanotube Science; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Zaikovski, V.I.; Chesnokov, V.V.; Buyanov, R.A. The relationship between the state of active species in a ni/al2o3 catalyst and the mechanism of growth of filamentous carbon. Kinet. Catal. 2001, 42, 803–820. [Google Scholar] [CrossRef]
- Latorre, N.; Romeo, E.; Villacampa, J.I.; Cazaña, F.; Royo, C.; Monzón, A. Kinetics of carbon nanotubes growth on a Ni-Mg-Al catalyst by CCVD of methane: Influence of catalyst deactivation. Catal. Today 2010, 154, 217–223. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of carbon nanotubes array growth kinetics during CVD. Appl. Phys. A 2005, 81, 223–240. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Investigation of growth dynamic of carbon nanotubes. Bielstein J. Nanotechnol. 2017, 8, 826–856. [Google Scholar] [CrossRef] [PubMed]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.; Mian, S.; Ali, G. An Overview of the recent progress in the synthesis and applications of carbon nanotubes. C J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef]
- Eres, G.; Kinkhabwala, A.A.; Cui, H.; Geohegan, D.B.; Puretzky, A.A.; Lowndes, D.H. Molecular beam-controlled nucleation and growth of vertically aligned single-wall carbon nanotube arrays. J. Phys. Chem. B 2005, 109, 16684–16694. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Gan, T.; Zheng, B.; Chu, X.; Yu, G.; Yan, W.; Zou, Y.; Zhang, W.; Liu, G. Synergism of Pt nanoparticles and iron oxide support for chemoselective hydrogenation of nitroarenes under mild conditions. Chin. J. Catal. 2019, 40, 214–222. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. Accelerated growth of carbon nanofibers using physical mixtures and alloys of Pd and Co in an ethelyne-hydrogen environment. Carbon 2011, 49, 1058–1066. [Google Scholar] [CrossRef]
- Teng, I.J.; Huang, C.S.; Hsu, H.L.; Chung, I.C.; Jian, S.R.; Kherani, N.P.; Kuo, C.Z.; Juang, J.Y. On the use of new oxidized Co-Cr-Pt-O catalysts for vertically aligned few-walled carbon nanotube forest synthesis in electron cyclotron resonance CVD. Carbon 2014, 80, 808–822. [Google Scholar] [CrossRef]
