A Tool to Design Bridging Oligos Used to Detect Pseudouridylation Sites on RNA after CMC Treatment
Abstract
1. Introduction
2. Results
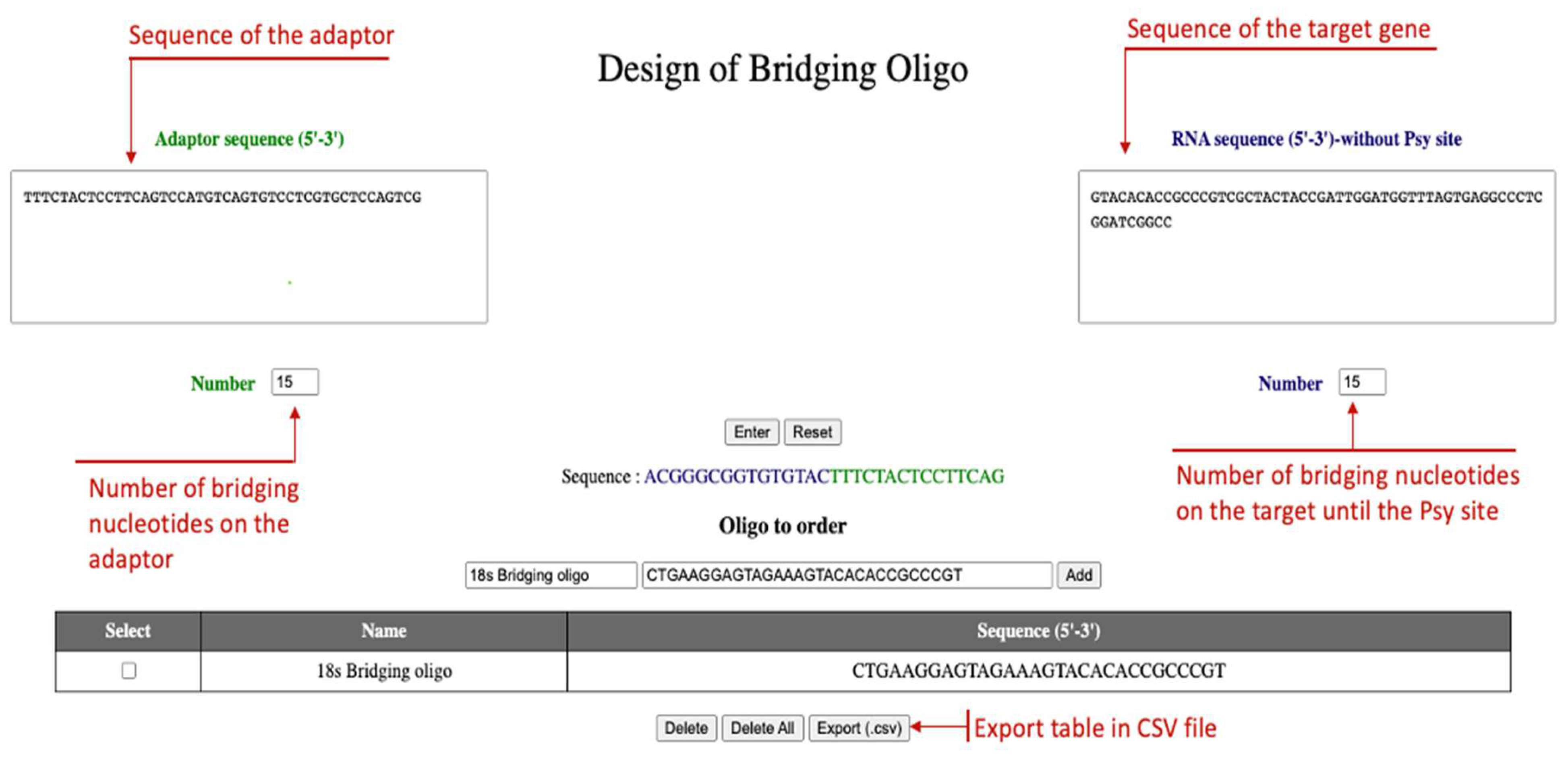
2.1. Use of the DBO Tool
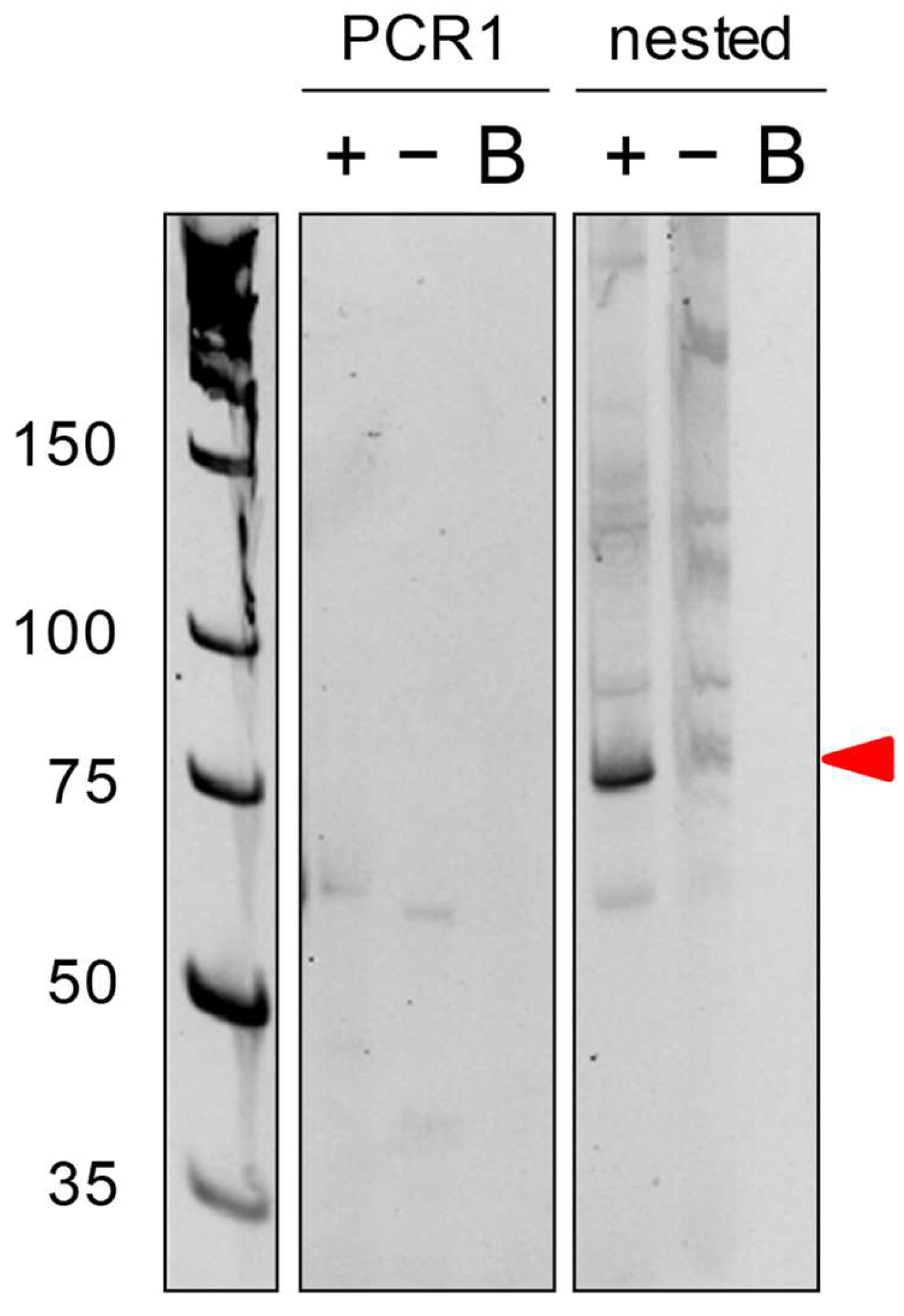
2.2. PCR Results
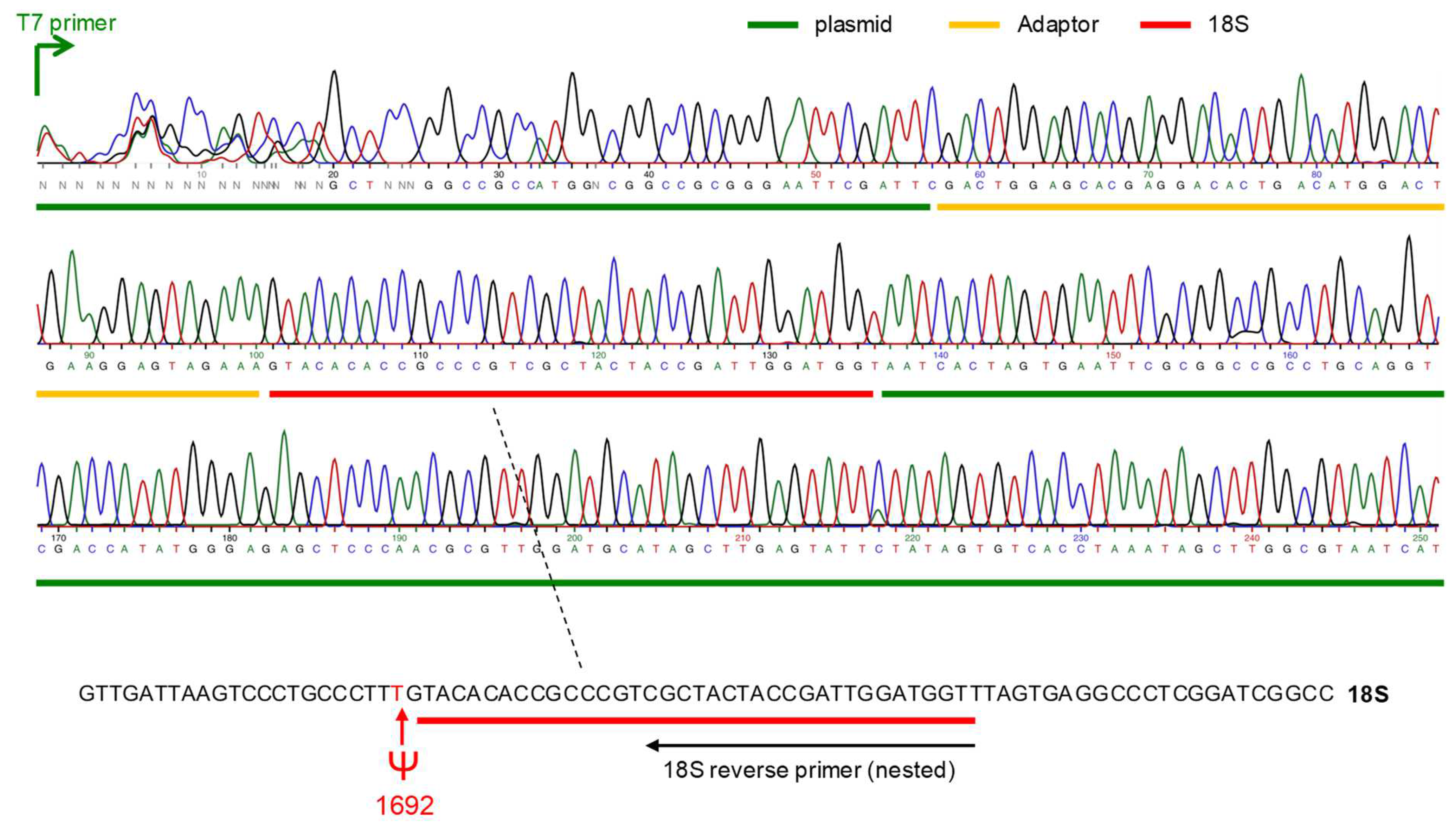
2.3. Sequencing Results
3. Conclusions
4. Materials and Methods
- Tri-reagent (Sigma-Aldrich, St. Louis, MO, USA, cat. no. T9424)
- Chloroform (Sigma-Aldrich, cat. no. C2432)
- Ethanol absolute (VWR chemicals, cat. no. 64-17-5)
- Glycogen (Roche, cat. no. 10901393001)
- 2-Propanol (isopropanol, Sigma-Aldrich, cat. no. 33539-M)
- Phenol:Chloroform:Iso-amyl alcohol (Sigma-Aldrich, cat. no. P1944)
- N-Cyclohexyl-N′-(2-morpholinoethyl)carbodiimide methyl-p-toluenesulfonate (CMCT, Sigma-Aldrich, cat. no. C106402)
- Tris base (Euromedex, Souffelweyersheim, France, cat. no. 200923)
- Hydrochloric acid (HCl, Sigma-Aldrich, cat no. 320331)
- Urea (Sigma-Aldrich, cat. no. U5378)
- EDTA, Tetrasodium Tetrahydrate Salt (Calbiochem, cat. no. 34103-M)
