Microbiological Properties of Microwave-Activated Carbons Impregnated with Enoxil and Nanoparticles of Ag and Se
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon
2.2. Activated Carbon Ozonizing
2.3. Thermal Analysis
2.4. Gas Adsorption Characterization
2.5. Infrared Spectroscopy (IR)
2.6. Impregnation of Activated Carbons with Biologically Active Substances
2.7. Microbiological Tests
3. Results and Discussion
3.1. Structural Parameters of Activated Carbons
3.2. Thermal Analysis
3.3. Infrared Spectroscopy Analysis
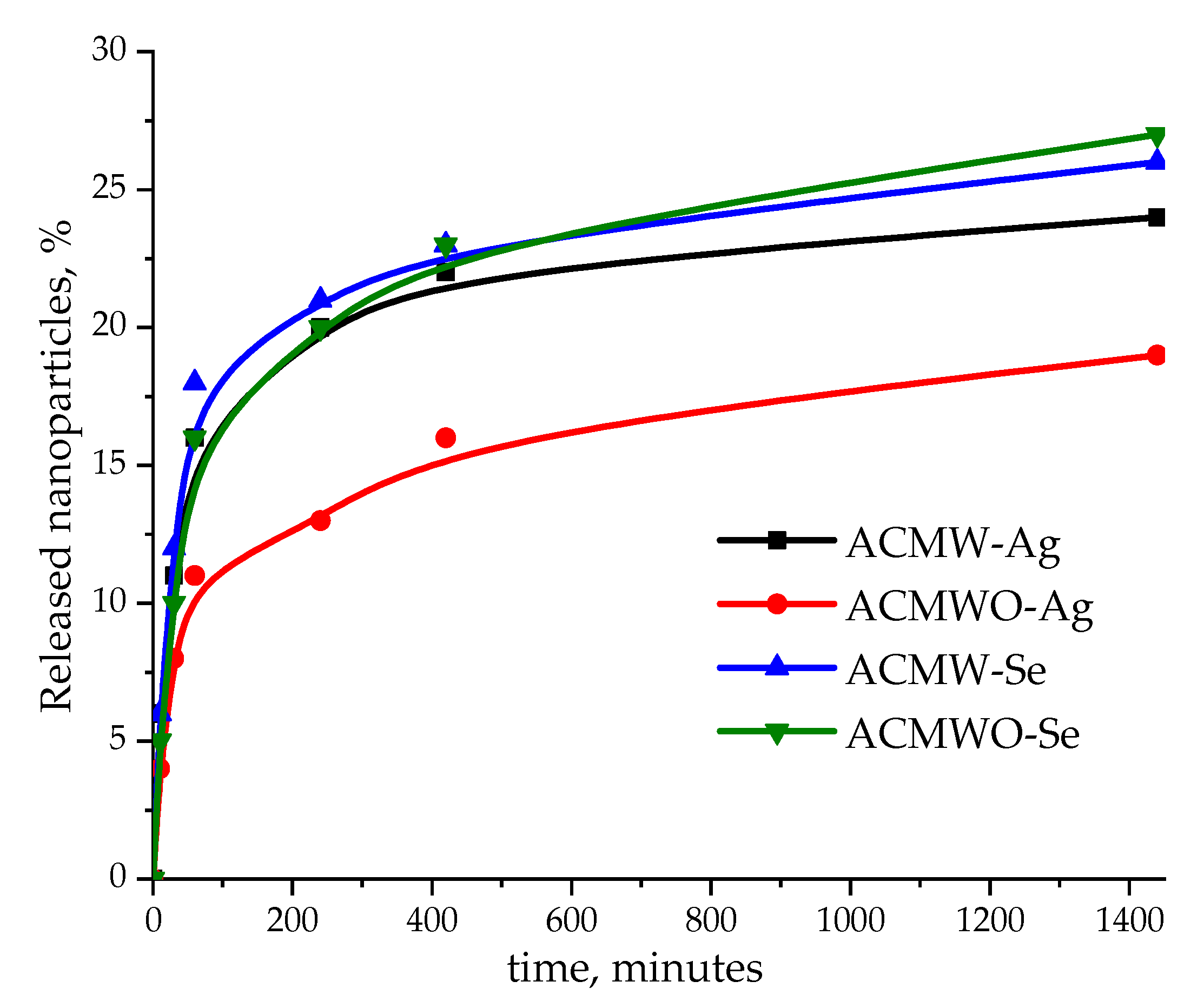
3.4. Microbiological Tests of Activated Carbons
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Acevedo, S.; Arevalo-Fester, J.; Galicia, L.; Atencio, R.; Plaza, E.; Gonzalez, E. Efficiency Study of Silver Nanoparticles (AgNPs) Supported on Granular Activated Carbon against Escherichia coli. J. Nanomed. Res. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Shankar, P.A.; Bandyopadhyaya, R. Plasma treated activated carbon impregnated with silver nanoparticles for improved