- Weigle, J.C.; Phillips, J. Modelling hydrogen spillover in dual bed catalytic reactors. AIChE J. 2004, 50, 821–828. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Rodriguez-Ramos, I. (Eds.) Spillover and Mobility of Species on Solid Surfaces. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Barrer, R.M. Diffusion in and Through Solids; Cambridge University Press: Cambridge, UK, 1941. [Google Scholar]
- Khoobiar, S. Particle to particle migration of hydrogen atoms on platinum-alumina catalysts from particle to neighboring particles. J. Phys. Chem. 1964, 68, 411. [Google Scholar] [CrossRef]
- Verhoeven, W.; Delmon, B. Actions des Metaux Etrangers Divisés sur la Reduction de l’Oxyde de Nickel par l’Hydrogène. C.R. Acad. Sci. 1966, 262, 33. [Google Scholar]
- Delmon, G.F. Froment. Remote control of catalytic sites by spillover species: a chemical reaction engineering approach. Catal. Rev: Sci. Eng. 1996, 38, 1. [Google Scholar] [CrossRef]
- Inui, T. (Ed.) Studies in Surface Science and Catalysis. In New Aspects of Spillover Effect in Catalysis; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Prinz, R. Hydrogen spillover. facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Alayoglu, S.; Specht, C.; Kruse, N.; Somorjai, G.A. A