Wool Carpet Dye Adsorption on Nanoporous Carbon Materials Derived from Agro-Product
Abstract
:1. Introduction
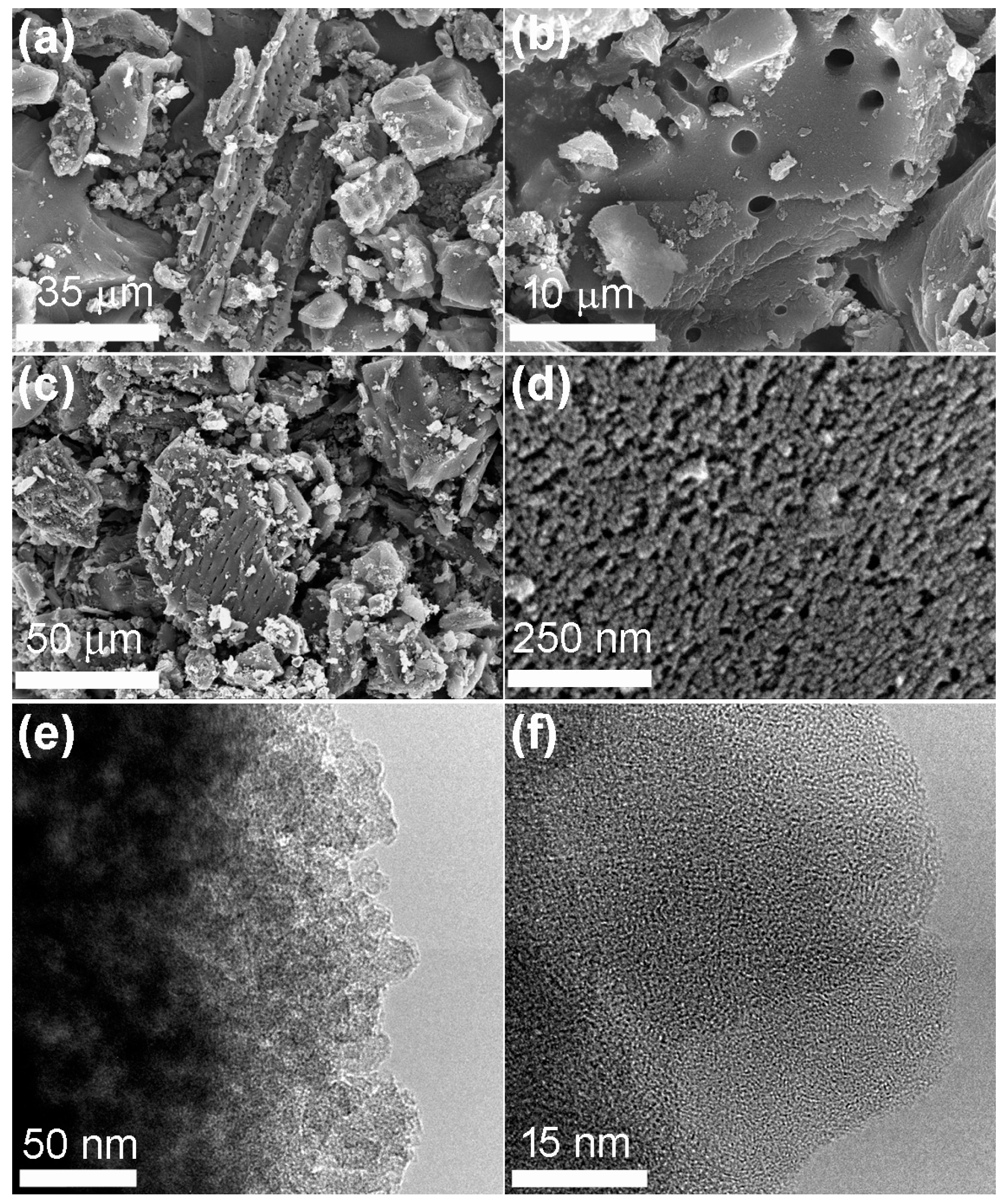
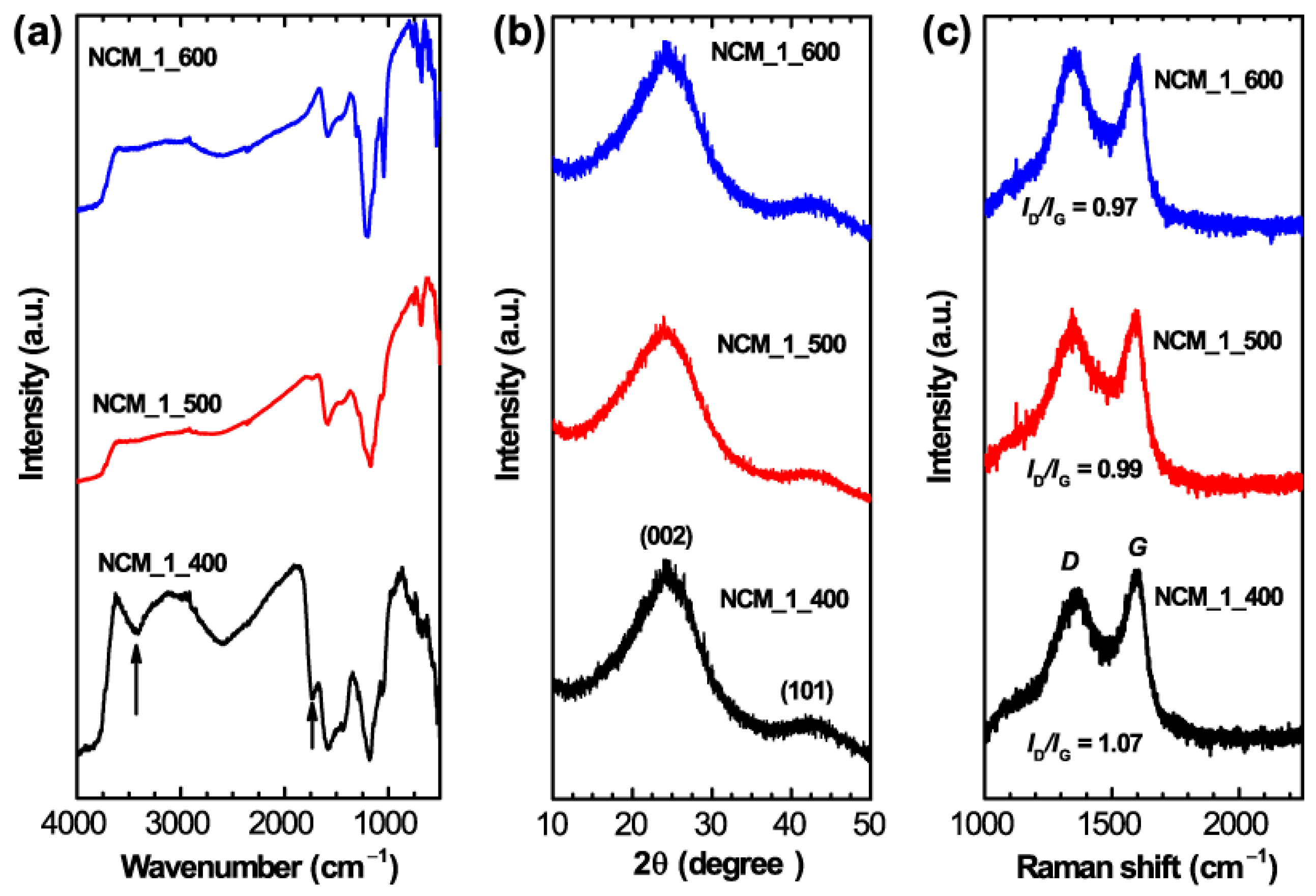
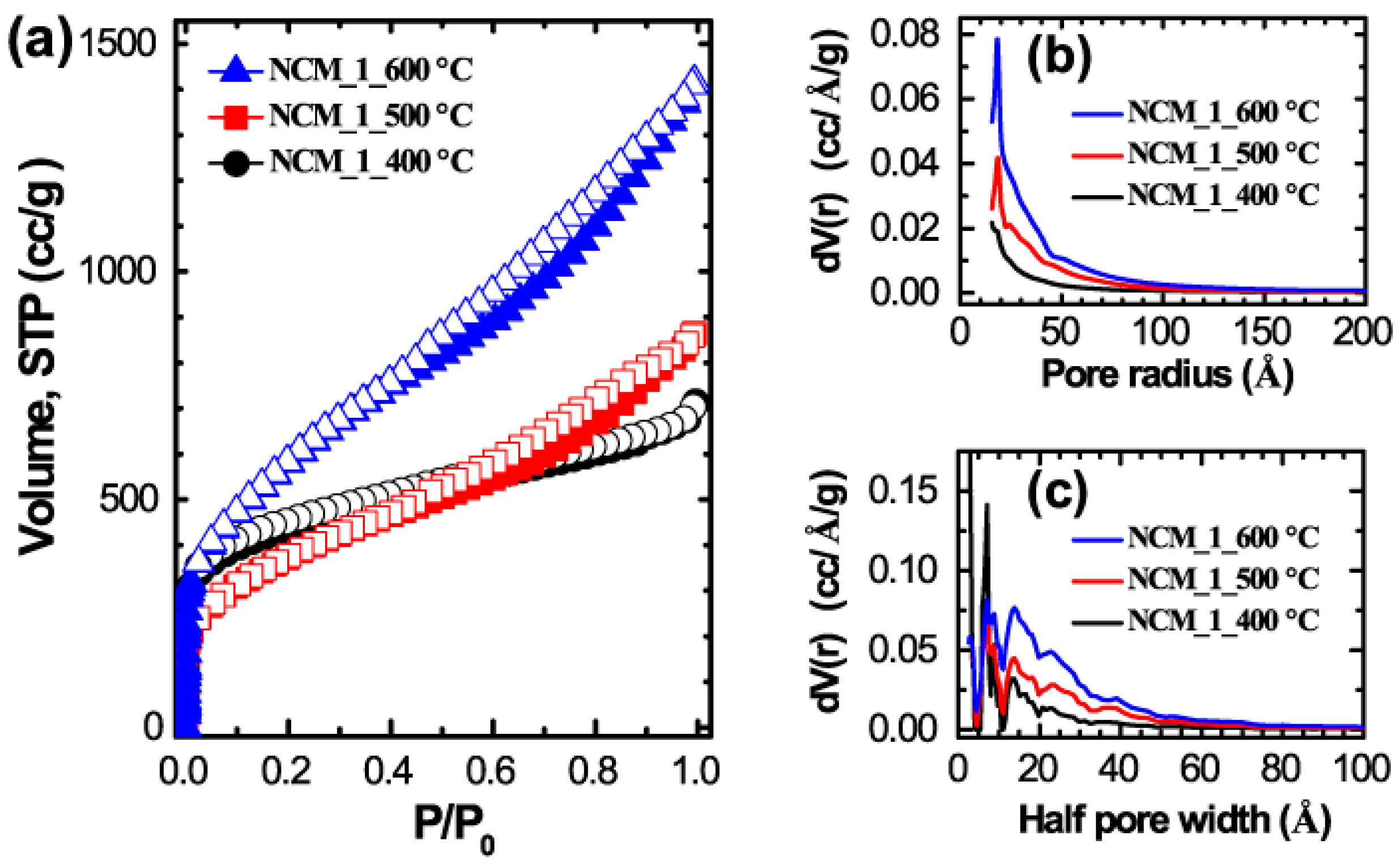
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Nanoporous Carbon Material (NCM)
3.3. Characterizations
3.4. Adsorption of Wool Carpet Dyes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peláez-Cid, A.-A.; Herra-González, A.-M.; Salazar-Villanueva, M.; Bautista-Hernández, A. Elimination of textile dyes using activated carbons prepared from vegetable residues and their characterization. J. Environ. Manag. 2017, 181, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.F.; Elhadidy, H. Production of activated carbons from waste carpets and its application in methylene blue adsorption: Kinetic and thermodynamic study. J. Environ. Chem. Eng. 2017, 5, 955–963. [Google Scholar] [CrossRef]
- Dong, P.; Maneerung, T.; Ng, W.C.; Zhen, X.; Dai, Y.; Tong, Y.W.; Ting, Y.-P.; Koh, S.N.; Wang, C.-H.; Neoh, K.G. Chemically treated carbon black waste and its potential applications. J. Hazard. Mater. 2017, 321, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Wei, M.; Gao, L.; Li, J.; Fang, J.; Cai, W.; Li, X.; Xu, A. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J. Hazard. Mater. 2016, 316, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye removal Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Huang, T.; Li, W.; Wang, Y.; Wang, Z. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism. J. Hazard. Mater. 2016, 320, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.O.; Foo, K.Y.; Asif, M.; Hameed, B.H. Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem. Eng. J. 2014, 250, 198–204. