Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures
Abstract
:1. Introduction
2. Results and Discussion
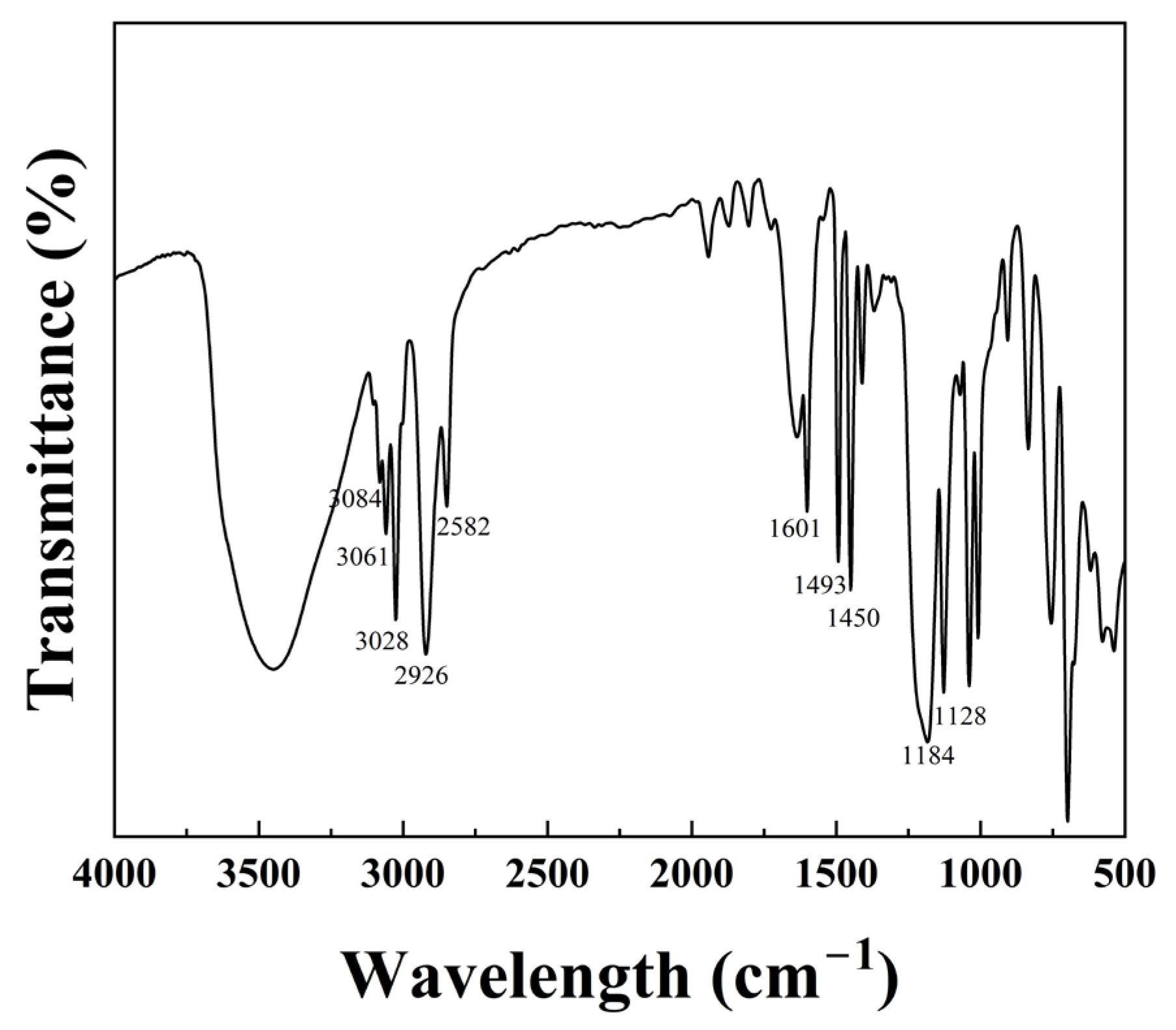
2.1. FTIR
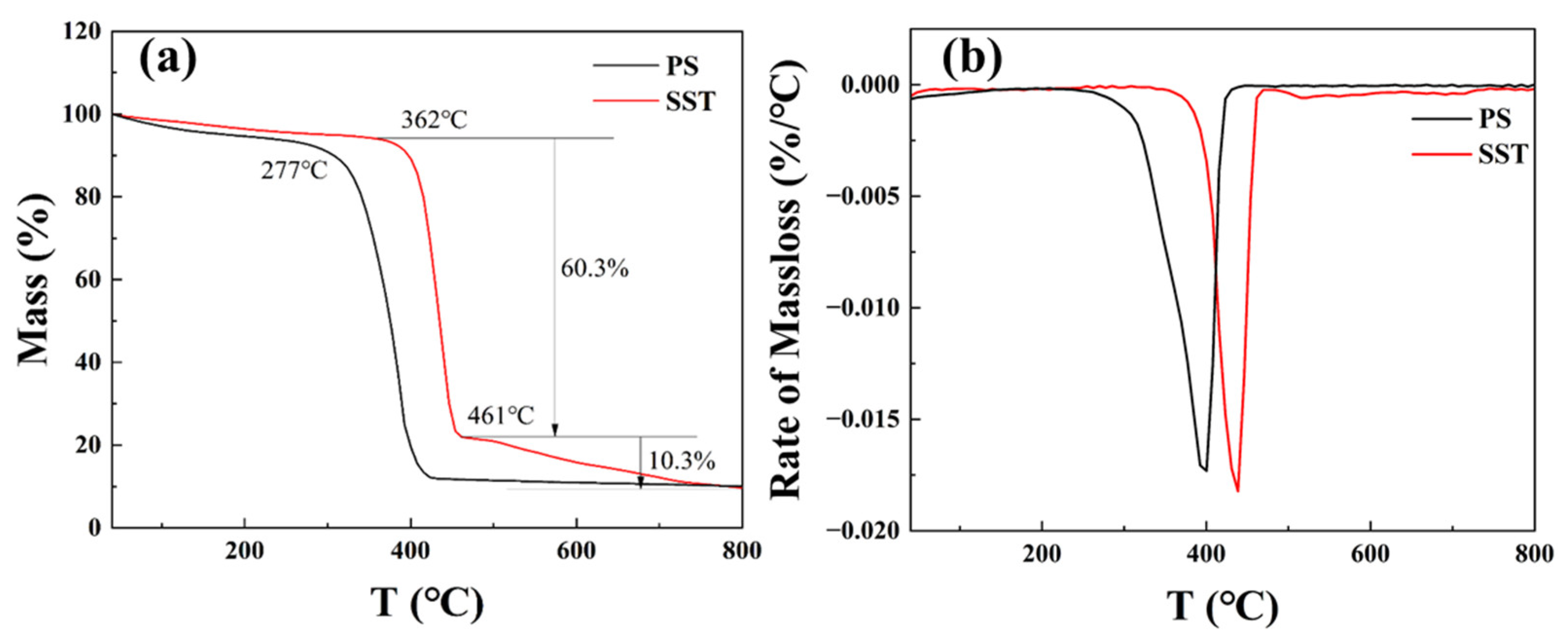
2.2. Thermal Gravimetric Analysis
2.3. Stability Analysis of Aqueous Dispersion Systems