Nanoscale demonstration of hydrogen atom spillover and surface diffusion across silica using the kinetics of co2 methanation catalyzed on spatially separate pt and co nanoparticles. Nano Lett. 2014, 14, 4792. [Google Scholar] [CrossRef]
- Budnikov, P.P.; Ginstling, A.M. Principles of Solid State Chemistry, Reactions in Solids; Gordon & Breach Science Publishers Inc.: Philadelphia, PA, USA, 1968. [Google Scholar]
- Erunal, E.; Ulusl, F.; Aslan, M.Y.; Guzel, B.; Uner, D. Enhancement of hydrogen storage capacity of MWCNTs with Pd doping prepared through supercritical CO2 deposition method. Int. J. Hydrog. Energy 2018, 43, 10755–10764. [Google Scholar] [CrossRef]
- Carraro, P.M.; Garcia Blanco, A.A.; Lener, G.; Barrera, D. Nanostructured carbons modified with nickel potential novel reversible hydrogen storage materials: Effects on nickel particle size. Microporous Mesoporous Mater. 2019, 273, 50–59. [Google Scholar] [CrossRef]
- Lobo, L.S. Intrinsic kinetics in carbon gasification. Understanding linearity, “nanoworms” and alloy catalysts. Appl. Catal. B: Environ. 2014, 148, 136–143. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Kinetics and mechanism of catalytic carbon gasification. Fuel 2016, 183, 157–469. [Google Scholar] [CrossRef]
- Lobo, L.S. Bamboo-like carbon fibers growth mechanism. Relevance of catalyst’s Tammann temperature. Submitted to C. 2019. [Google Scholar]
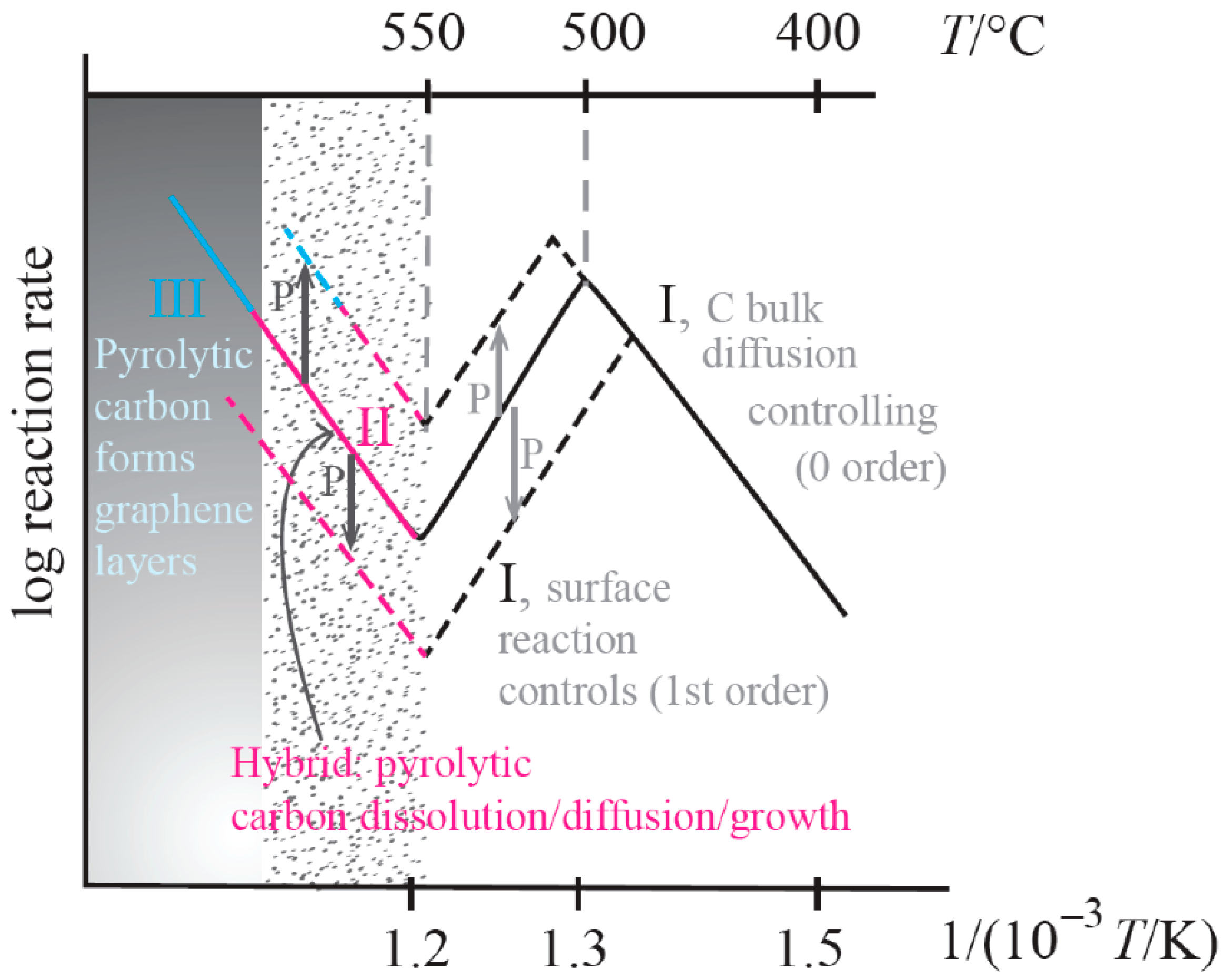
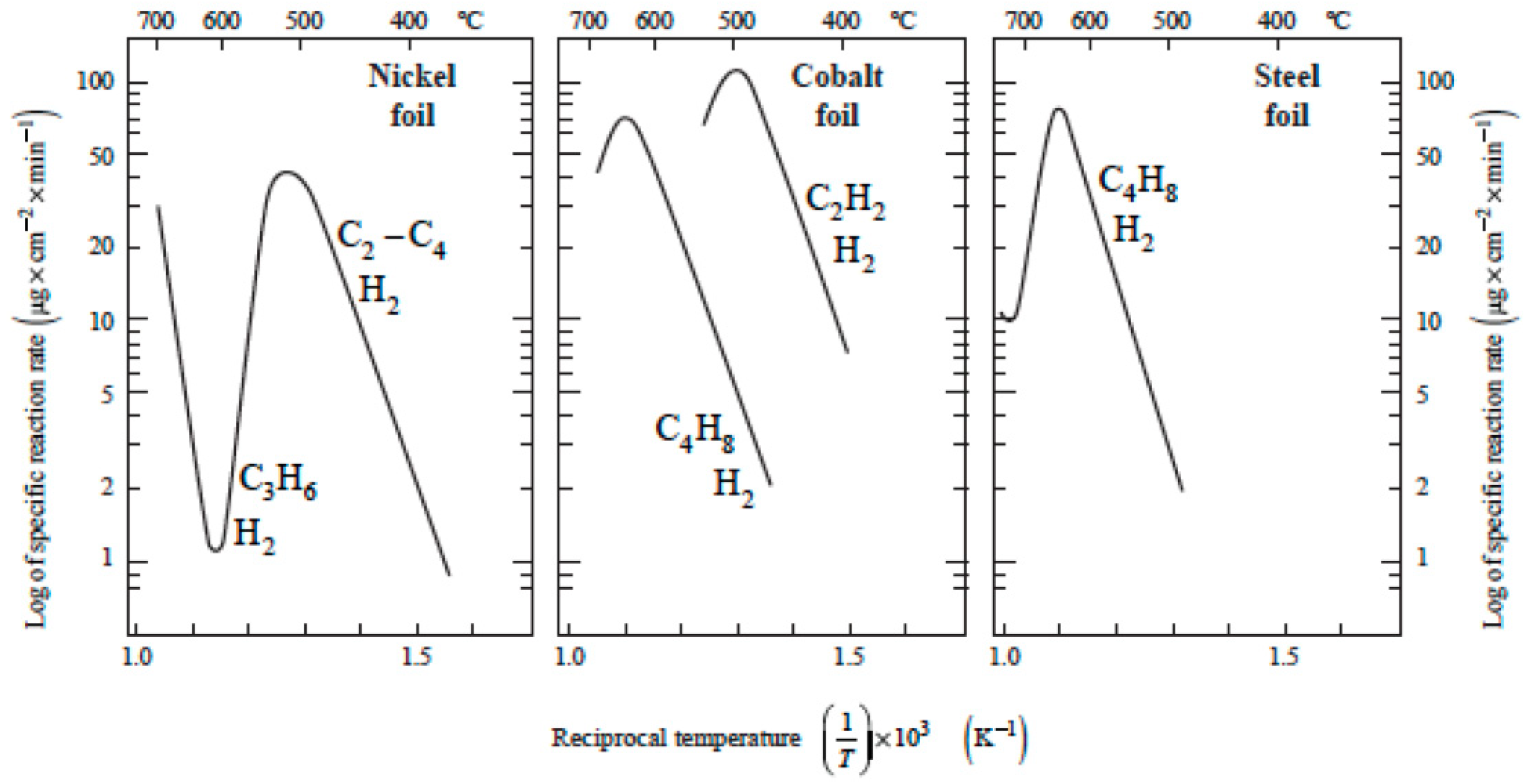
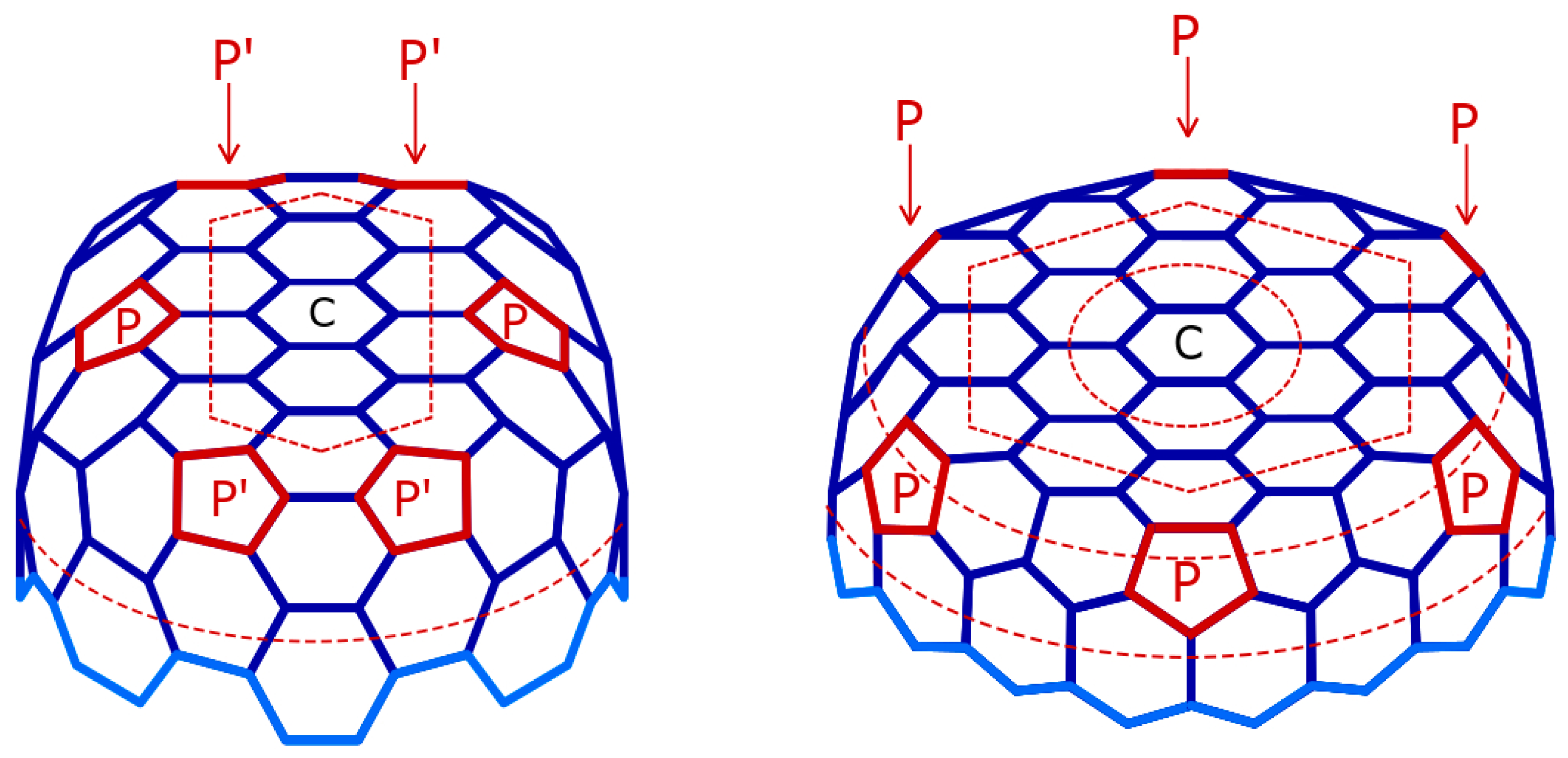
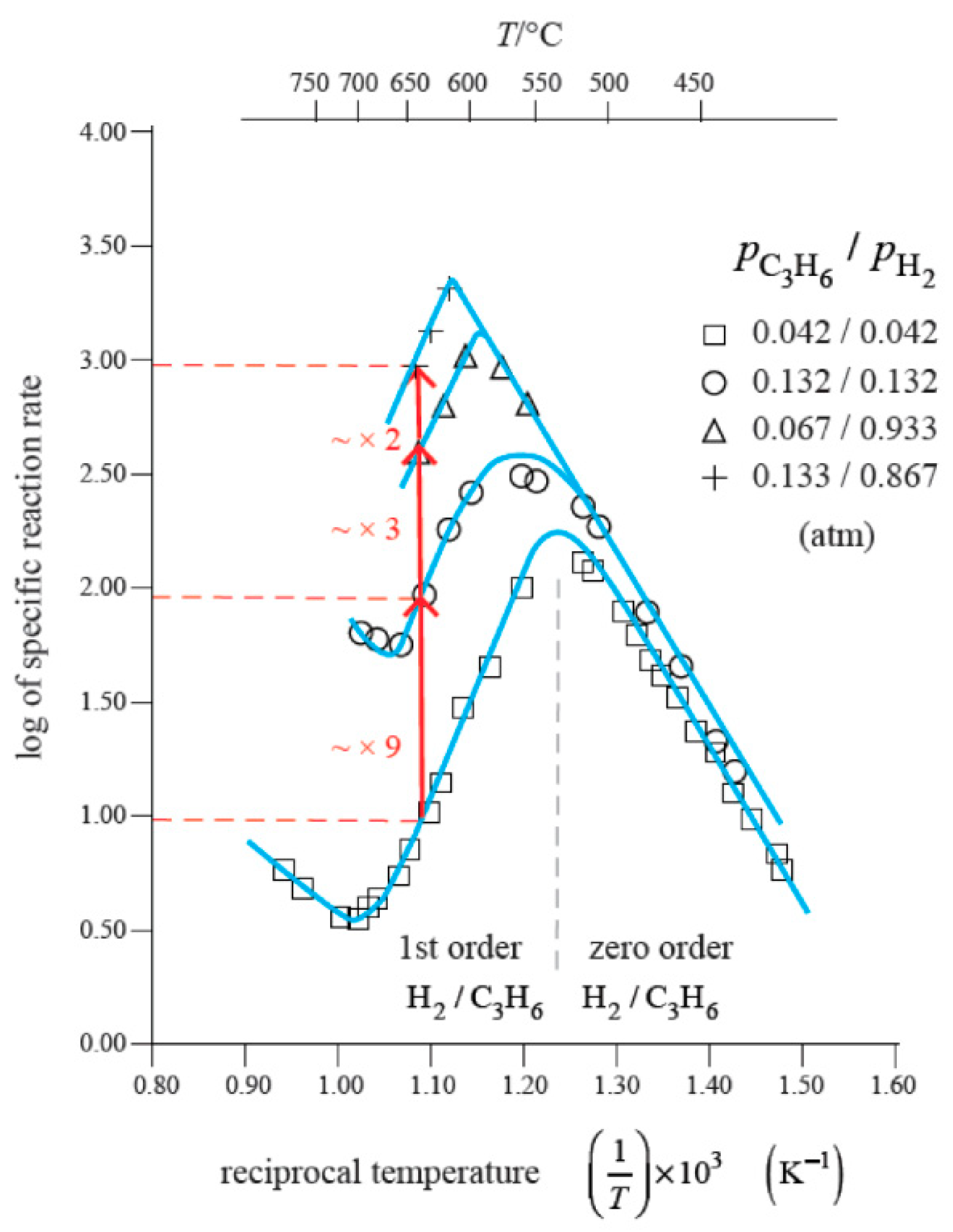

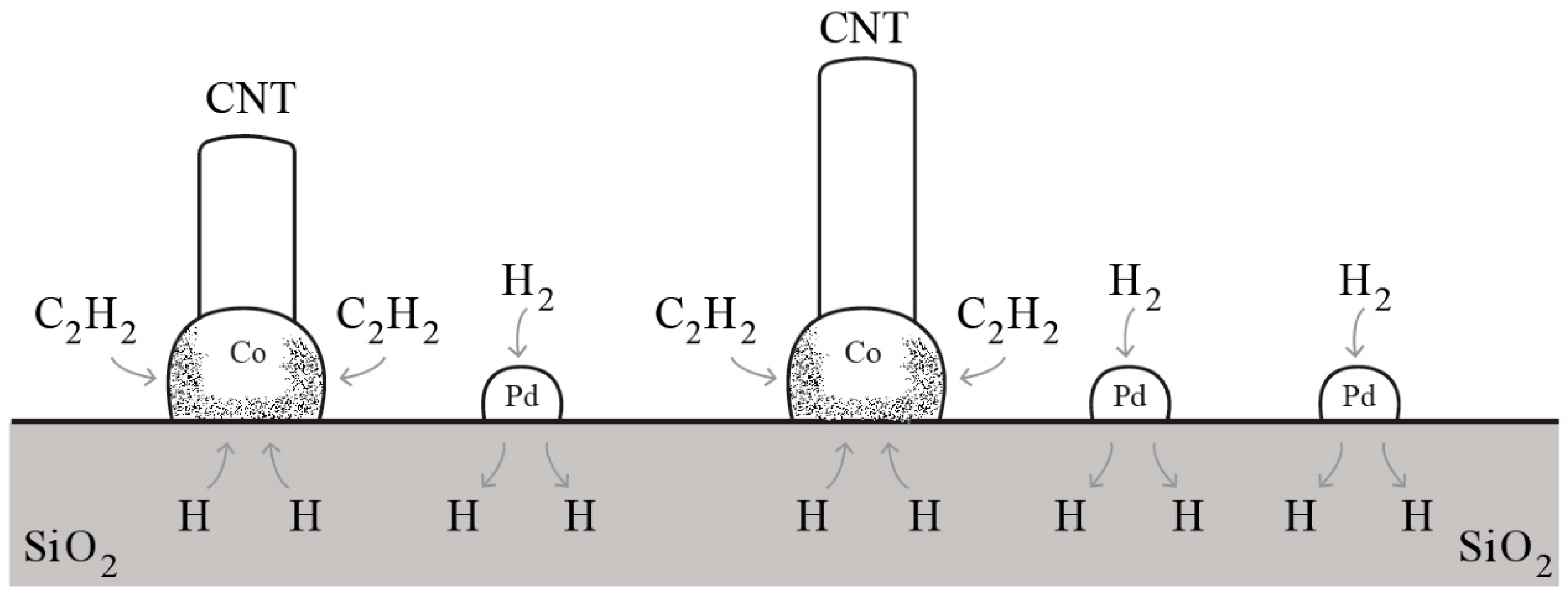
| Catalytic Routes | T Range | Gas Phase Reactions | Surf Catalysis | Carbon Diffusion | Nucleation, Initial Growth |
|---|---|---|---|---|---|
| I Catalytic | Low T 300–550 °C | None or negligible | Yes | Through catalyst nanoparticle | On catalyst surface Ex: Ni (111) |
| II Hybrid | Medium T 550–700 °C | Pyrolysis: C black. No gas changes | No | Through catalyst nanoparticle | On catalyst surface Ex: Mo |
| III Pirolytic | High T >700 (*) °C | Pyrolysis: C black. Gas changes | No | Moving over previous layer of graphene | Over previous graphene layer |
| Reactant Gas | P, Gas (torr) | P, H2 (torr) | Ea (Kcal/mole) |