- Potassium acetate (KOAc, Sigma-Aldrich, cat. no. P1190)
- Potassium chloride (KCl, Sigma-Aldrich, cat. no. P9541)
- Sodium carbonate (Na2CO3, Sigma-Aldrich, cat. no. S2127)
- Murine RNase inhibitor (New England BioLabs, Ipswich, M, USA, cat. no. M0314L)
- T4 Polynucleotide kinase (PNK, New England BioLabs, cat. no. M0201L)
- T4 Polynucleotide Kinase Reaction Buffer, 10x (supplied with T4 Polynucleotide kinase)
- Adenosine 5′-Triphosphate (supplied with T4 RNA Ligase 1)
- Dimethyl sulfoxide (DMSO, Sigma-Aldrich, cat. no. D4540)
- T4 RNA Ligase 1 (New England BioLabs, cat. no. M0204S)
- T4 RNA Ligase Reaction Buffer, 10x (supplied with T4 RNA Ligase 1)
- Blocking oligo: 5′-[AmC6]ACCCA-3′ (Eurofins Genomics, Luxembourg)
- RevertAid H Minus Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA, cat. no. EP0452)
- RevertAid H Minus Reverse Transcriptase Reaction Buffer, 5× (supplied with RevertAid H Minus Reverse Transcriptase)
- Random hexamer primer (Thermo Scientific, cat. no. SO142)
- Deoxynucleotide (dNTP) Solution Set (New England BioLabs, cat. no. N0446S)