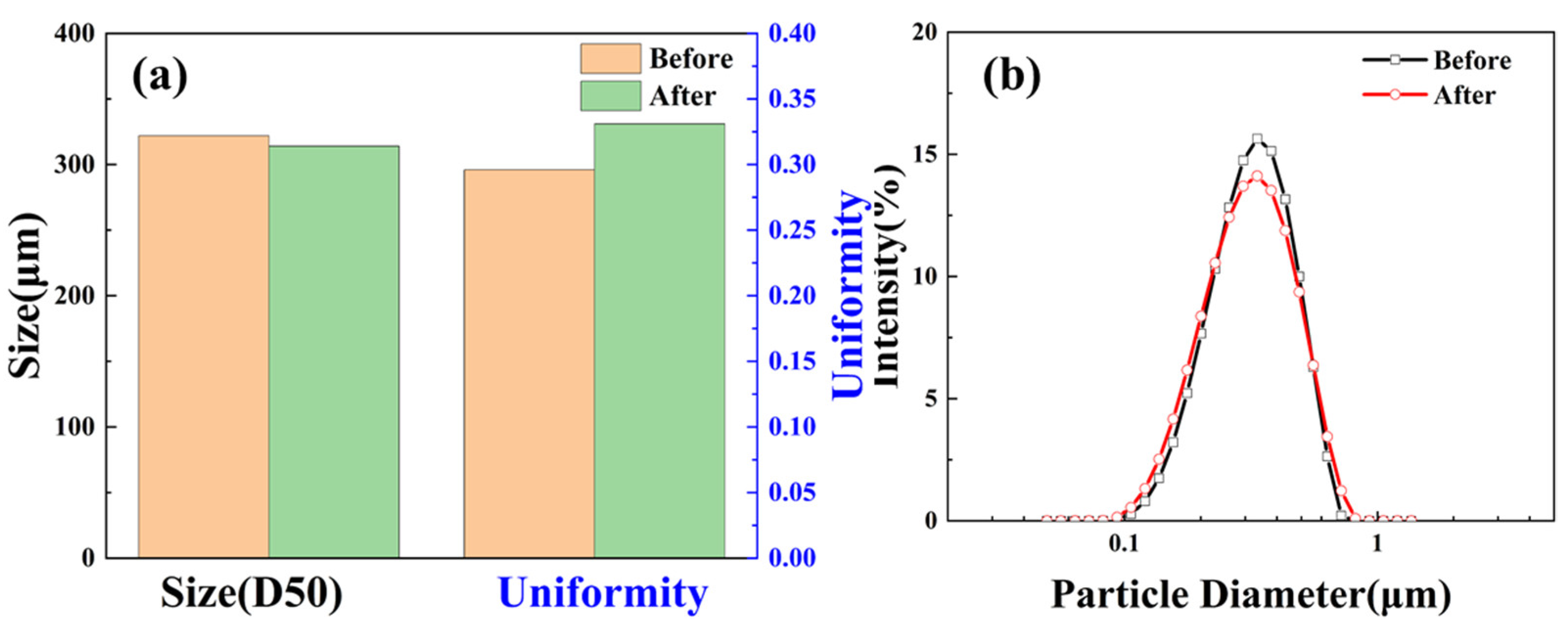
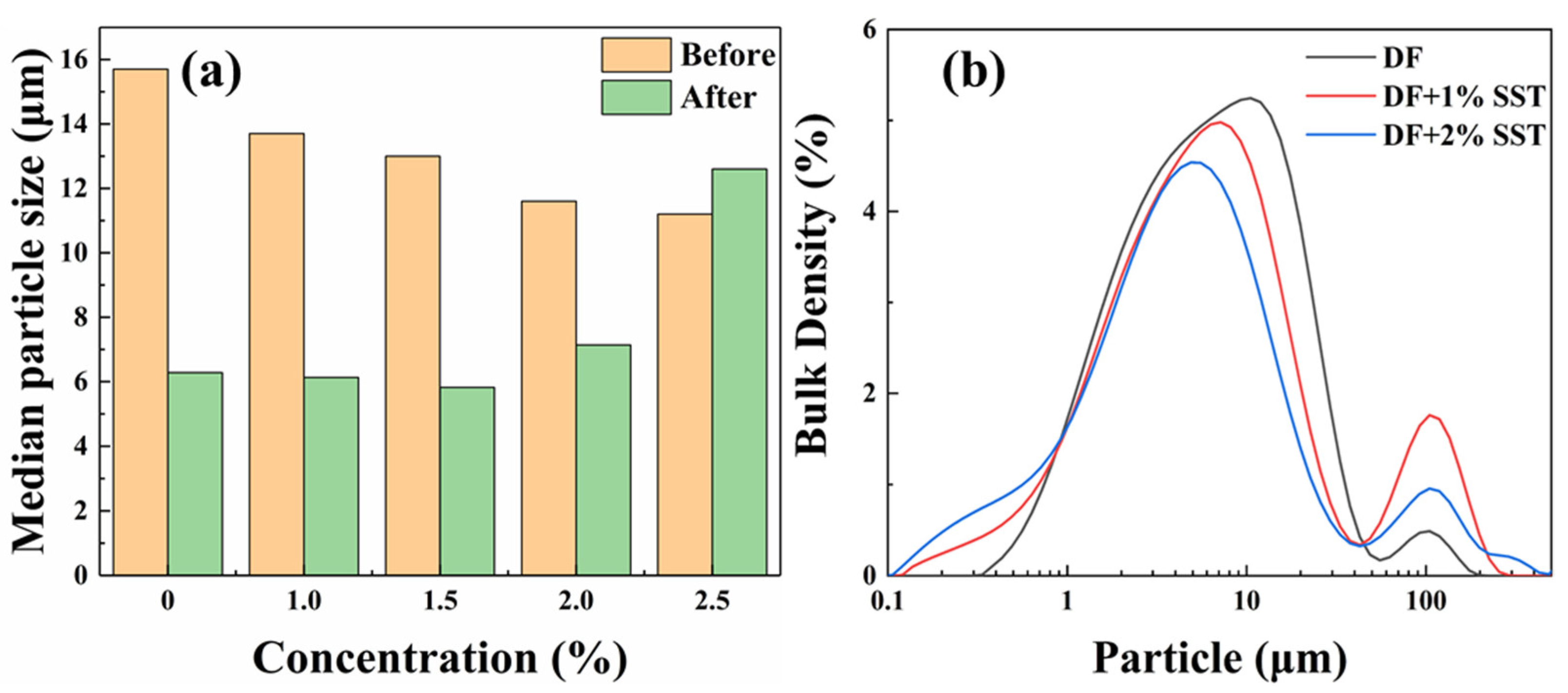
2.3.1. Particle Size
2.3.2. Micromorphology
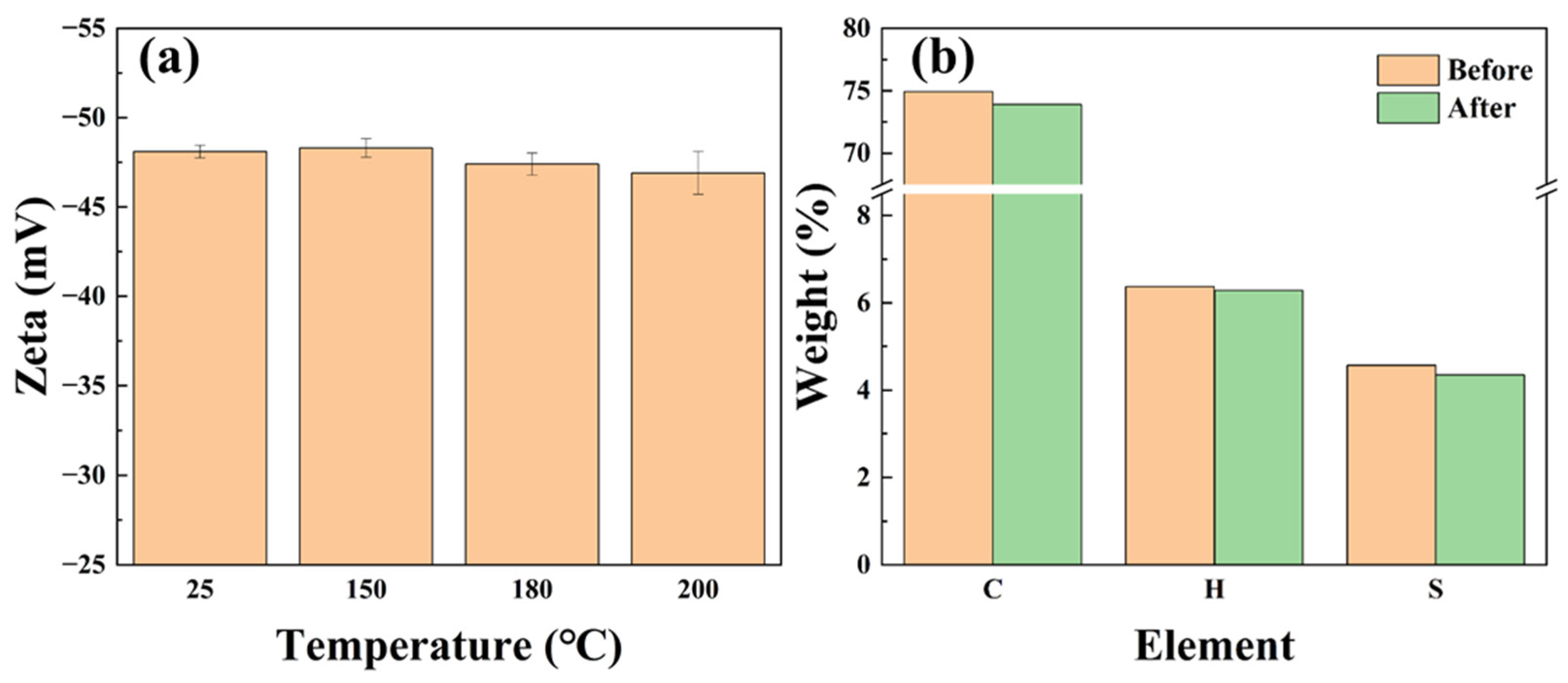
2.3.3. Composition and Dispersion Stability
2.4. Application in Drilling Fluids
