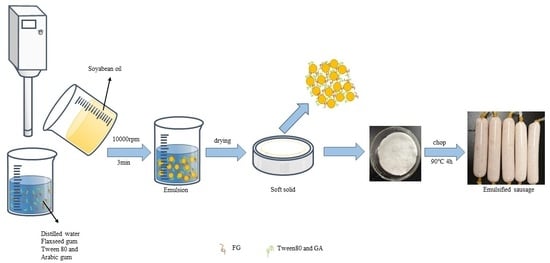
Flaxseed Gum/Arabic Gum/Tween 80-Based Oleogel as a Fat Substitute Applied in Emulsified Sausage: Physicochemical Properties, Sensory Attributes and Nutritional Quality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size and Microstructure of O/W Emulsions
2.2. Rheological Characterization of Emulsions and Oleogels
2.3. Microstructure and Oil Loss of Oleogels
2.4. Chemical Characterization by ATR-FTIR Spectroscopy
2.5. Characterization of Emulsified Sausage
2.5.1. Proximate Composition and Color
2.5.2. Texture Profile Analysis (TPA)
2.5.3. Water-Holding Capacity, Cooking Loss and Microstructure of Emulsified Sausages
2.5.4. Fatty Acid Profiles of Emulsified Sausages
3. Conclusions
4. Material and Methods
4.1. Materials
4.2. Preparation of Emulsions
4.3. Characterization of Emulsions
4.3.1. Droplet Size Distribution
4.3.2. Microstructural Observation
4.3.3. Rheological Properties of Emulsions
4.4. Preparation and Characterization of Oleogels
4.4.1. Preparation of Oleogels by Emulsion-Templated Approach
4.4.2. Microstructural Observation
4.4.3. Viscoelastic Properties
4.4.4. ATR-FTIR
4.4.5. Oil loss Analysis
4.5. Preparation of Emulsified Sausage
4.6. Proximate Composition and Color Determination
4.7. Texture Profile Analysis of Emulsified Sausages
4.8. Water-Holding Capacity
4.9. Cooking Loss
4.10. Microstructure of Emulsified Sausage
4.11. Fatty Acid Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bascuas, S.; Morell, P.; Hernando, I.; Quiles, A. Recent trends in oil structuring using hydrocolloids. Food Hydrocoll. 2021, 118, 106612. [Google Scholar] [CrossRef]
- Bolger, Z.; Brunton, N.P.; Monahan, F.J. Impact of inclusion of flaxseed oil (pre-emulsified or encapsulated) on the physical characteristics of chicken sausages. J. Food Eng. 2018, 230, 39–48. [Google Scholar] [CrossRef]
- Wolfer, T.L.; Acevedo, N.C.; Prusa, K.J.; Sebranek, J.G.; Tarté, R. Replacement of pork fat in frankfurter-type sausages by soybean oil oleogels structured with rice bran wax. Meat Sci. 2018, 145, 352–362. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Alejandre, M.; Astiasarán, I.; Ansorena, D.; Barbut, S. Using canola oil hydrogels and organogels to reduce saturated animal fat in meat batters. Food Res. Int. 2019, 122, 129–136. [Google Scholar] [CrossRef]
- Ferro, A.C.; de Souza Paglarini, C.; Pollonio, M.A.R.; Cunha, R.L. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2020, 174, 108424. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Dewettinck, K. Comparative evaluation of structured oil systems: Shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid Sci. Technol. 2015, 117, 1772–1781. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT 2020, 126, 109277. [Google Scholar] [CrossRef]
- Oh, I.K.; Lee, S. Utilization of foam structured hydroxypropyl methylcellulose for oleogels and their application as a solid fat replacer in muffins. Food Hydrocoll. 2018, 77, 796–802. [Google Scholar] [CrossRef]