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Bunmahotama, W.; Hung, W.-N.; Lin, T.-F. Prediction of the adsorption capacities of four typical pollutants on activated carbons in natural waters. Water Res. 2017, 111, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, E.; Turkoglu, S.; Banford, A.; Aktas, Z. The relative performance of microwave regenerated activated carbons on the removal of phenolic pollutants. J. Clean. Prod. 2017, 149, 1109–1117. [Google Scholar] [CrossRef]
- Machida, M.; Chensun, S.; Amano, Y.; Iamzeki, F. Adsorptive removal of Pb(II) ions from aqueous solution by (NH4)2S2O8 oxidized activated carbons. Bull. Chem. Soc. Jpn. 2015, 88, 127–132. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; Lazaridis, N.K. Multi-parametric adsorption effects of the reactive dye removal with commercial activated carbons. J. Mol. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Tong, K.; Lin, A.; Ji, G.; Wang, D.; Wang, X. The effects of adsorbing organic pollutants from super heavy oil wastewater by lignite activated coke. J. Hazard. Mater. 2016, 308, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Yeung, K.Y.; Guo, J.; Wang, H.; Mckey, G. Sustainable development of tyre char-based activated carbons with different textural properties for value-added applications. J. Environ. Manag. 2016, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thue, P.S.; Adebayo, M.A.; Lima, E.C.; Sieliechi, J.M.; Machado, F.M.; Dotto, G.L.; Vaghetti, J.C.P.; Dias, S.L.P. Preparation, characterization and application of microwave-assisted activated carbons from wood chips for removal of phenol from aqueous solution. J. Mol. Liq. 2016, 223, 1067–1080. [Google Scholar] [CrossRef]