- 18s RT primer: 5′-ATCCGAGGGCCTCACTAAAC-3′
- RNase H, recombinant (New England BioLabs, cat. no. M0297L)
- Adaptor: 5′-TTTCTACTCCTTCAGTCCATGTCAGTGTCCTCGTGCTCCAGTCG-3′ (Eurofins Genomics)
- 18s Bridging oligo: 5′-CTGAAGGAGTAGAAAGTACACACCGCCCGT[SpC3]-3′ (Eurofins Genomics)
- T4 DNA Ligase (New England BioLabs, cat. no. M0202S)
- T4 DNA Ligase Reaction Buffer, 10× (supplied with T4 DNA Ligase)
- OneTaq® DNA Polymerase (New England BioLabs, cat. no. M0480L)
- OneTaq® Standard Reaction Buffer, 5× (supplied with OneTaq® DNA Polymerase)
- Adaptor primer: 5′-CGACTGGAGCACGAGGACACTGA-3′ (Eurofins Genomics)
- Adaptor nested primer: 5′-GGACACTGACATGGACTGAAGGAGTA-3′ (Eurofins Genomics)
- 18s nested primer: 5′-ACCATCCAATCGGTAGTAGCG-3′ (Eurofins Genomics)
- Ethidium Bromide (BET, Euromedex, cat. no. EU0070)
- Acrylamide/Bis-acrylamide (19:1) (Sigma-Aldrich, cat. no. A3449)
- Ammonium persulfate (APS, Sigma-Aldrich, cat. no. A3678)
- N,N,N′,N′-Tetramethylethylenediamine (TEMED, Sigma-Aldrich, cat. no. T9281)