- Espert, M.; Hernández, M.J.; Sanz, T.; Salvador, A. Rheological properties of emulsion templated oleogels based on xanthan gum and different structuring agents. Curr. Res. Food Sci. 2022, 5, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.M.; Wei, W.; Zhang, L.J.; Meng, J.; Sui, W.J.; Wu, T.; Li, J.L.; Wang, P.; Zhang, M. Fabrication and characterization of gel-in-oil-water (G/O/W) double emulsion stabilized by flaxseed gum/whey protein isolate complexes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129566. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, M.; Sharma, H. Span 80-Tween 80 based fluid-filled organogel for topical delivery of fluconazole. Int. J. Sci. Res. Rev. 2014, 3, 29–46. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.Y.; Feng, M.Q.; Xu, X.L.; Zhou, G.H. Characterization of olive oil emulsions stabilized by flaxseed gum. J. Food Eng. 2019, 247, 74–79. [Google Scholar] [CrossRef]
- Liu, W.Y.; Feng, M.Q.; Wang, M.; Wang, P.; Sun, J.; Xu, X.L.; Zhou, G.H. Influence of flaxseed gum and NaCl concentrations on the stability of oil-in-water emulsions. Food Hydrocoll. 2018, 79, 371–381. [Google Scholar] [CrossRef]
- Walstra, P. Food emulsions: Principles, practice, and techniques. Trends Food Sci. Technol. 1999, 10, 714. [Google Scholar] [CrossRef]
- Ikeda, S.; Nishinari, K. “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated κ-carrageenan helices. J. Agric. Food Chem. 2001, 49, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, P.; Ji, M.; Du, L.; Li, S.; Liu, Y.; Meng, Z. Synergetic effects of water-soluble polysaccharides for intensifying performances of oleogels fabricated by oil-absorbing cryogels. Food Chem. 2022, 372, 131357. [Google Scholar] [CrossRef]
- Lupi, F.R.; Greco, V.; Baldino, N.; Cindio, B.D.; Fischer, P.; Gabriele, D. The effects of intermolecular interactions on the physical properties of organogels in edible oils. J. Colloid Interface Sci. 2016, 483, 154–164. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Ye, F.Y.; Chen, J.; Ming, J.; Zhou, C.Q.; Zhao, G.H.; Lei, L. The microstructure and gel properties of linseed oil and soy protein isolate based-oleogel constructed with highland barley β-glucan and its application in luncheon meat. Food Hydrocoll. 2023, 140, 108666. [Google Scholar] [CrossRef]
- Cynthia, F.C.; Marta, M.S.; Pilar, G.C.; Visitación, C.M.; Samuel, V.; Raúl, G.; Amparo, L.R. Polysaccharide-based emulsion gels as fat replacers in Frankfurter sausages: Physicochemical, nutritional and sensorial evaluation. LWT 2023, 180, 114705. [Google Scholar] [CrossRef]
- Silva, S.L.D.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; Franzen, F.D.L.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2018, 149, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.; Barbut, S. Fat reduction in comminuted meat products-effects of beef fat, regular and pre-emulsified canola oil. Meat Sci. 2010, 87, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.T.D.O.; Lorenzo, J.M.; Santos, B.A.D.; Fagundes, M.B.; Heck, R.T.; Cichoski, A.; Wagner, R.; Campagnol, P.C.B. Pork skin and canola oil as strategy to confer technological and nutritional advantages to burgers. Czech J. Food Sci. 2017, 36, 352–359. [Google Scholar] [CrossRef]