antibacterial effect in water disinfection. Carbon 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, K.; He, T.; Zhu, L. Synthesis and characterization of Ag nanoparticles decorated mesoporous sintered activated carbon with antibacterial and adsorptive properties. J. Alloys Compd. 2015, 647, 1007–1012. [Google Scholar] [CrossRef]
- Egorova, E.M.; Kubatiev, A.A.; Schvets, V.I. The Effect of Metal Nanoparticles on Biological Objects. In Biological Effects of Metal Nanoparticles; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–218. [Google Scholar]
- Li, X.; Gao, G.; Sun, C.; Yaoyao, Z.; Lingyun, Q.; Fenghua, J.; Haibing, D. Preparation and antibacterial performance testing of Ag nanoparticles embedded biological materials. Appl. Surf. Sci. 2015, 330, 237–244. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, X.; Li, W.; Liu, S. Antibacterial action of silver-doped activated carbon prepared by vacuum impregnation. Appl Surf. Sci. 2013, 266, 67–72. [Google Scholar] [CrossRef]
- Tran, Q.T.; Van Nguyen, S.; Hoang, T.K.D.; Nguyen, H.L.; Bui, T.T.; Nguyen, T.; Van, A.; Nguyen, D.H.; Nguyen, H.H. Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J. Hazard. Mater. 2011, 192, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A.O. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.L.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Lupaşcu, L.; Rudic, V.; Cotos, V.; Lupaşcu, T. Antimicrobial activity of the autochthonous compound Enoxil. J. Biomed. Sci. Eng. 2010, 3, 758–762. [Google Scholar] [CrossRef]
- Duca, G.; Lupascu, T.; Vlad, P.; Kulcitki, V.; Nastas, R. Studies on the sollubilization processes in water of enotannins and their phisico-chemical properties. Chem. J. Mold. 2006, 1, 60–64. [Google Scholar]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N.K. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Piriz-Almeida, F.; Durán-Valle, C.J.; Pastor-Villegas, J. Formation of oxygen structures by air activation. A study by FT-IR spectroscopy. Carbon 1999, 37, 1517–1528. [Google Scholar] [CrossRef]