- Tris Borate EDTA (TBE) Buffer, 10× (Euromedex, cat. no. ET020-B)
- TriDye™ Ultra Low Range DNA Ladder (New England BioLabs, cat. no. N0558S)
- Gel Loading Dye, Purple (6×), no SDS (New England BioLabs, cat. no. B7025S)
- pGEM®-T Easy Vector System I (Promega, Madison, WI, USA, cat. no. A1360)
- NEB® 5-alpha Competent E. coli (New England BioLabs, C2987)
- List of equipment
- Filter units to sterilize buffer solutions (Thermo Scientific, Nalgene rapid flow filters)
- Thermoblock with shaking (Eppendorf Thermomixer comfort, Eppendorf, Montesson, France)
- Refrigerated centrifuge (Eppendorf, cat. no. 5415R)
- Vortex
- Luria broth (LB) agar plates with ampicillin for bacterial selection
- Scalpels
- Vertical electrophoresis system (Hoefer Inc., Holliston, MA, USA, cat. no. SE400)
- Power supply units
- Gel imaging system (Bio-Rad, Hercules, CA, USA, Gel Doc XR+)
- Reagent buffers
- Tris-EDTA-Urea (TEU) buffer: Mix 50 mM Tris-HCl (pH 8.3), 4 mM EDTA and 7 M urea. Store at room temperature
- CMC solution: 1M CMC prepared in TEU buffer. To be prepared extemporaneously.