- Ruifan, Z.; Tao, Z.; Mengyue, H.; Yong, X.; Changhu, X. Effects of oleogels prepared with fish oil and beeswax on the gelation behaviors of protein recovered from Alaska Pollock. LWT 2020, 137, 110423. [Google Scholar] [CrossRef]
- Asuming-Bediako, N.; Jaspal, M.H.; Hallett, K.; Bayntun, J.; Baker, A.; Sheard, P.R. Effects of replacing pork backfat with emulsified vegetable oil on fatty acid composition and quality of UK-style sausages. Meat Sci. 2014, 96, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Xie, Y.Y.; Li, X.M.; Liu, Y.; Yan, W.J. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef]
- Deray, S.; Hüdayi, E.; Ekin, Ş. Hazelnut as functional food component and fat replacer in fermented sausage. J. Food Sci. Technol. 2018, 55, 3385–3390. [Google Scholar] [CrossRef]
- Geng, X.Y.; Zhao, Y.; Zhao, N.; Zhu, Q.M.; Zhang, M. Quality characteristics and gastrointestinal fate of low fat emulsified sausage formulated with konjac glucomannan/oat β-glucan composite hydrogel. Int. J. Biol. Macromol. 2023, 239, 124251. [Google Scholar] [CrossRef] [PubMed]
- Juhui, C.; Hack-Youn, K. Quality characteristics of reduced fat emulsion-type chicken sausages using chicken skin and wheat fiber mixture as fat replacer. Poult. Sci. 2019, 98, 2662–2669. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, H.Y.; Lee, J.M.; Kim, Y.J.; Kim, C.J. Quality of frankfurter-type sausages with added pig skin and wheat fiber mixture as fat replacers. Meat Sci. 2013, 93, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Berker, N.; Burcu, Ö.-K.; Dilay, Y.; Özlem, Ç.; Meltem, S. Peanut and linseed oil emulsion gels as potential fat replacer in emulsified sausages. Meat Sci. 2021, 176, 108464. [Google Scholar] [CrossRef]









| Control | R-25 | R-50 | R-75 | R-100 | |
|---|---|---|---|---|---|
| Moisture(%) | 60.15 ± 0.33 e | 60.73 ± 0.18 d | 61.63 ± 0.31 c | 62.74 ± 0.20 b | 63.24 ± 0.25 a |
| Protein(%) | 30.25 ± 0.30 c | 31.15 ± 0.26 ab | 30.80 ± 0.14 b | 31.33 ± 0.32 a | 30.71 ± 0.26 bc |
| Fat(%) | 28.53 ± 0.24 e | 21.55 ± 0.25 d | 14.55 ± 0.07 c | 7.55 ± 0.12 b | 1.52 ± 0.06 a |
| L* | 66.47 ± 0.38 d | 68.24 ± 0.40 c | 73.39 ± 0.12 b | 74.76 ± 0.09 a | 75.04 ± 0.18 a |
| a* | 7.27 ± 0.13 a | 6.75 ± 0.11 b | 6.48 ± 0.10 bc | 6.57 ± 0.17 bc | 6.52 ± 0.11 bc |
| b* | 12.67 ± 0.41 c | 12.50 ± 0.08 c | 12.77 ± 0.07 bc | 13.39 ± 0.27 a | 13.12 ± 0.12 ab |
| Whiteness | 63.42 ± 0.56 d | 65.20 ± 0.43 c | 69.78 ± 0.11 b | 70.68 ± 0.21 a | 71.05 ± 0.16 a |
| Control | R-25 | R-50 | R-75 | R-100 | |
|---|---|---|---|---|---|
| C4:0 | 1.22 ± 0.11 a | 1.18 ± 0.17 a | 0.95 ± 0.07 b | 0.93 ± 0.09 b | 0.71 ± 0.04 c |
| C10:0 | 0.15 ± 0.04 a | 0.12 ± 0.03 ab | 0.11 ± 0.01 ab | 0.09 ± 0.01 b | 0.07 ± 0.01 b |
| C11:0 | 0.14 ± 0.03 a | 0.13 ± 0.03 a | 0.10 ± 0.02 ab | 0.09 ± 0.01 bc | 0.06 ± 0.01 ac |
| C13:0 | 0.09 ± 0.00 b | 0.12 ± 0.05 b | 0.08 ± 0.01 b | 0.22 ± 0.07 a | 0.06 ± 0.00 b |
| C14:0 | 1.10 ± 0.07 a | 0.41 ± 0.09 b | 0.27 ± 0.01 c | 0.18 ± 0.03 cd | 0.13 ± 0.06 d |
| C16:0 | 12.11 ± 1.04 a | 8.64 ± 0.45 b | 5.23 ± 0.52 c | 4.31 ± 0.56 c | 2.09 ± 0.16 d |