- Sutherland, I.; Sheng, E.; Bradley, R.H.; Freakley, P.K. Effects of ozone oxidation on carbon black surfaces. J. Mater. Sci. 1996, 31, 5651–5655. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2016, 44, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, L.; Baraniak, M.; Buchwald, T. The application of activated carbon modified by ozone treatment for energy storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef]
- Valdes, H.; Sanchez-Polo, M.; Zaror, A. Effect of ozonation on the activated carbon surface chemical properties and on 2-mercaptobenzothiazole adsorption. Lat. Am. Appl. Res. 2003, 33, 219–223. [Google Scholar]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of Ozone Treatment on Surface Properties of Activated Carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J. Adsorbent−Adsorbate Interactions in the Adsorption of Cd(II) and Hg(II) on Ozonized Activated Carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- De Oliveira, G.F.; de Andrade, R.C.; Trindade, M.A.G.; Andrade, H.M.C.; Teodoro de Carvalho, C. Thermogravimetric and spectroscopic study (TG–DTA/FT–IR) of activated carbon from the renewable biomass source Babassu. Quim. Nova 2016, 40, 284–292. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Álvarez, P.M.; Jaramillo, J.; Beltrán, F.J. Formation of oxygen complexes by ozonation of carbonaceous materials prepared from cherry stones I. Thermal effects. Carbon 2002, 40, 513–522. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Yates, J.T. FTIR study of the oxidation of amorphous carbon by ozone at 300 K—Direct COOH formation. Carbon 2001, 39, 1167–1173. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Chiang, P.C.; Huang, C.P. Ozonation of activated carbon and its effects on the adsorption of VOCs exemplified by methylethylketone and benzene. Chemosphere 2002, 47, 267–275. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 56, 5232–5236. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Wiley: Chichester, UK, July 2004; pp. 71–94. [Google Scholar]
- Kuptsov, A.H.; Zhizhin, G.N. Handbook of Fourier Transform. Raman and Infrared Spectra of Polymers; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1–571. [Google Scholar]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–239. [Google Scholar]
