Development and Gelation Mechanism of Ultra-High-Temperature-Resistant Polymer Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization of the Synthesized Polymer
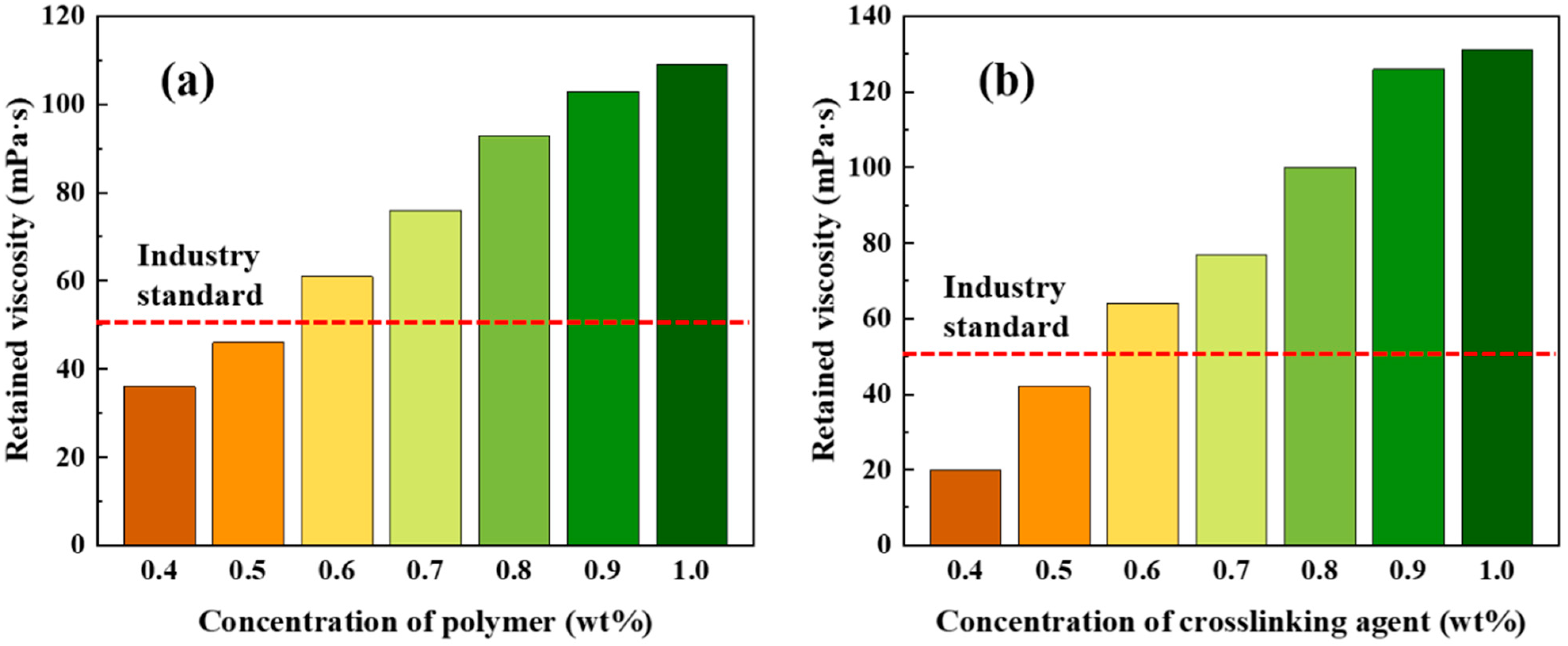
2.2. Concentration Optimization of Polymer and Crosslinking Agent
2.3. Rheological Properties of the Polymer Gel
2.3.1. Steady Shear Property
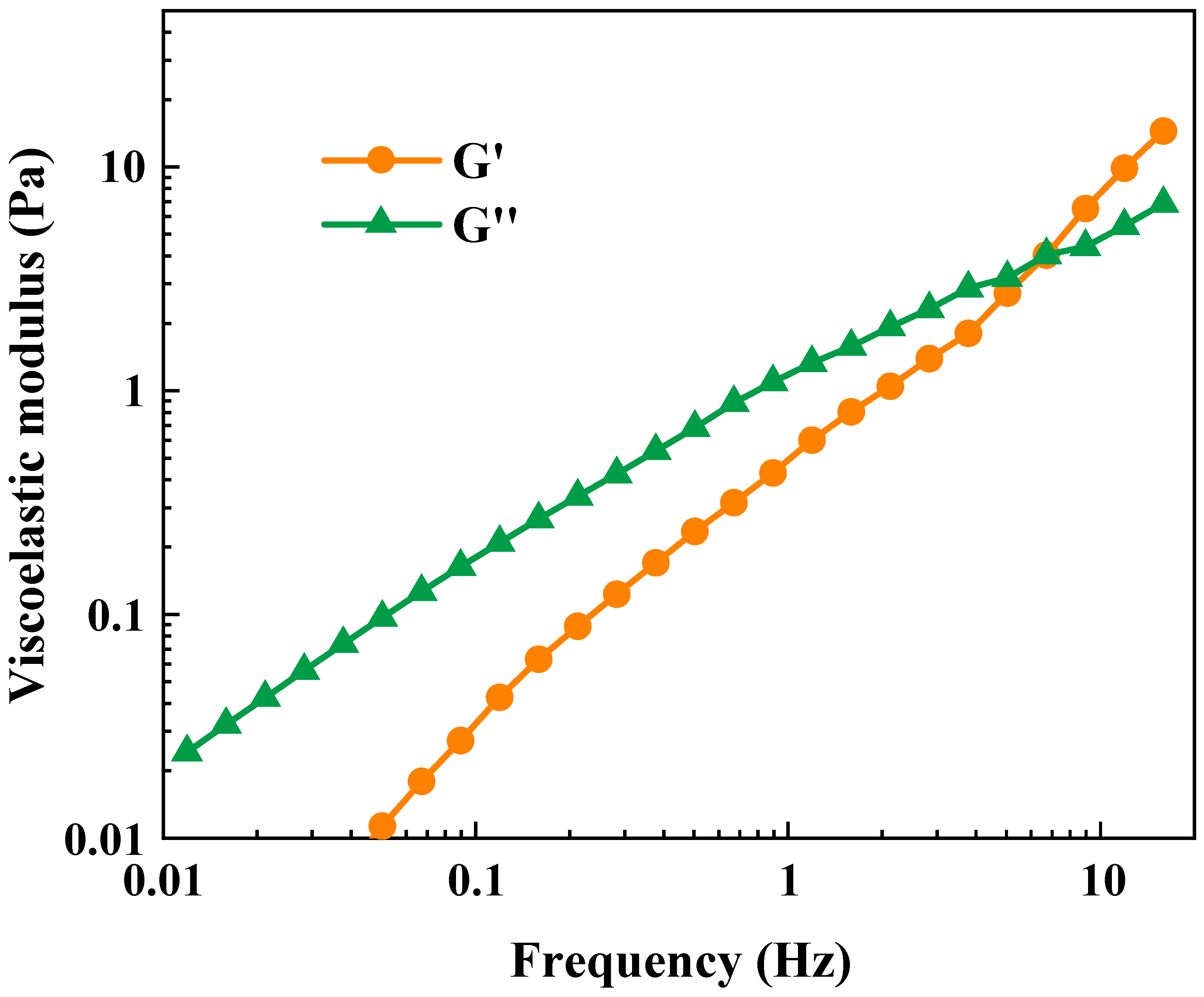
2.3.2. Dynamic Viscoelasticity
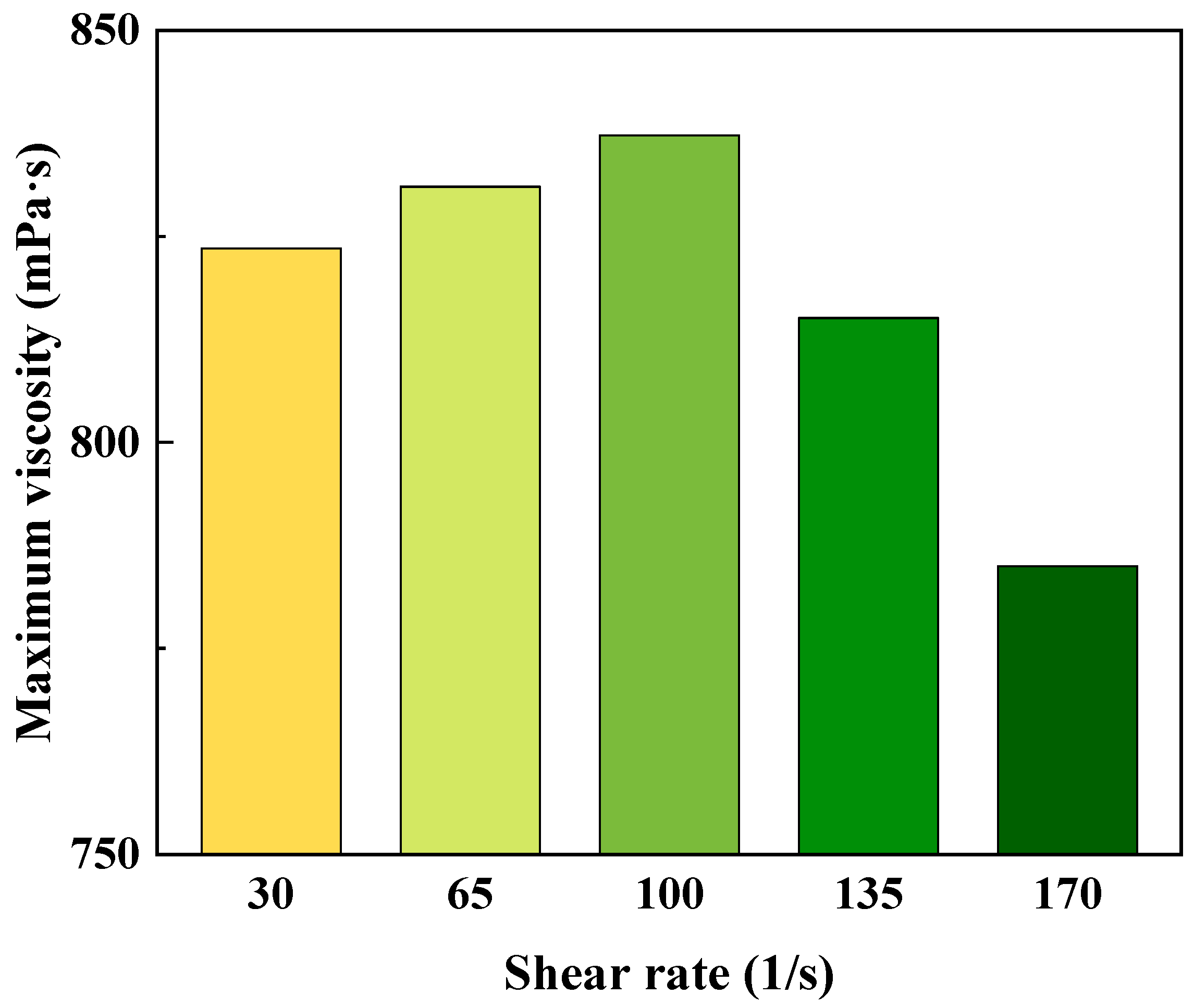
2.3.3. Temperature and Shear Resistance
2.4. Core Damage Performance
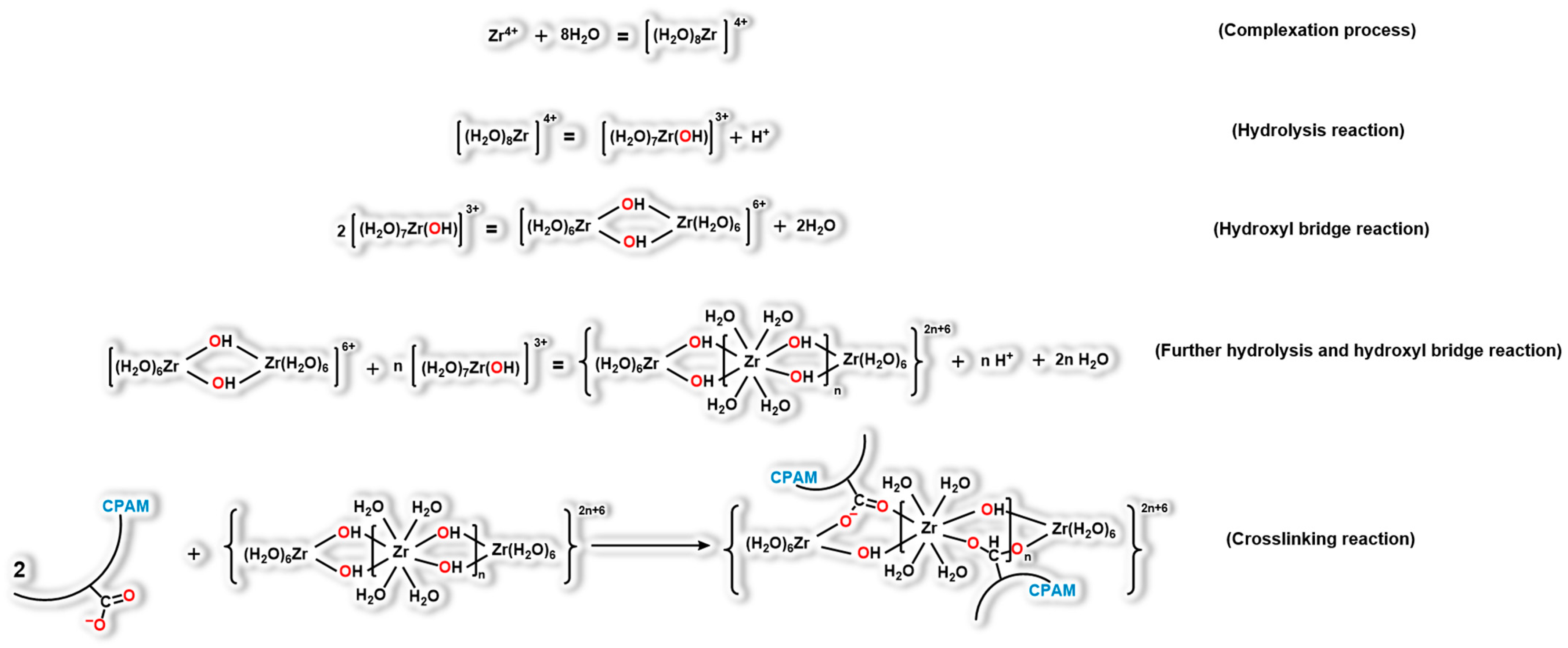