| C17:0 | 21.56 ± 4.67 b | 29.54 ± 1.91 a | 22.74 ± 1.28 b | 13.38 ± 0.77 c | 8.29 ± 1.40 d |
| C18:1n9t | 3.75 ± 0.43 a | 2.19 ± 0.21 b | 1.58 ± 0.19 c | 1.30 ± 0.05 c | 0.66 ± 0.14 d |
| C18:0 | 14.11 ± 1.85 a | 9.43 ± 0.37 b | 8.87 ± 0.55 b | 5.39 ± 0.65 c | 3.70 ± 0.37 c |
| C18:2n6c | 5.01 ± 0.86 e | 15.97 ± 0.99 d | 27.69 ± 1.54 c | 38.43 ± 0.88 b | 50.26 ± 3.07 a |
| C20:0 | 27.19 ± 1.68 a | 17.73 ± 1.16 b | 13.91 ± 0.80 c | 8.96 ± 0.75 d | 2.45 ± 0.58 d |
| C18:3n6 | 9.35 ± 0.38 a | 5.44 ± 0.03 b | 4.09 ± 0.26 c | 3.62 ± 0.30 c | 2.42 ± 0.34 e |
| C18:1n9c | 2.15 ± 0.37 e | 6.84 ± 0.22 d | 11.87 ± 0.66 c | 20.69 ± 1.47 b | 27.42 ± 1.25 a |
| C22:0 | 1.16 ± 0.07 c | 1.61 ± 0.11 c | 2.29 ± 0.18 b | 3.16 ± 0.42 a | 2.90 ± 0.45 a |
| C22:6n3 | 0.92 ± 0.04 a | 0.65 ± 0.12 b | 0.65 ± 0.11 b | 0.62 ± 0.05 b | 0.33 ± 0.14 c |
| ∑SFA | 57.04 ± 2.95 a | 39.13 ± 0.79 b | 31.64 ± 1.45 c | 23.03 ± 0.98 d | 12.05 ± 0.88 e |
| ∑MUFA | 5.09 ± 0.75 e | 9.03 ± 0.51 d | 13.44 ± 0.80 c | 21.99 ± 0.52 b | 28.08 ± 1.21 a |
| ∑PUFA | 14.35 ± 1.13 e | 21.40 ± 1.02 d | 31.78 ± 1.29 c | 42.05 ± 1.09 b | 52.68 ± 2.74 a |
| ∑UFA | 21.17 ± 1.92 a | 31.09 ± 1.50 b | 45.44 ± 2.41 c | 63.30 ± 1.53 d | 79.54 ± 2.13 e |
| PUFA/SFA | 0.25 ± 0.01 d | 0.55 ± 0.02 d | 1.01 ± 0.09 c | 1.83 ± 0.12 b | 4.37 ± 0.53 a |
| n-6/n-3 | 0.53 ± 0.08 d | 2.94 ± 0.17 d | 6.76 ± 0.81 c | 10.61 ± 0.79 b | 20.80 ± 4.29 a |
| IA | 1.51 ± 0.01 a | 0.65 ± 0.05 b | 0.34 ± 0.04 c | 0.16 ± 0.01 d | 0.08 ± 0.00 e |
| IT | 0.57 ± 0.02 a | 0.29 ± 0.02 b | 0.16 ± 0.02 c | 0.08 ± 0.00 d | 0.04 ± 0.00 e |
| Control | R-25 | R-50 | R-75 | R-100 | |
|---|---|---|---|---|---|
| Lean meat | 40 g | 40 g | 40 g | 40 g | 40 g |
| Pork back fat | 10 g | 7.5 g | 5 g | 2.5 g | 0 |
| Fat replacer | 0 g | 2.5 g | 5 g | 7.5 g | 10 g |
| Ice water | 15 g | 14.25 g | 13.5 g | 12.75 g | 12 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Chen, F.; Li, P.; Wu, T.; Pan, Y.; Zhang, M. Flaxseed Gum/Arabic Gum/Tween 80-Based Oleogel as a Fat Substitute Applied in Emulsified Sausage: Physicochemical Properties, Sensory Attributes and Nutritional Quality. Gels 2023, 9, 759. https://doi.org/10.3390/gels9090759
Zhu Q, Chen F, Li P, Wu T, Pan Y, Zhang M. Flaxseed Gum/Arabic Gum/Tween 80-Based Oleogel as a Fat Substitute Applied in Emulsified Sausage: Physicochemical Properties, Sensory Attributes and Nutritional Quality. Gels. 2023; 9(9):759. https://doi.org/10.3390/gels9090759
Chicago/Turabian StyleZhu, Qiaomei, Fu Chen, Peiyang Li, Tao Wu, Yijun Pan, and Min Zhang. 2023. "Flaxseed Gum/Arabic Gum/Tween 80-Based Oleogel as a Fat Substitute Applied in Emulsified Sausage: Physicochemical Properties, Sensory Attributes and Nutritional Quality" Gels 9, no. 9: 759. https://doi.org/10.3390/gels9090759
APA StyleZhu, Q., Chen, F., Li, P., Wu, T., Pan, Y., & Zhang, M. (2023). Flaxseed Gum/Arabic Gum/Tween 80-Based Oleogel as a Fat Substitute Applied in Emulsified Sausage: Physicochemical Properties, Sensory Attributes and Nutritional Quality. Gels, 9(9), 759. https://doi.org/10.3390/gels9090759