- Burg, P.; Cagniant, D. Characterization of Carbon Surface Chemistry. In Chemistry and Physics of Carbon; Radovic, L.R., Ed.; CRC Press: Boca Raton, FI, USA, 2007; Volume 30, pp. 129–176. [Google Scholar]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic carbons activated with phosphoric acid. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Álvarez, P.M.; Jaramillo, J.; Beltrán, F.J. Formation of oxygen structures by ozonation of carbonaceous materials prepared from cherry stones II. Kinetic study. Carbon 2002, 40, 523–529. [Google Scholar] [CrossRef]
- Kim, J.-H.; Min, B.-G. Functionalization of Multi-walled Carbon Nanotube by Treatment with Dry Ozone Gas for the Enhanced Dispersion and Adhesion in Polymeric Composites. Carbon Lett. 2010, 11, 298–303. [Google Scholar] [CrossRef]
- Alvarez, P.M.; Garcia-Araya, J.F.; Beltran, F.J.; Masa, F.J.; Medina, F. Ozonation of activated carbons: Effect on the adsorption of selected phenolic compounds from aqueous solutions. J. Colloid Interface Sci. 2005, 283, 503–512. [Google Scholar] [CrossRef] [PubMed]
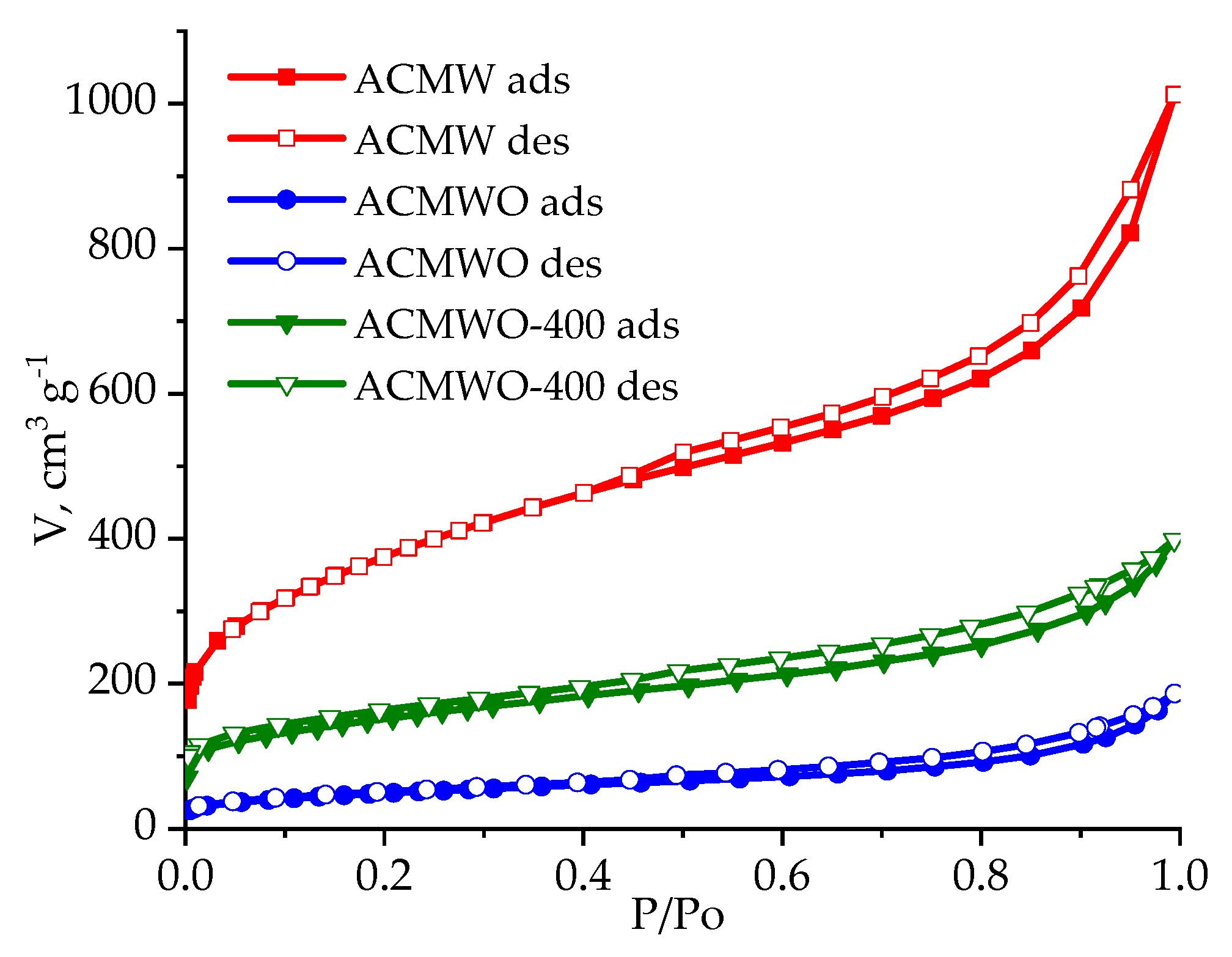
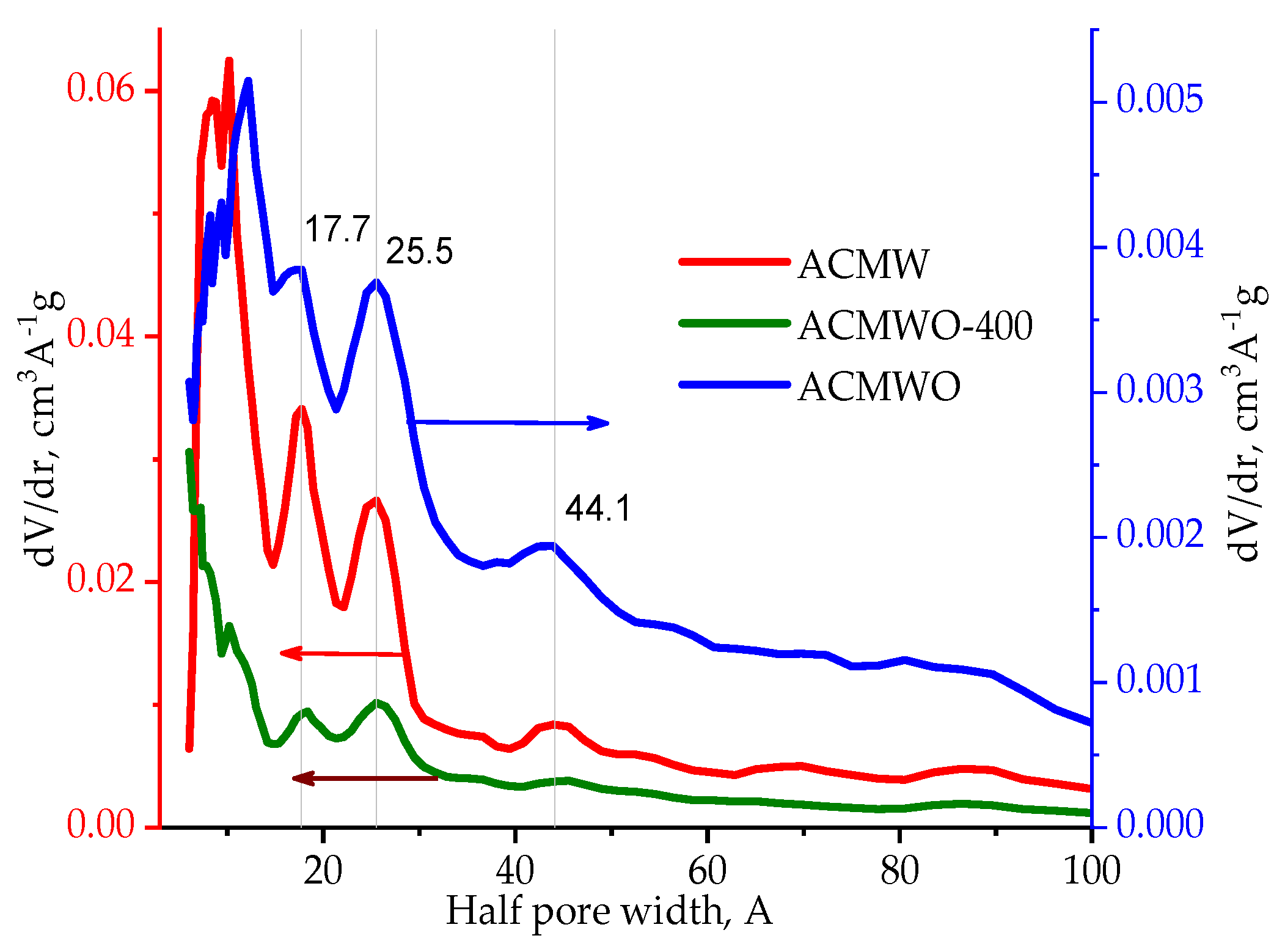
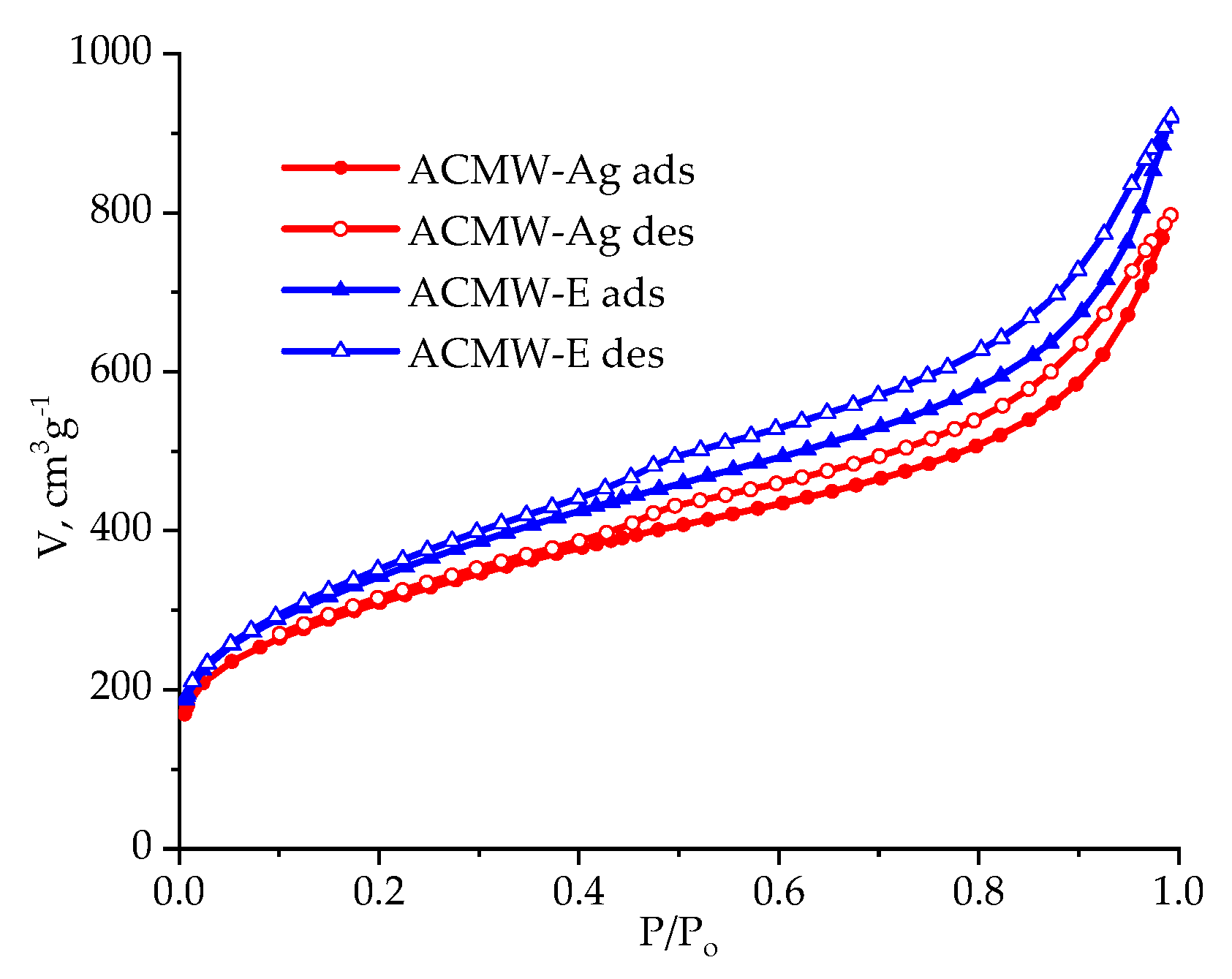
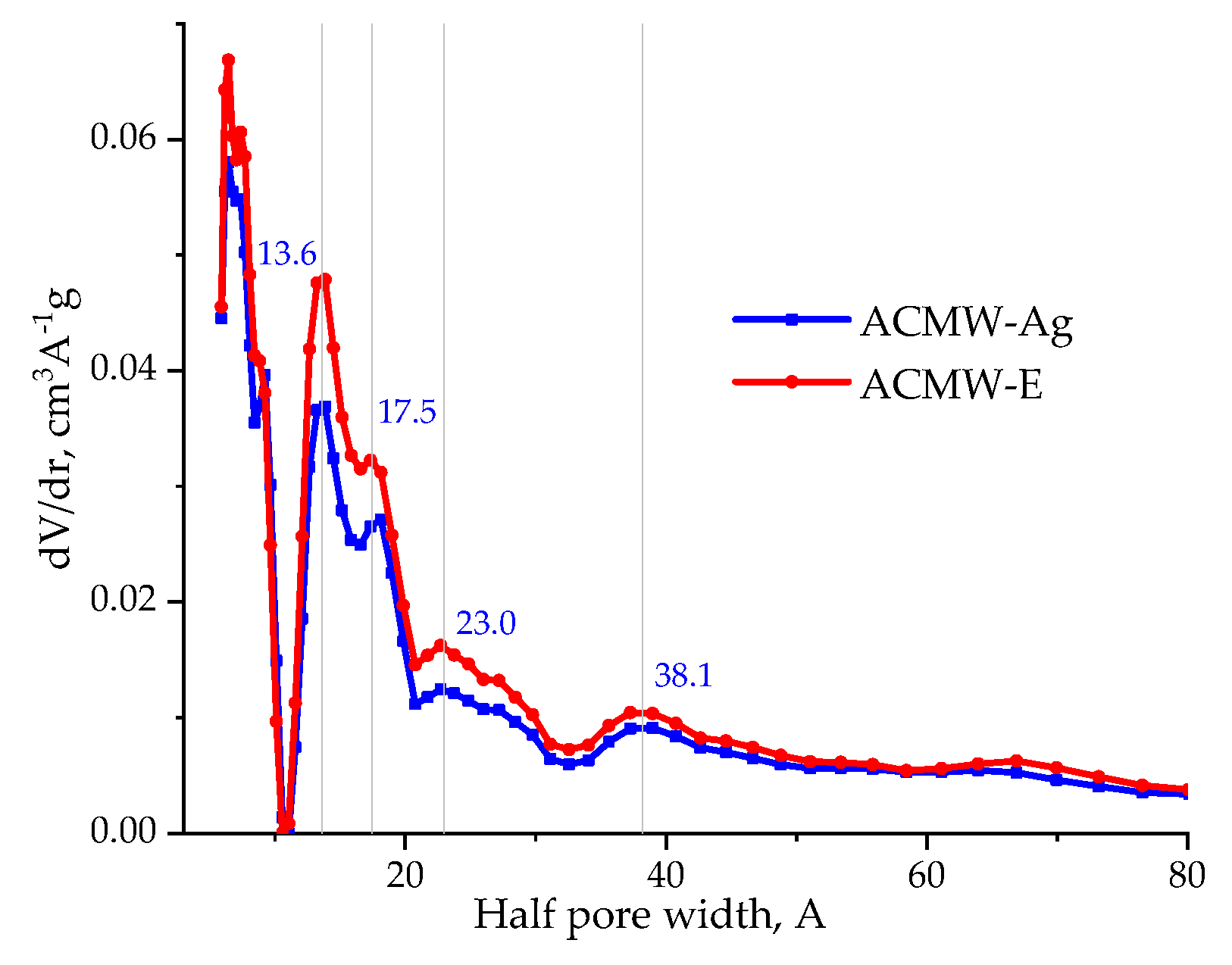
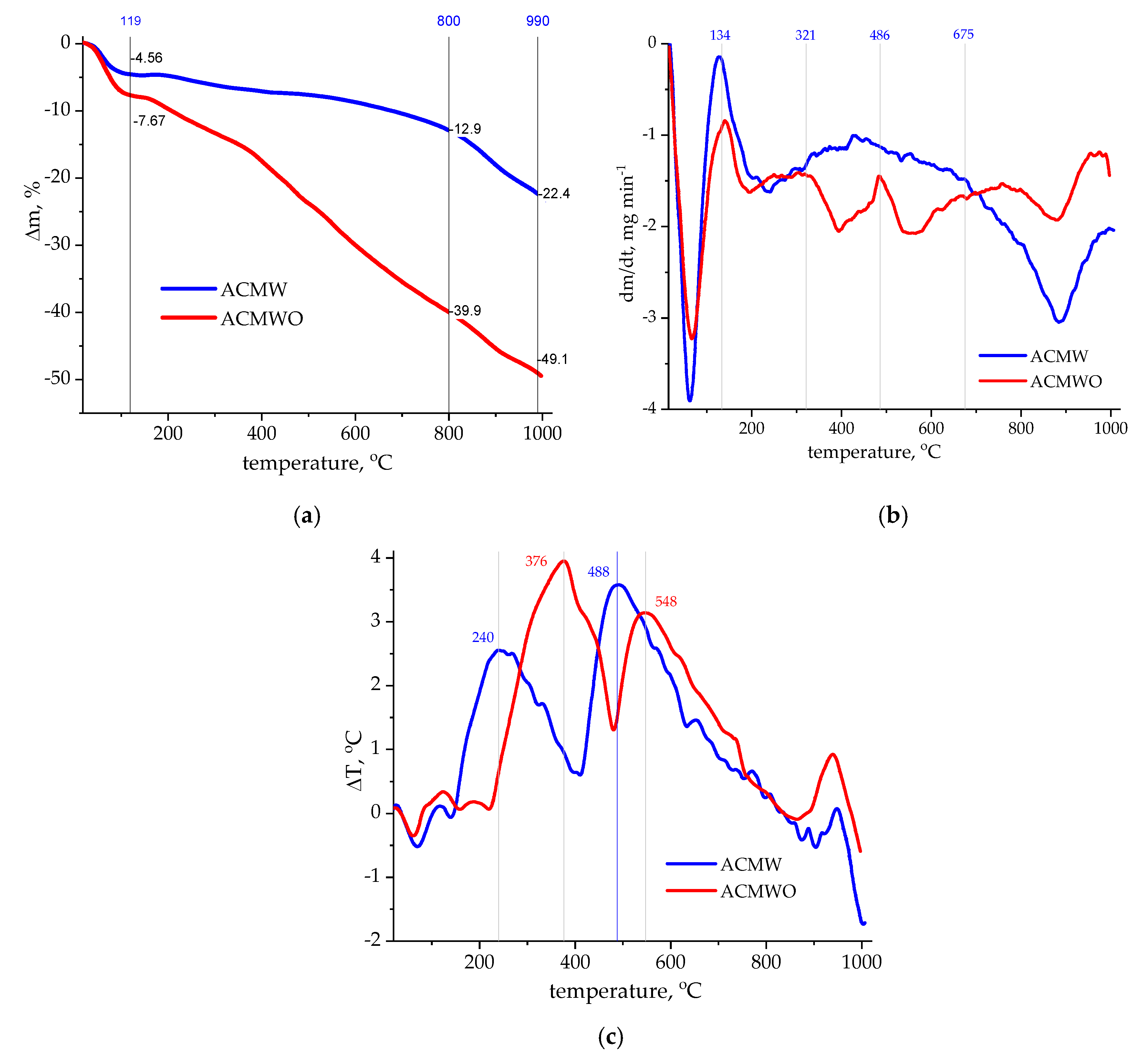
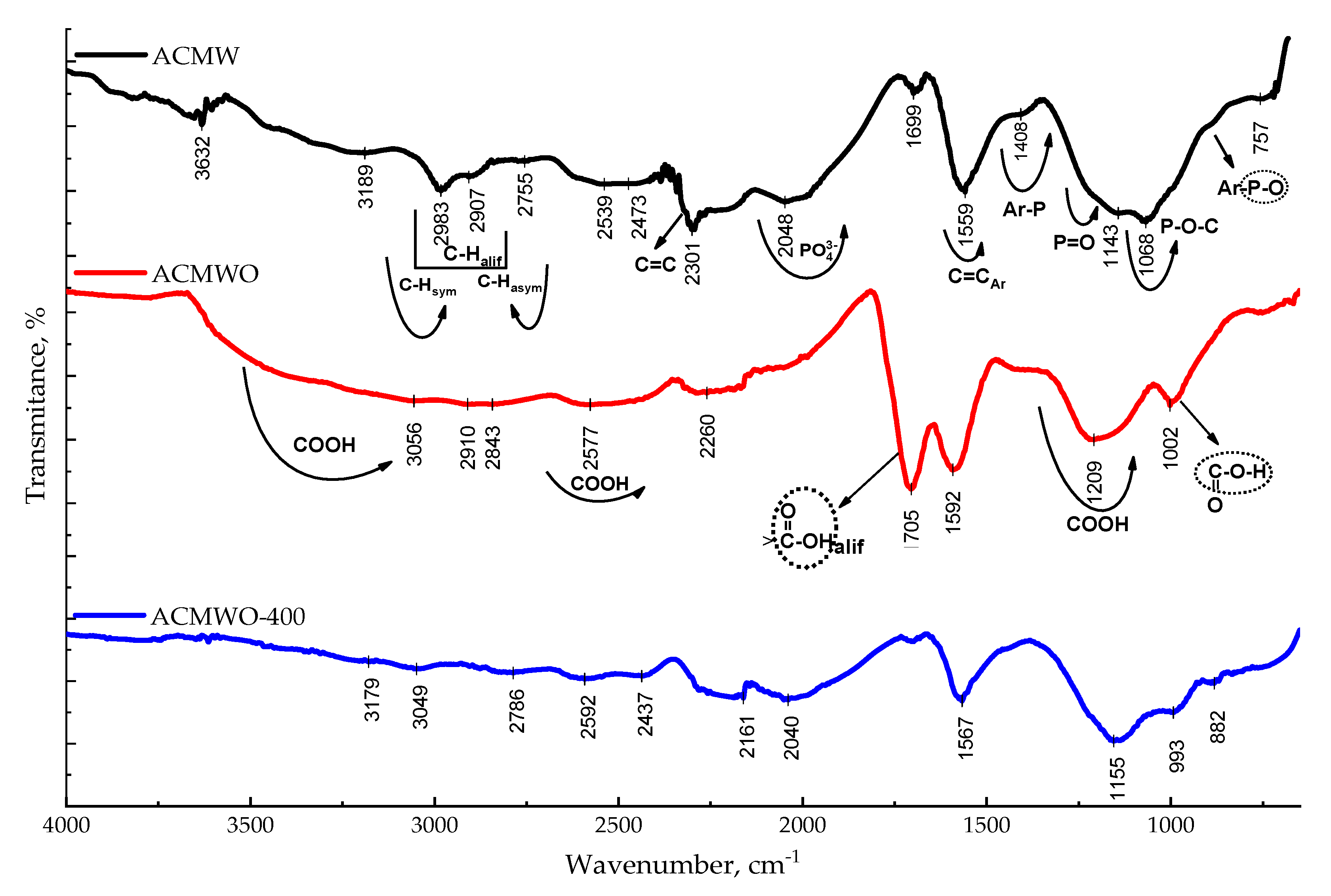
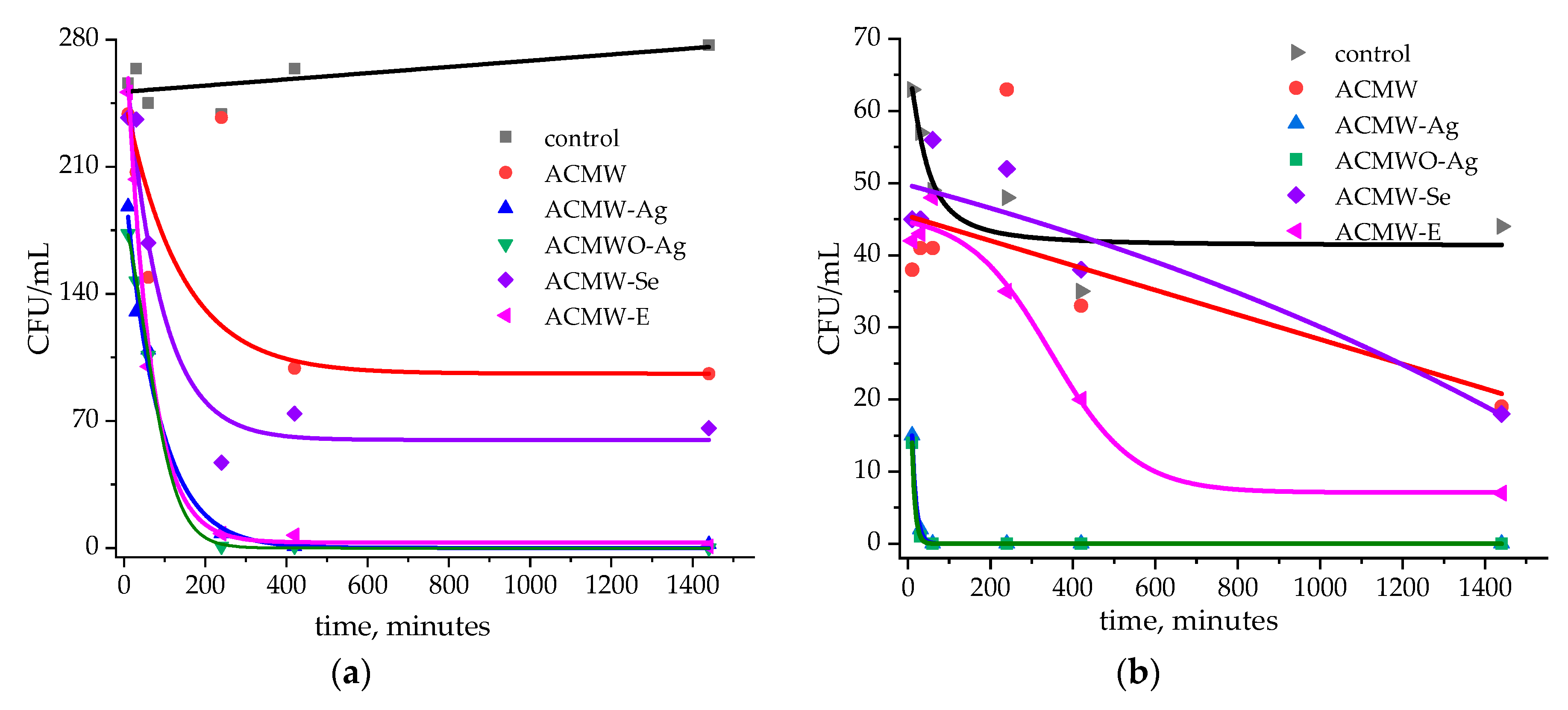

| AC | SBET, m2/g | Sme, m2/g | Vmi, cm3/g | Vme, cm3/g | Vt, cm3/g | Ea, kJ/mol | ref, Å |
|---|---|---|---|---|---|---|---|
| ACMW | 1369 | 788 | 0.260 | 1.360 | 1.566 | 12.9 | 10.2 |
| ACMWO | 172 | 111 | 0.029 | 0.259 | 0.288 | 6.4 | 12.4 |
| ACMWO-400 | 530 | 229 | 0.157 | 0.459 | 0.616 | 14.1 | 6.4 |
| ACMW-Ag | 1062 | 627 | 0.224 | 1.009 | 1.233 | 11.3 | 6.4 |
| ACMW-E | 1188 | 763 | 0.216 | 1.208 | 1.424 | 11.3 | 6.4 |
| Sample | C(Ag), mg/g | C(Se), µg/g |
|---|---|---|
| ACMW | <0.001 | <0.05 |
| ACMWO | <0.001 | <0.05 |
| ACMW-Ag | 1.48 | <0.05 |
| ACMWO-Ag | 1.02 | <0.05 |
| ACMW-Se | <0.001 | 17.14 |