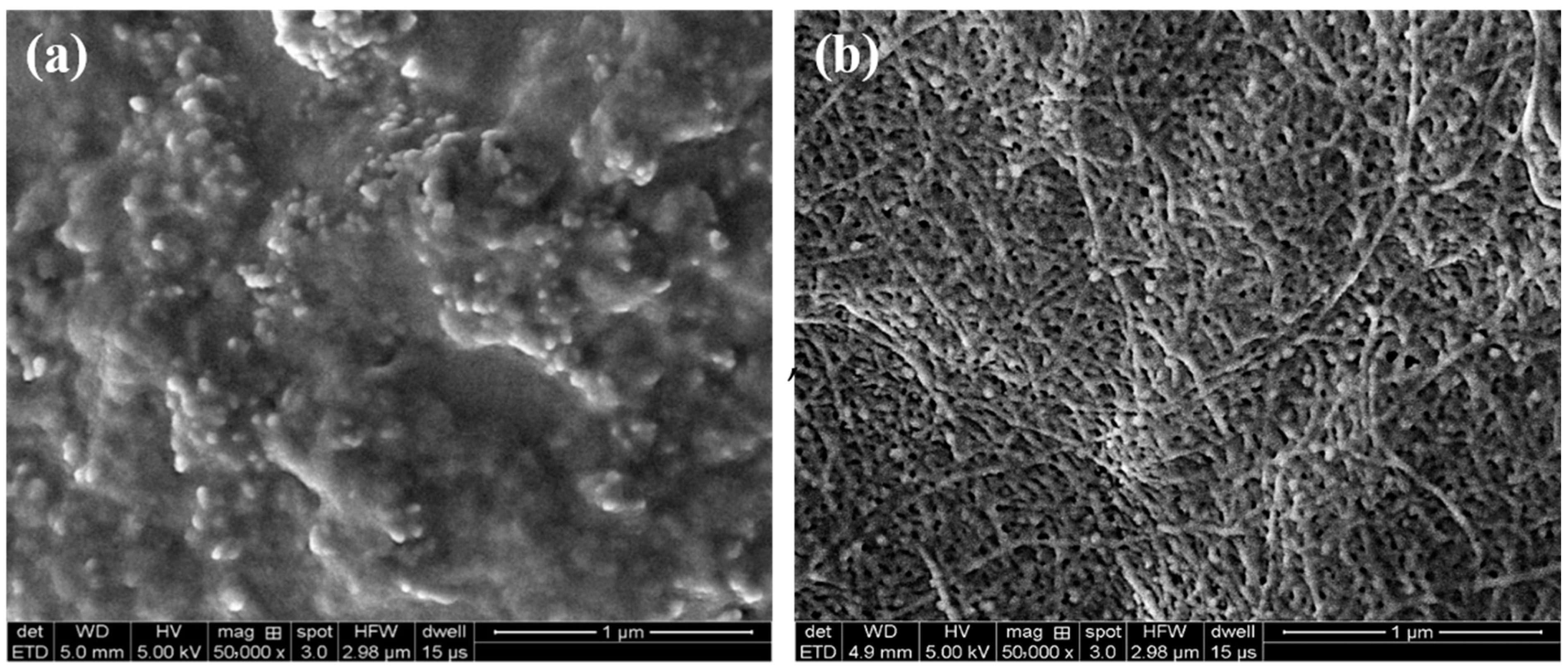
2.5. Gelation Mechanism of the Polymer Gel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Temperature-Resistant Polymer
4.3. Structure Characterization
4.4. Concentration Optimization of Polymer and Crosslinking Agent
4.5. Rheological Properties Test
4.6. Core Damage Test
- (1)
- A low-permeability sandstone core measuring 10 cm in length was extracted and subsequently divided into two equal-length sections, which were then appropriately labeled. The cut cores underwent ultrasonic vibration cleaning to effectively