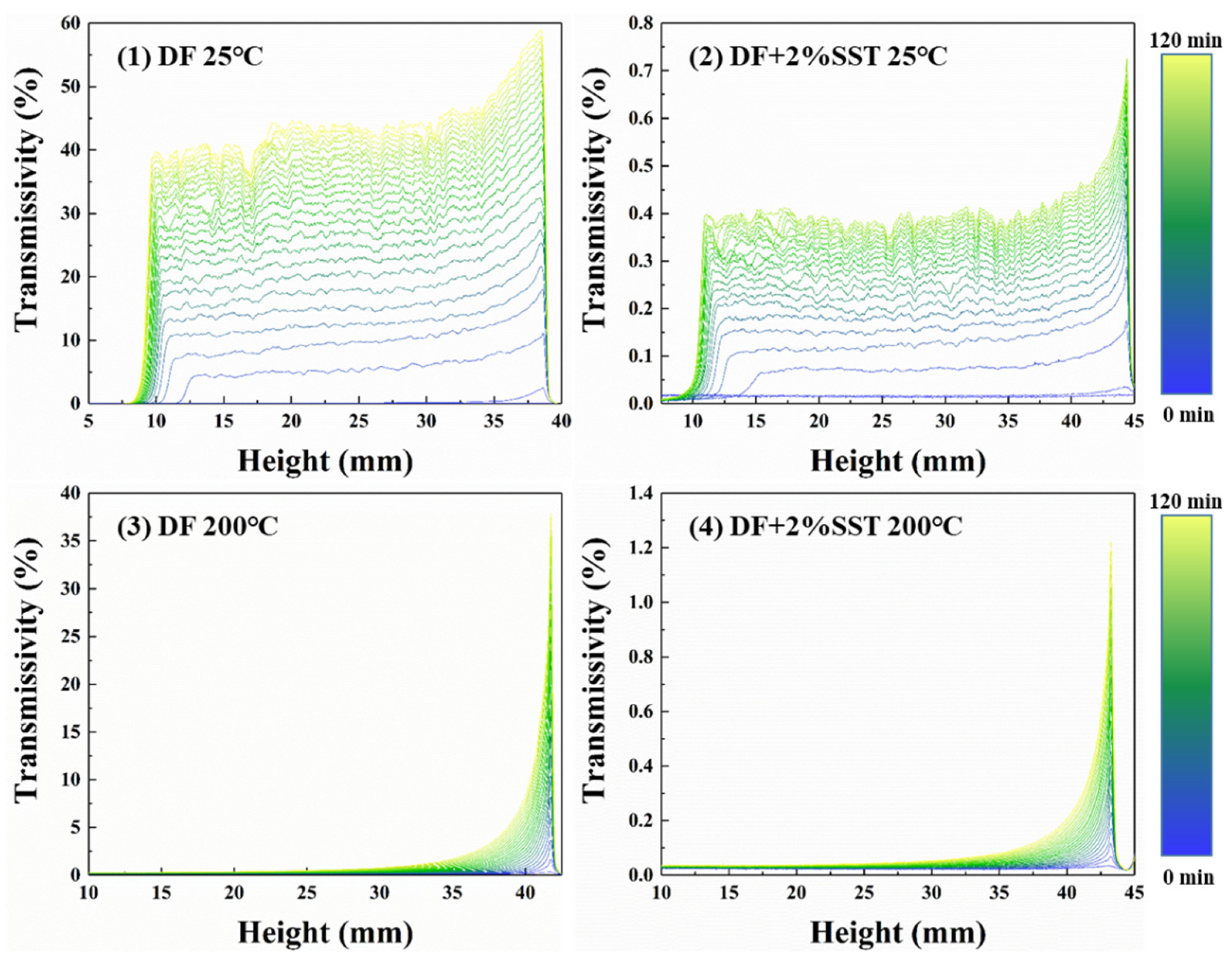
2.4.1. Plugging Performance
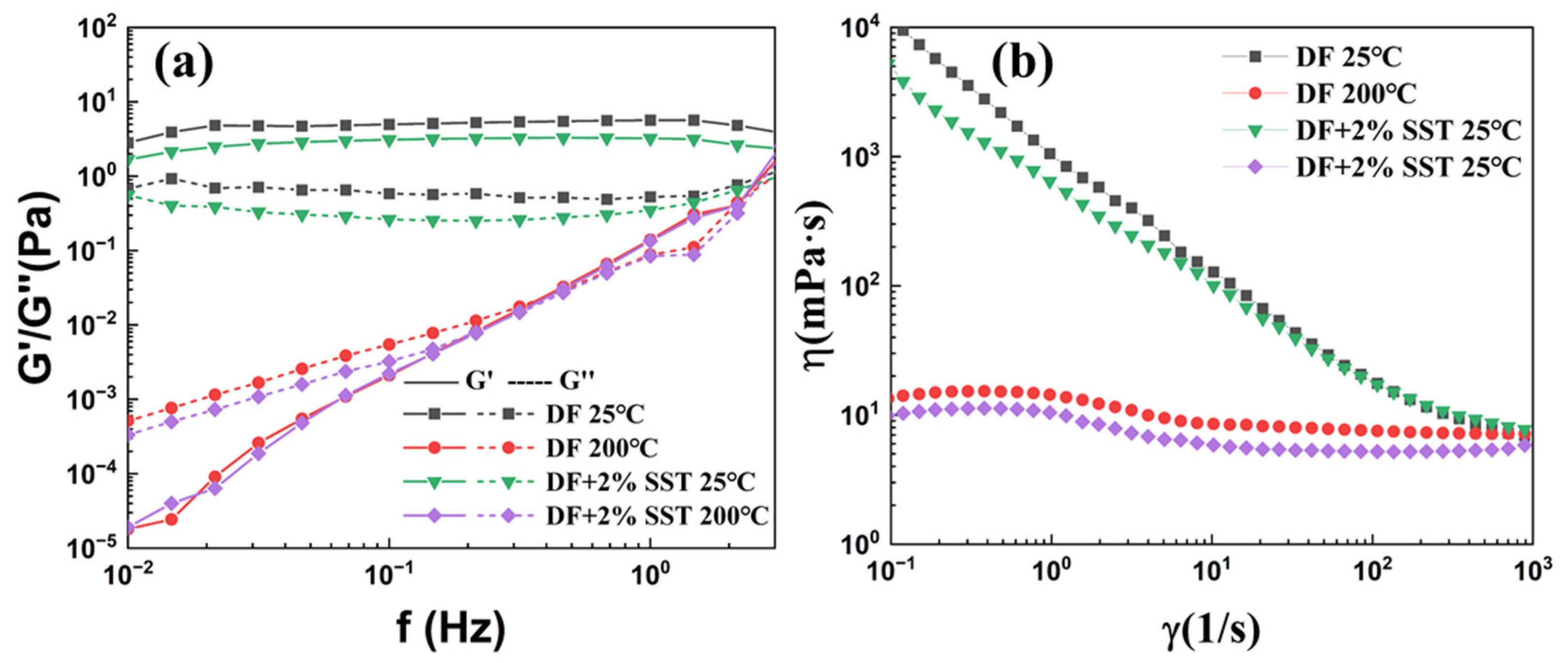
2.4.2. Rheological Properties
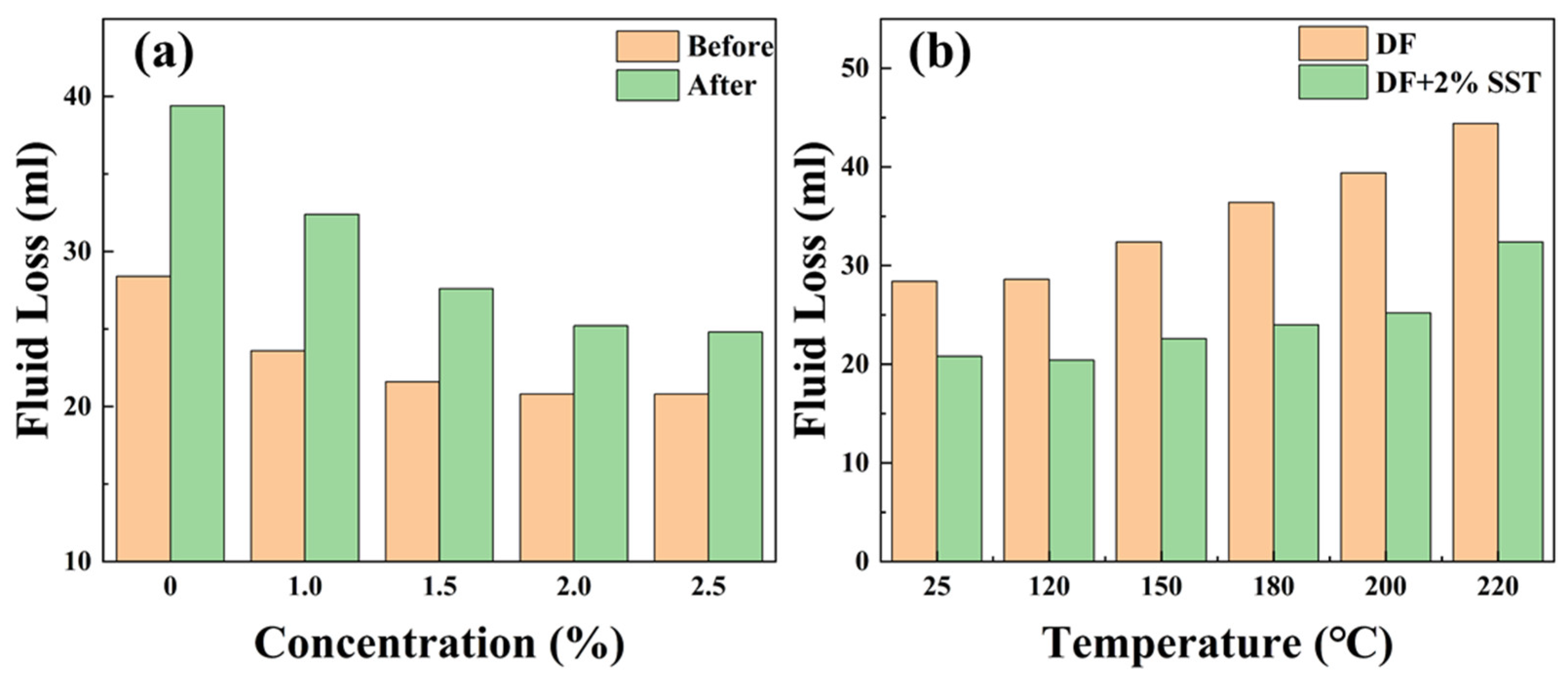
2.4.3. Fluid Loss Reduction
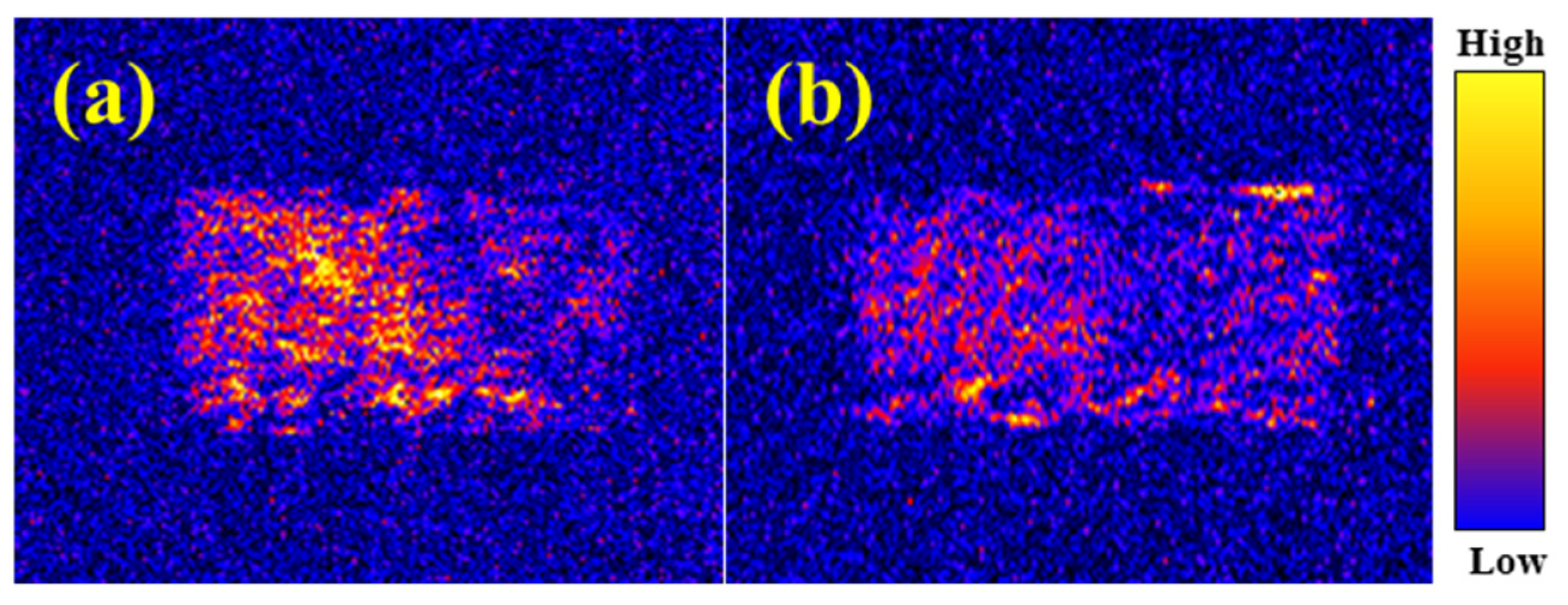