- Donald, J.; Ohtsuka, Y.; Xu, C.C. Effects of activation agents and intrinsic minerals on development in activated carbons derived from a Canadian peat. Mater. Lett. 2011, 65, 744–747. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Mesoporous activated carbon from wood sawdust by K2CO3 activation using microwave heating. Bioresour. Technol. 2012, 111, 425–432. [Google Scholar] [CrossRef] [PubMed]
- González, J.F.; Román, S.; González-García, C.M.; Nabais, J.M.V.; Ortiz, A.L. Porosity development in activated carbons prepard from walnut shells by carbon dioxide or steam activation. Ind. Eng. Chem. Res. 2009, 48, 7474–7481. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.V. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Rajbhandari, R.; Shrestha, L.K.; Pradhananga, R.R. Nanoporous activated carbon derived from Lapsi (Choerospondias Axillaris) seed stone for the removal of arsenic from water. J. Nanosci. Nanotechnol. 2012, 12, 7002–7009. [Google Scholar] [CrossRef] [PubMed]
- Okman, I.; Karagöz, S.; Tay, T.; Erdem, M. Activated carbon from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl. Surf. Sci. 2014, 293, 138–142. [Google Scholar] [CrossRef]
- Hayashi, J.; Horikawa, T.; Takeda, I.; Muroyama, K.; Nasir-Ani, F. Preparing activated carbons from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Rafatullaha, M.; Sulaimana, O.; Hashima, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J. Hazard. Mater. 2009, 162, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Yuen, F.K.; Hameed, B.H. Recent developments in the preparation and regeneration of activated carbons my microwaves. Adv. Colloid Interface Sci. 2009, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, M.; Lin, Z.; Li, N.; Liu, Y.; Zhao, B.; Pang, H.; Cao, J.; He, P.; Shi, Y. Activated carbon with ultrahigh specific surface area synthesized from natural plant material for lithium-sulfur batteries. J. Mater. Chem. A 2014, 2, 15889–15896. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Adhikari, L.; Shrestha, R.G.; Adhikari, M.P.; Adhikari, R.; Hill, J.P.; Pradhananga, R.R.; Ariga, K. Nanoporous carbon materials with enhanced supercapacitance performance and non-aromatic chemical sensing with C1/C2 alcohol discrimination. Sci. Technol. Adv. Mater. 2016, 17, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.P.; Adhikari, R.; Shrestha, R.G.; Rajendran, R.; Adhikari, L.; Bairi, P.; Pradhananga, R.R.; Shrestha, L.K.; Ariga, K. Nanoporous activated carbon derived from agro-waste corncob for enhanced electrochemical and sensing performance. Bull. Chem. Soc. Jpn. 2015, 88, 1108–1115. [Google Scholar] [CrossRef]
- Joshi, S.; Shrestha, L.K.; Kamachi, Y.; Malgras, V.; Pradhananga, M.A.; Pokharel, B.P.; Naata, T.; Pradhananga, R.R.; Ariga, K.; Yamauchi, Y. Synthesis and characterizations of nanoporous carbon derived from Lapsi (Choerospondias axillaris) seed: Effect of carbonization conditions. Adv. Powder Technol. 2015, 26, 894–900. [Google Scholar] [CrossRef]
- Joshi, S.; Shrestha, L.K.; Kamachi, Y.; Yamauchi, Y.; Pradhananga, M.A.; Pokhrel, B.P.; Ariga, K.; Pradhananga, R.R. Sodium hydroxide activated nanoporous carbons based on Lapsi seed stone. J. Nanosci. Nanotechnol. 2015, 15, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Shrestha, R.G.; Joshi, S.; Rajbhandari, R.; Shrestha, N.; Adhikari, M.P.; Pradhananga, R.R.; Ariga, K. Nanoarchitectonics of nanoporous carbon materials from natural resource for supercapacitor application. J. Inorg. Organomet. Polym. 2017. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, A.; Yan, L.; Liu, F.; Zhang, Q. Preparation and characterization of highly mesoporous spherical activated carbons from divinylbenzene-derived polymer by ZnCl2 activation. J. Colloid Interface Sci. 2007, 316, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous carbon tubes from fullerene crystal as the π-electron carbon source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Shrestha, R.G.; Hill, J.P.; Nishimura, T.; Ariga, K.; Shrestha, L.K. Mesoporous graphitic carbon microtubes derived from fullerene C70 tubes as a high performance electrode material for advanced supercapacitors. J. Mater. Chem. A 2016, 4, 13899–13906. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Tsuruoka, T.; Ji, Q.; Nishimura, T.; Ariga, K. Surfactant-Triggered Nanoarchitectonics of Fullerene C60 Crystals at a Liquid–Liquid Interface. Langmuir 2016, 32, 12511–12519. [Google Scholar] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Dynamic adsorption behavior of methylene blue onto oil palm shell granular activated carbon prepared by microwave heating. Chem. Eng. J. 2012, 203, 81–87. [Google Scholar] [CrossRef]
- Hameeda, B.H.; El-Khaiary, M.I. Removal of basic dye from aqueous medium using a novel agricultural waste material: Pumpkin seed hull. J. Hazard. Mater. 2008, 155, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Qada, E.N.E.; Allen, S.J.; Walker, G.M. Adsorption of basic dyes from aqueous solution onto activated carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- Rodríguez, A.; García, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mater. 2009, 172, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Zheng, T.; Wang, N.L.P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, B.S.; Zheng, C. Preparation and adsorption properties of corncob-derived activated carbon with high surface area. J. Chem. Eng. Data 2010, 55, 4669–4676. [Google Scholar] [CrossRef]