cleanse the core surface, followed by drying in an oven at 110 °C for a duration of 48 h. Essential parameters, including length, diameter, and permeability of the cores, were meticulously measured and documented. To ensure consistency, simulated formation water was prepared, and the cores were subjected to vacuum saturation with simulated formation water for a span of 24 h. Following this, the polymer gel was broken using 0.1 wt% (NH4)2S2O8. Once the gel was completely broken, the lower layer of the gel-breaking liquid was isolated for subsequent use by placing it in a water bath at 100 °C.
- (2)
- The experimental temperature was maintained at 100 °C. The core sample was securely positioned within the core gripper, with the surrounding pressure meticulously adjusted to 3.5 MPa. To introduce simulated formation water into the core, a fixed-flow pump in the form of an advection plunger pump was employed, operating at a consistent flow rate of 0.1 mL/min. To ensure reliable results, the system was allowed to reach a stable state for a minimum duration of 60 min, during which the pressure difference was carefully monitored and recorded.
- (3)
- The core gripper was carefully opened, and the core was rotated back and forth before being placed back into the core gripper. A consistent surrounding pressure of 3.5 MPa was meticulously applied, and the plunger pump was engaged to administer the gel-breaking liquid into the core at a precisely controlled flow rate of 1 mL/min. This injection process was maintained for a duration of 36 min. Subsequent to this, the valves situated at both ends of the gripper were securely closed, and a waiting period of 2 h was meticulously observed.