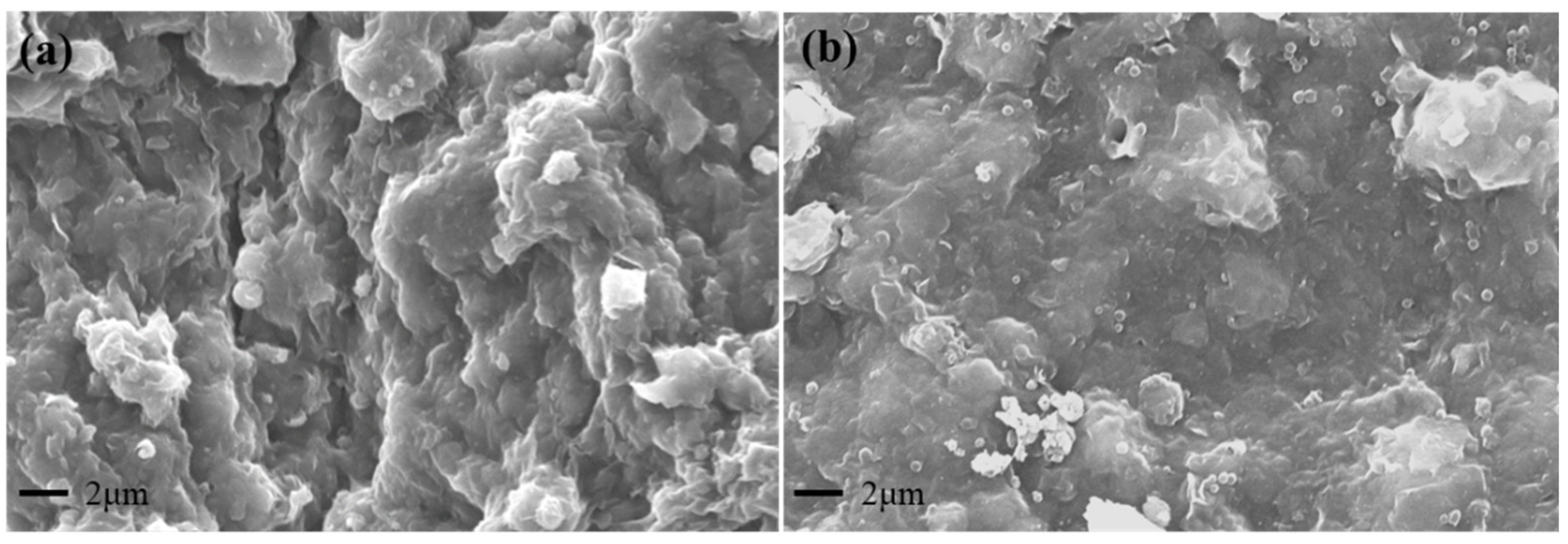
2.4.4. Microscopic Morphology of the Filter Cake
2.4.5. Stability Analysis
2.4.6. Particle Size
3. Conclusions
- A kind of monodisperse hydrophilic microsphere with a particle size of 322 nm was prepared by the copolymerization of sodium styrene sulfonate and styrene, which exhibited a significant core-plugging efficiency of 78.9% and effectively reduced the rock pores.
- Unlike general polystyrene-based microspheres, SST has excellent thermal stability, and it can maintain its stability of particle size, morphology, elemental composition, and zeta potential in an aqueous solution at 200 °C.
- For drilling fluids under 200 °C, SST still improved the filter cake quality, enhanced suspension and dispersion stability, and reduced fluid loss by more than 36%.