- Liou, T. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]

| Parameters | LO | LG |
|---|---|---|
| qm (mg·g−1) | 2.60 × 103 | 3.04 × 103 |
| KL (L·mg−1) | 7.83 | 4.59 |
| RL | 4.3 × 10−4 | 7.3 × 10−3 |
| R2 | 0.9841 | 0.976 |
| 1/n | 0.6986 | 0.7651 |
| n | 1.43 | 1.31 |
| KF (mg·g−1) | 42.93 | 29.54 |
| R2 | 0.9908 | 0.9899 |
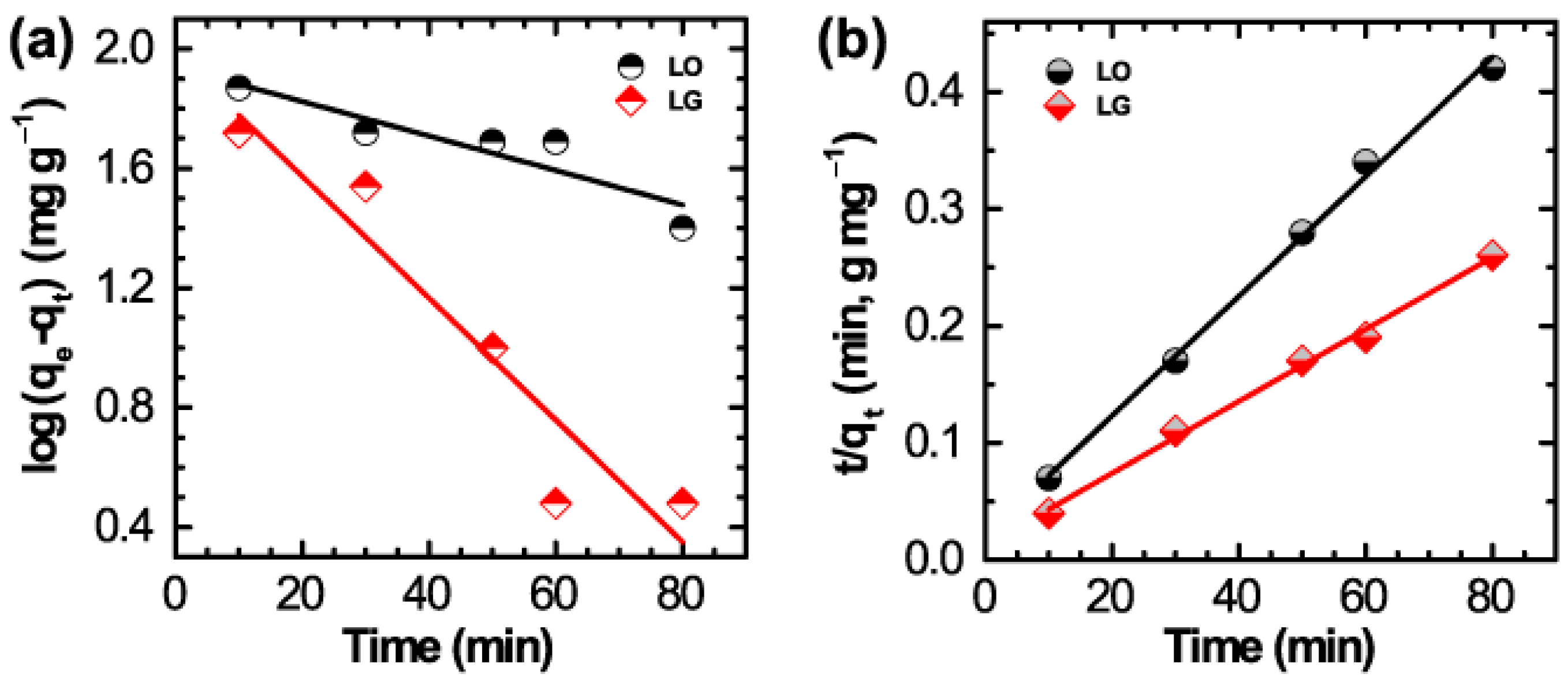
| Dye | Pseudo 1st Order | Pseudo 2nd Order |
|---|---|---|
| LO | k1 = 1.36 × 10−3 L·min−1 | k2 = 1.24 × 10−3 g·mg−1·min−1 |
| qe = 86.98 mg·g−1 | qe = 196.1 mg·g−1 | |
| R2 = 0.8648 | R2 = 0.9963 | |
| LG | k1 = 4.70 × 10−2 L·min−1 | k2 = 7.69 ×10−4 g·mg−1·min−1 |
| qe = 95.85 mg·g−1 | qe = 323 mg·g−1 | |
| R2 = 0.9046 | R2 = 0.9963 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhananga, R.R.; Adhikari, L.; Shrestha, R.G.; Adhikari, M.P.; Rajbhandari, R.; Ariga, K.; Shrestha, L.K. Wool Carpet Dye Adsorption on Nanoporous Carbon Materials Derived from Agro-Product. C 2017, 3, 12. https://doi.org/10.3390/c3020012
Pradhananga RR, Adhikari L, Shrestha RG, Adhikari MP, Rajbhandari R, Ariga K, Shrestha LK. Wool Carpet Dye Adsorption on Nanoporous Carbon Materials Derived from Agro-Product. C. 2017; 3(2):12. https://doi.org/10.3390/c3020012
Chicago/Turabian StylePradhananga, Raja Ram, Laxmi Adhikari, Rekha Goswami Shrestha, Mandira Pradhananga Adhikari, Rinita Rajbhandari, Katsuhiko Ariga, and Lok Kumar Shrestha. 2017. "Wool Carpet Dye Adsorption on Nanoporous Carbon Materials Derived from Agro-Product" C 3, no. 2: 12. https://doi.org/10.3390/c3020012
APA StylePradhananga, R. R., Adhikari, L., Shrestha, R. G., Adhikari, M. P., Rajbhandari, R., Ariga, K., & Shrestha, L. K. (2017). Wool Carpet Dye Adsorption on Nanoporous Carbon Materials Derived from Agro-Product. C, 3(2), 12. https://doi.org/10.3390/c3020012