- (4)
- Subsequently, the core gripper was reopened, and the core was once again rotated back and forth before being placed back into the core gripper. The previously delineated procedures were systematically replicated, and the resultant stable pressure difference was meticulously logged. The assessment of core permeability, both prior to and post the inflicted damage, was quantitatively determined utilizing Darcy’s formula:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D.; Peng, X.; Peng, Q.; Wang, T.; Qiao, C.; Zhao, Z.; Gong, L.; Liu, Y.; Zhang, H.; Zeng, H. Probing the Interfacial Forces and Surface Interaction Mechanisms in Petroleum Production Processes. Engineering 2022, 18, 49–61. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Dai, C.; Yan, R.; Li, Y.; Liu, P. Development and Drag Reduction Behaviors of a Water-in-Water Emulsion Polymer Drag Reducer. ACS Appl. Polym. Mater. 2023, 5, 3707–3716. [Google Scholar] [CrossRef]
- Guo, X.; Hu, D.; Li, Y.; Duan, J.; Zhang, X.; Fan, X.; Duan, H.; Li, W. Theoretical Progress and Key Technologies of Onshore Ultra-Deep Oil/Gas Exploration. Engineering 2019, 5, 458–470. [Google Scholar] [CrossRef]
- Hu, D.; Ren, L.; Li, Z.; Zhao, J.; Lin, R.; Jiang, T. Simulation of fracture control during temporary plugging at fracture openings in deep and ultra-deep shale-gas horizontal wells. Nat. Gas Ind. B 2022, 9, 487–496. [Google Scholar] [CrossRef]
- Lei, Q.; Xu, Y.; Yang, Z.; Cai, B.; Wang, X.; Zhou, L.; Liu, H.; Xu, M.; Wang, L.; Li, S. Progress and development directions of stimulation techniques for ultra-deep oil and gas reservoirs. Pet. Explor. Dev. 2021, 48, 221–231. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Ma, W.; Wang, D.; Ma, C.; Li, Z. Evaluation on occluded hydrocarbon in deep–ultra deep ancient source rocks and its cracked gas resources. Nat. Gas Ind. B 2015, 2, 499–505. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Ping, J.; Ying, Y. Recent advances in water-driven triboelectric nanogenerators based on hydrophobic interfaces. Nano Energy 2021, 90, 106592. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Zhang, J.; Meng, S.; Duan, Y. Fracturing with carbon dioxide: Application status and development trend. Pet. Explor. Dev. 2014, 41, 513–519. [Google Scholar] [CrossRef]
- Osiptsov, A.A. Fluid Mechanics of Hydraulic Fracturing: A Review. J. Pet. Sci. Eng. 2017, 156, 513–535. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, S.; Cheng, W.; Wang, G. Fracture permeability damage and recovery behaviors with fracturing fluid treatment of coal: An experimental study. Fuel 2020, 282, 118809. [Google Scholar] [CrossRef]
- Wang, J.; Elsworth, D.; Wu, Y.; Liu, J.; Zhu, W.; Liu, Y. The Influence of Fracturing Fluids on Fracturing Processes: A Comparison Between Water, Oil and SC-CO2. Rock Mech. Rock Eng. 2018, 51, 299–313. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Lin, W.; Kang, Z.; You, Q. Formula optimization and rheology study of clean fracturing fluid. J. Mol. Liq. 2017, 241, 563–569. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.; Mao, J.; Tan, H.; Zhang, Y.; Song, Z. Review of friction reducers used in slickwater fracturing fluids for shale gas reservoirs. J. Nat. Gas Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Wang, X.; Liu, P.; Fan, M.; Yan, X.; Ma, Z.; Zhang, Y.; Dai, C. Synergistic effect of dual hydrogen-donor deep eutectic solvent for performance improvement of fracturing-oil expulsion fluids. Chem. Eng. J. 2023, 468, 143728. [Google Scholar] [CrossRef]