- It was found that in drilling fluids, SST achieved improvement in drilling fluid performance in multi-functional ways such as by plugging the micropores of the filter cake, improving the dispersion of the clay, and promoting the particle size grading of the drilling fluids.
4. Materials and Methods
4.1. Materials
4.2. Preparation of SST
4.3. Methods
4.3.1. Drilling Fluid and Hot Rolling
4.3.2. Characterization of SST
4.3.3. Particle Size and Zeta Potential
4.3.4. Evaluation of Plugging Performance
4.3.5. Evaluation of Drilling Fluid Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Dai, Z.; Li, C.; Lv, K.; Huang, X.; Sun, J.; Wei, B. Inhibition of the Hydration Expansion of Sichuan Gas Shale by Adsorption of Compounded Surfactants. Energy Fuels 2019, 33, 6020–6026. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Huang, X.; Bai, Y.; Wang, J.; Jin, J. Synthesis of hydrophobic associative polymers to improve the rheological and filtration performance of drilling fluids under high temperature and high salinity conditions. J. Pet. Sci. Eng. 2022, 209, 109808. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Kalantariasl, A.; Keshavarz, A. Borehole stability in shale formations: Effects of Thermal-Mechanical-Chemical parameters on well design. J. Nat. Gas Sci. Eng. 2021, 88, 103852. [Google Scholar] [CrossRef]
- Gholami, R.; Elochukwu, H.; Fakhari, N.; Sarmadivaleh, M. A review on borehole instability tin active shale formations: Interactions; mechanisms and inhibitors. Earth-Sci. Rev. 2018, 177, 2–13. [Google Scholar] [CrossRef]
- Yan, C.; Dong, L.; Zhao, K.; Cheng, Y.; Li, X.; Deng, J.; Li, Z.; Chen, Y. Time-dependent borehole stability in hard-brittle shale. Pet. Sci. 2022, 19, 663–677. [Google Scholar] [CrossRef]
- Kanfar, M.F.; Chen, Z.; Rahman, S.S. Analyzing wellbore stability in chemically-active anisotropic formations under thermal, hydraulic, mechanical and chemical loadings. J. Nat. Gas Sci. Eng. 2017, 41, 93–111. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Yu, P.; Chen, Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl. Clay Sci. 2017, 150, 267–274. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Liu, J.; Lv, K.; Zhang, F.; Shao, Z.; Dong, X.; Dai, Z.; Zhang, X. Notoginsenoside as an environmentally friendly shale inhibitor in water-based drilling fluid. Pet. Sci. 2022, 19, 608–618. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Chang, X.; Xu, Z.; Zhang, X.; Huang, X.; Liu, J.; Lv, K. A Novel Environment-Friendly Natural Extract for Inhibiting Shale Hydration. Energy Fuels 2019, 33, 7118–7126. [Google Scholar] [CrossRef]
- Cao, Q.; Zhou, W.; Deng, H.; Chen, W. Classification and controlling factors of organic pores in continental shale gas reservoirs based on laboratory experimental results. J. Nat. Gas Sci. Eng. 2015, 27, 1381–1388. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Nguyen, T.A.H.; Bai, B.; Ding, W.; Bao, M. Morphology and Surface Chemistry of Gas-Wetting Nanoparticles and Their Effect on the Liquid Menisci in Porous Media. Ind. Eng. Chem. Res. 2019, 58, 6747–6755. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Nguyen, T.A.H.; Nguyen, A.V.; Wei, M.; Bai, B. The effect of gas-wetting nano-particle on the fluid flowing behavior in porous media. Fuel 2017, 196, 431–441. [Google Scholar] [CrossRef]
- Huang, T.; Cao, L.; Cai, J.; Xu, P. Experimental investigation on rock structure and chemical properties of hard brittle shale under different drilling fluids. J. Pet. Sci. Eng. 2019, 181, 106185. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, J.; Liu, X. Experimental study on crack propagation in shale formations considering hydration and wettability. J. Nat. Gas Sci. Eng. 2015, 23, 492–499. [Google Scholar] [CrossRef]
- Yan, X.; Kang, Y.; You, L.; Xu, C.; Lin, C.; Zhang, J. Drill-in fluid loss mechanisms in brittle gas shale: A case study in the Longmaxi Formation, Sichuan Basin, China. J. Pet. Sci. Eng. 2019, 174, 394–405. [Google Scholar] [CrossRef]
- Khodja, M.; Canselier, J.P.; Bergaya, F.; Fourar, K.; Khodja, M.; Cohaut, N.; Benmounah, A. Shale problems and water-based drilling fluid optimisation in the Hassi Messaoud Algerian oil field. Appl. Clay Sci. 2010, 49, 383–393. [Google Scholar] [CrossRef]
- Feng, Y.; Gray, K.E. Review of fundamental studies on lost circulation and wellbore strengthening. J. Pet. Sci. Eng. 2017, 152, 511–522. [Google Scholar] [CrossRef]
- Ghanbari, S.; Naderifar, A. Enhancing the physical plugging behavior of colloidal silica nanoparticles using binomial size distribution. J. Nat. Gas Sci. Eng. 2016, 30, 213–220. [Google Scholar] [CrossRef]
- Al-Yasiri, M.; Awad, A.; Pervaiz, S.; Wen, D. Influence of silica nanoparticles on the functionality of water-based drilling fluids. J. Pet. Sci. Eng. 2019, 179, 504–512. [Google Scholar] [CrossRef]
- Cao, X.C.; Li, Y.Y.; Ma, G.K.; Ke, K. Application Research of Fibrous Ultrafine TiO2 Particles in Drilling Fluid. Adv. Mater. Res. 2012, 578, 179–182. [Google Scholar] [CrossRef]
- Ghasemi, N.; Mirzaee, M.; Aghayari, R.; Maddah, H. Investigating Created Properties of Nanoparticles Based Drilling Mud. Heat Mass Transf. 2017, 54, 1381–1393. [Google Scholar] [CrossRef]