- Reverse-reaction buffer: Mix 50 mM Na2CO3 and 2 mM EDTA. Adjust pH at 10.4. Store at room temperature
- Non-denaturing polyacrylamide gel: Gel prepared with 10% acrylamide (19:1), 1× TBE in water
- BET working solution: Prepare fresh, dilute stock solution with 1× TBE to use at 0.5 µg/mL
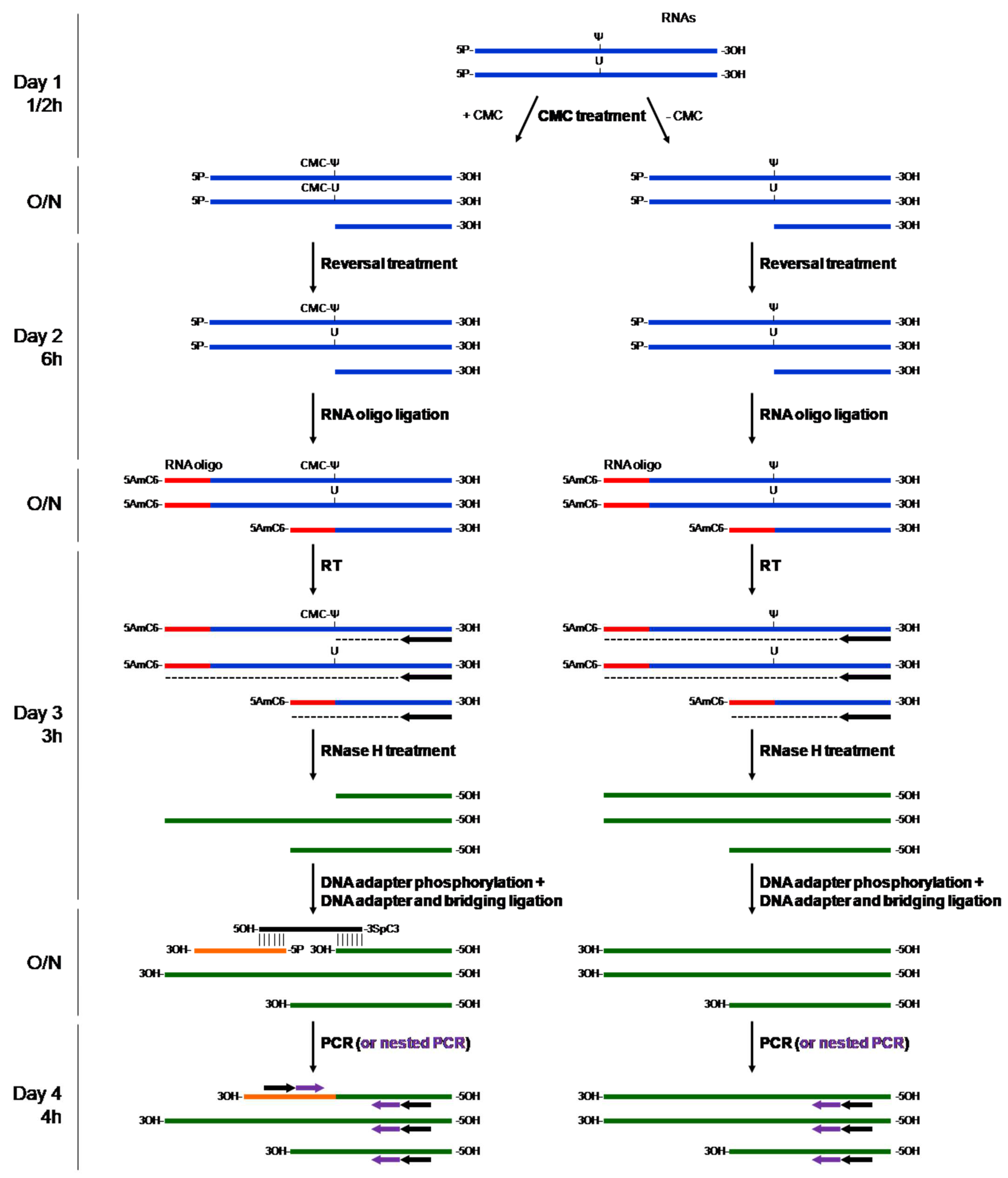
4.1. CMC Treatment
- The CMC buffer must be made extemporaneously (TEU can be kept at RT). The CMC should be resuspended in TEU buffer.