- Xie, K.; Mei, J.; Cao, W.; Cao, B.; Yao, L.; Zhang, B.; Wang, H.; Guo, K.; Wu, Z.; Yan, K.; et al. Improving oil mechanism of polymer gel fracturing fluid based on filtration displacement. J. Pet. Sci. Eng. 2022, 218, 111030. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Bai, H.; Li, Y.; Yang, H. A Comprehensive method to evaluate the viscous slickwater as fracturing fluids for hydraulic fracturing applications. J. Pet. Sci. Eng. 2020, 193, 107359. [Google Scholar] [CrossRef]
- Liu, P.; Dai, C.; Gao, M.; Wang, X.; Liu, S.; Jin, X.; Li, T.; Zhao, M. Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C. Gels 2022, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilirad, N.; White, S.; Terry, C.; Prior, A.; Carlson, K. Influence of inorganic ions in recycled produced water on gel-based hydraulic fracturing fluid viscosity. J. Pet. Sci. Eng. 2016, 139, 104–111. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Chen, S.; Li, S.; Li, T.; Ni, G.; Fu, Y.; Zhou, W. Experimental Evaluation of the Rheological Properties and Influencing Factors of Gel Fracturing Fluid Mixed with CO2 for Shale Gas Reservoir Stimulation. Gels 2022, 8, 527. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.-i.; Fujiwara, F.; Usui, S.; Tominaga, T. Effect of inorganic salt on the dose sensitivity of polymer gel dosimeter. Radiat. Phys. Chem. 2012, 81, 884–888. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Khan, F.; Tandon, A.; Dhar, V.; Beg, M.; Saxena, A.; Sharma, S. Cement Slurry Design for High-Pressure, High-Temperature Wellbores Using Polymer Nanocomposite: A Laboratory Scale Study Based on the Compressive Strength and Fluid Loss. Energy Fuels 2022, 36, 6820–6830. [Google Scholar] [CrossRef]
- Dastan, S.; Hassnajili, S.; Abdollahi, E. Hydrophobically associating terpolymers of acrylamide, alkyl acrylamide, and methacrylic acid as EOR thickeners. J. Polym. Res. 2016, 23, 175. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ramazani, S.A.A.; Haddadi, S.A. Applications of highly salt and highly temperature resistance terpolymer of acrylamide/styrene/maleic anhydride monomers as a rheological modifier: Rheological and corrosion protection properties studies. J. Mol. Liq. 2019, 294, 111635. [Google Scholar] [CrossRef]
- Van Mastrigt, F.; Stoffelsma, T.; Wever, D.A.Z.; Picchioni, F. Thermoresponsive comb polymers as thickeners for high temperature aqueous fluids. Mater. Today Commun. 2017, 10, 34–40. [Google Scholar] [CrossRef]
- Zheng, C.; Hou, Z.; Xu, K.; Weng, D.; Hou, Z.; Shi, Y.; Lai, J.; Liu, C.; Wang, T. Preparation and rheological properties of acrylamide-based penta-polymer for ultra-high temperature fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131386. [Google Scholar] [CrossRef]
- Mao, J.; Xue, J.; Zhang, H.; Yang, X.; Lin, C.; Wang, Q.; Li, C.; Liao, Z. Investigation of a hydrophobically associating polymer’s temperature and salt resistance for fracturing fluid thickener. Colloid Polym. Sci. 2022, 300, 569–582. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Zhao, L.; Liu, P.; Chen, X.; Wang, Q.; Yu, M. Water-soluble polymers for high-temperature resistant hydraulic fracturing: A review. J. Nat. Gas Sci. Eng. 2022, 104, 104673. [Google Scholar] [CrossRef]
- He, Y.; Gou, S.; Zhou, Y.; Zhou, L.; Tang, L.; Liu, L.; Fang, S. Thermoresponsive behaviors of novel polyoxyethylene-functionalized acrylamide copolymers: Water solubility, rheological properties and surface activity. J. Mol. Liq. 2020, 319, 114337. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Wong, R.S.; Ashton, M.; Dodou, K. Effect of Crosslinking Agent Concentration on the Properties of Unmedicated Hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef]