- Zhai, K.; Yi, H.; Liu, Y.; Geng, Y.; Fan, S.; Zhu, D. Experimental Evaluation of the Shielded Temporary Plugging System Composed of Calcium Carbonate and Acid-Soluble Preformed Particle Gels (ASPPG) for Petroleum Drilling. Energy Fuels 2020, 34, 14023–14033. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Qi, Y.; Ge, Q.; Zhang, L. Nano-fluid loss agent based on an acrylamide based copolymer “grafted” on a modified silica surface. RSC Adv. 2016, 6, 17246–17255. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Lv, K.; Liu, J.; Shen, H.; Zhang, F. Application of core-shell structural acrylic resin/nano-SiO2 composite in water based drilling fluid to plug shale pores. J. Nat. Gas Sci. Eng. 2018, 55, 418–425. [Google Scholar] [CrossRef]
- Lei, S.; Sun, J.; Lv, K.; Zhang, Q.; Yang, J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels 2022, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, J.; Bai, Y.; Lv, K.; Zhang, G.; Li, Y. Status and Prospect of Drilling Fluid Loss and Lost Circulation Control Technology in Fractured Formation. Gels 2022, 8, 260. [Google Scholar] [CrossRef]
- Ma, L.; Luo, P.; He, Y.; Zhang, L.; Fan, Y.; Jiang, Z. Ultra-Stable Silica Nanoparticles as Nano-Plugging Additive for Shale Exploitation in Harsh Environments. Nanomaterials 2019, 9, 1683. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Ning, T.; Luo, Y.; Zhou, S. Preparation, characterization and properties of SiO2 expansible composite microspheres for water-based drilling fluid. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1172–1183. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Qiu, Z.; Zhao, C.; Zhang, X.; Guo, B.; Li, G. Formulation and evaluation of β-cyclodextrin polymer microspheres for improved HTHP filtration control in water-based drilling fluids. J. Mol. Liq. 2020, 313, 113549. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, Z.; Huang, W.; Zhao, X. Preparation and performance properties of polymer latex SDNL in water-based drilling fluids for drilling troublesome shale formations. J. Nat. Gas Sci. Eng. 2017, 37, 462–470. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, Z.; Zhao, X.; Mou, T.; Zhong, H.; Huang, W. A polymer microsphere emulsion as a high-performance shale stabilizer for water-based drilling fluids. Rsc Adv. 2018, 8, 20852–20861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, G.; Dong, T.; Wang, L.; Li, X.; Wang, G. An amphoteric polymer as a shale borehole stabilizer in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 170, 112–120. [Google Scholar] [CrossRef]
- Huang, X.; Meng, X.; Lv, K.; Zhang, Z.; Cao, L.; Wang, R.; Feng, J.; Wu, Y.; Sheng, W. Development of a high temperature resistant nano-plugging agent and the plugging performance of multi-scale micropores. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128275. [Google Scholar] [CrossRef]
- Cheng, R.; Lei, Z.; Bai, Y.; Zhang, J.; Hao, H.; Xie, G. Preparation of the Tetrameric Poly(VS-St-BMA-BA) Nano-Plugging Agent and Its Plugging Mechanism in Water-Based Drilling Fluids. ACS Omega 2022, 7, 28304–28312. [Google Scholar] [CrossRef]
- Li, H.; Lv, K.; Huang, X.; Lu, Z.; Dong, X. The Synthesis of Polymeric Nanospheres and the Application as High-Temperature Nano-Plugging Agent in Water Based Drilling Fluid. Front. Chem. 2020, 8, 247. [Google Scholar] [CrossRef]
- Caroline, L.; Patrick, C.; Christine, V.; Dominique, C.; Ruxandra, G. Study of emulsion stabilization by graft copolymers using the optical analyzer Turbiscan. Int. J. Pharm. 2003, 254, 77–82. [Google Scholar]
- Li, X.; Huang, W.; Sun, J.; Li, X.; Jia, J.; Duan, W.; Sun, J. Wettability alteration and mitigating aqueous phase trapping damage in tight gas sandstone reservoirs using mixed cationic surfactant/nonionic fluoro-surfactant solution. J. Pet. Sci. Eng. 2020, 195, 107490. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, H.; Kang, X.; Jiang, H.; Li, M.; Kang, W.; Sarsenbekuly, B. Study on the types and formation mechanisms of residual oil after two surfactant imbibition. J. Pet. Sci. Eng. 2020, 195, 107904. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhang, W.; Lv, K.; Bai, Y.; Wang, J.; Huang, X.; Jin, J.; Sun, J. Carbon nanotube enhanced water-based drilling fluid for high temperature and high salinity deep resource development. Pet. Sci. 2022, 19, 916–926. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Jin, J.; Zhou, X.; Dai, Z. Environmentally friendly and salt-responsive polymer brush based on lignin nanoparticle as fluid-loss additive in water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126482. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, J.; Sun, J.; Lv, K.; Wang, Z.; Xu, Z.; Sun, Y. Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures. Gels 2023, 9, 763. https://doi.org/10.3390/gels9090763
Zhang X, Liu J, Sun J, Lv K, Wang Z, Xu Z, Sun Y. Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures. Gels. 2023; 9(9):763. https://doi.org/10.3390/gels9090763
Chicago/Turabian StyleZhang, Xianfa, Jingping Liu, Jinsheng Sun, Kaihe Lv, Zonglun Wang, Zhe Xu, and Yuanwei Sun. 2023. "Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures" Gels 9, no. 9: 763. https://doi.org/10.3390/gels9090763
APA StyleZhang, X., Liu, J., Sun, J., Lv, K., Wang, Z., Xu, Z., & Sun, Y. (2023). Novel Modified Styrene-Based Microspheres for Enhancing the Performance of Drilling Fluids at High Temperatures. Gels, 9(9), 763. https://doi.org/10.3390/gels9090763