| ACMWO-Se | <0.001 | 2.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petuhov, O.; Lupascu, T.; Behunová, D.; Povar, I.; Mitina, T.; Rusu, M. Microbiological Properties of Microwave-Activated Carbons Impregnated with Enoxil and Nanoparticles of Ag and Se. C 2019, 5, 31. https://doi.org/10.3390/c5020031
Petuhov O, Lupascu T, Behunová D, Povar I, Mitina T, Rusu M. Microbiological Properties of Microwave-Activated Carbons Impregnated with Enoxil and Nanoparticles of Ag and Se. C. 2019; 5(2):31. https://doi.org/10.3390/c5020031
Chicago/Turabian StylePetuhov, Oleg, Tudor Lupascu, Dominika Behunová, Igor Povar, Tatiana Mitina, and Maria Rusu. 2019. "Microbiological Properties of Microwave-Activated Carbons Impregnated with Enoxil and Nanoparticles of Ag and Se" C 5, no. 2: 31. https://doi.org/10.3390/c5020031
APA StylePetuhov, O., Lupascu, T., Behunová, D., Povar, I., Mitina, T., & Rusu, M. (2019). Microbiological Properties of Microwave-Activated Carbons Impregnated with Enoxil and Nanoparticles of Ag and Se. C, 5(2), 31. https://doi.org/10.3390/c5020031