- Add glycogen (20 µg) during the phenol/chloroform procedure.
- If the target to be recovered is an mRNA, use 10 µg of mRNA instead of the 10 µg of total RNA.
4.2. RNA 5′ Phosphorylation
4.3. Ligation of the 5′ RNA Blocking Oligo
4.4. Reverse Transcription
4.5. Adaptor Ligation
- Adaptor, as almost all commercial oligos, is not phosphorylated in 5′-end. Treat the adaptor with T4 PNK before use, as previously described (Section 4.2).
- Use the DBO tool provided to design bridging oligos (see Figure 2).
4.6. PCR Amplification
- To observe a Psi site in 18S or 28S rRNA, apply a PCR of 15 cycles only. To view Psi site in mRNA, perform 35 cycles PCR.
- If the first PCR does not produce a PCR product or non-specific product, use nested PCR to amplify the PCR products more specifically (see Figure 1).
4.7. Sequencing
4.8. Troubleshooting
| No conversion/modification using CMC |
|
| No RT product |
|
| No adaptor ligation |
|
| No PCR product |
|
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Availability of the tool
Conflicts of Interest
References
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic. Acids. Res. 2022, 50, D231–D235. [Google Scholar] [CrossRef] [PubMed]
- Charette, M.; Gray, M.W. Pseudouridine in RNA: What, where, how, and why. IUBMB Life 2000, 49, 341–351. [Google Scholar] [PubMed]
- Nachtergaele, S.; He, C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017, 14, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cohn, W.E.; Volkin, E.L.L.I. Nucleoside-5’-Phosphates from Ribonucleic Acid. Nature 1954, 167, 483–484. [Google Scholar] [CrossRef]
- Zhang, W.; Eckwahl, M.J.; Zhou, K.; Pan, T. Sensitive and quantitative probing of pseudouridine modification in mRNA and long noncoding RNA. RNA 2019, 25, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, T. Probing RNA Modification Status at Single-Nucleotide Resolution in Total RNA. Methods Enzymol. 2015, 560, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, A.; Limbach, P.A. Improving CMC-derivatization of pseudouridine in RNA for mass spectrometric detection. Anal. Chim. Acta 2008, 612, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ganot, P.; Bortolin, M.-L.; Kiss, T. Site-Specific Pseudouridine Formation in Preribosomal RNA Is Guided by Small Nucleolar RNAs. Cell 1997, 89, 799–809. [Google Scholar] [CrossRef]
- Hubé, F.; Velasco, G.; Rollin, J.; Furling, D.; Francastel, C. Steroid receptor RNA activator protein binds to and counteracts SRA RNA-mediated activation of MyoD and muscle differentiation. Nucleic Acids Res. 2010, 39, 513–525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogard, B.; Tellier, G.; Francastel, C.; Hubé, F. A Tool to Design Bridging Oligos Used to Detect Pseudouridylation Sites on RNA after CMC Treatment. Non-Coding RNA 2022, 8, 63. https://doi.org/10.3390/ncrna8050063
Bogard B, Tellier G, Francastel C, Hubé F. A Tool to Design Bridging Oligos Used to Detect Pseudouridylation Sites on RNA after CMC Treatment. Non-Coding RNA. 2022; 8(5):63. https://doi.org/10.3390/ncrna8050063
Chicago/Turabian StyleBogard, Baptiste, Gilles Tellier, Claire Francastel, and Florent Hubé. 2022. "A Tool to Design Bridging Oligos Used to Detect Pseudouridylation Sites on RNA after CMC Treatment" Non-Coding RNA 8, no. 5: 63. https://doi.org/10.3390/ncrna8050063
APA StyleBogard, B., Tellier, G., Francastel, C., & Hubé, F. (2022). A Tool to Design Bridging Oligos Used to Detect Pseudouridylation Sites on RNA after CMC Treatment. Non-Coding RNA, 8(5), 63. https://doi.org/10.3390/ncrna8050063