|---|---|---|---|
| C3H6 | 100 | 25 | −79 |
| C3H6 | 25 | 25 | −33 |
| C2H2 | 82 | 345 | −53 |
| C2H4 | 82 | 68 | −75 |
| 1-C4H8 | 30 | 250 | −64 |
| Temp | Gas Pressure, Torr | PH2 (torr) | Orders | |||
|---|---|---|---|---|---|---|
| /°C | C2H2 | C2H4 | C4H8 | H2 | Hydr. | |
| 600 | 3–12 | - | - | 50 | - | 1 |
| 600 | 12.0 | - | - | 44–52 | ~0 | - |
| 625 | - | 8–13 | - | 9.5 | - | 1 |
| 625 | - | 13.0 | - | 9–14 | 1 | - |
| 625 | - | 13.0 | - | 50–66 | ~0 | - |
| 625 | - | - | 31–62 | 8 | 0 | - |
| 625 | - | - | 32.5 | 4–25 | - | 1 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, L.S. Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe. C 2019, 5, 42. https://doi.org/10.3390/c5030042
Lobo LS. Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe. C. 2019; 5(3):42. https://doi.org/10.3390/c5030042
Chicago/Turabian StyleLobo, Luis Sousa. 2019. "Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe" C 5, no. 3: 42. https://doi.org/10.3390/c5030042
APA StyleLobo, L. S. (2019). Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe. C, 5(3), 42. https://doi.org/10.3390/c5030042