- Nianyin, L.; Yu, J.; Daocheng, W.; Chao, W.; Jia, K.; Pingli, L.; Chengzhi, H.; Ying, X. Development status of crosslinking agent in high-temperature and pressure fracturing fluid: A review. J. Nat. Gas Sci. Eng. 2022, 107, 104369. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Xu, Z.; Wang, K.; Gao, M.; Lv, W.; Dai, C. Dynamic cross-linking mechanism of acid gel fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125471. [Google Scholar] [CrossRef]
- Kamal, M.S.; Mohammed, M.; Mahmoud, M.; Elkatatny, S. Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies 2018, 11, 1663. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, L.; Wang, Y.; Ji, Y.; Horvat, J.; Cheng, M.-L.; Jia, X.; Wang, G. Manganese-Based Layered Coordination Polymer: Synthesis, Structural Characterization, Magnetic Property, and Electrochemical Performance in Lithium-Ion Batteries. Inorg. Chem. 2013, 52, 2817–2822. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.-R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Chen, W.-H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Loganathan, S.; Valapa, R.B.; Mishra, R.K.; Pugazhenthi, G.; Thomas, S. Chapter 4—Thermogravimetric Analysis for Characterization of Nanomaterials. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Thomas, S., Thomas, R., Zachariah, A.K., Mishra, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Bair, S.; Yamaguchi, T.; Brouwer, L.; Schwarze, H.; Vergne, P.; Poll, G. Oscillatory and steady shear viscosity: The Cox–Merz rule, superposition, and application to EHL friction. Tribol. Int. 2014, 79, 126–131. [Google Scholar] [CrossRef]
- Jasso, M.; Polacco, G.; Zanzotto, L. Shear Viscosity Overshoots in Polymer Modified Asphalts. Materials 2022, 15, 7551. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, M.; Wu, Y.; Gao, Z.; Dai, C.; Liu, P. Development and Performance Evaluation of a Novel Silica Nanoparticle-Reinforced CO2-Sensitive Fracturing Fluid with High Temperature and Shear Resistance Ability. Energy Fuels 2022, 36, 7177–7185. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Yang, J.; Bai, Y.; Sun, J.; Lv, K.; Han, J.; Dai, L. Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels 2022, 8, 229. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, Z.; Wang, H.; Liang, S.; Xu, N.; Li, D. Mechanism Analysis of Enhancing the Temperature and Shear Resistance of Hydroxypropyl Guar Gum Fracturing Fluid by Boron-Functionalized Nanosilica Colloidal Crosslinker. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132154. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Wang, Y.-n.; Zhou, J.; Shi, B. Chrome-free synergistic tanning system based on biomass-derived hydroxycarboxylic acid–zirconium complexes. J. Clean. Prod. 2022, 336, 130428. [Google Scholar] [CrossRef]
- Fu, L.; Liao, K.; Ge, J.; Huang, W.; Chen, L.; Sun, X.; Zhang, S. Study on the damage and control method of fracturing fluid to tight reservoir matrix. J. Nat. Gas Sci. Eng. 2020, 82, 103464. [Google Scholar] [CrossRef]
- Lufeng, Z.; Fujian, Z.; Shicheng, Z.; Zhun, L.; Jin, W.; Yuechun, W. Evaluation of permeability damage caused by drilling and fracturing fluids in tight low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2019, 175, 1122–1135. [Google Scholar] [CrossRef]
- Tang, H.; Tang, H.; He, J.; Zhao, F.; Zhang, L.; Liao, J.; Wang, Q.; Yuan, X. Damage mechanism of water-based fracturing fluid to tight sandstone gas reservoirs: Improvement of The Evaluation Measurement for Properties of Water-based Fracturing Fluid: SY/T 5107-2016. Nat. Gas Ind. B 2021, 8, 163–172. [Google Scholar] [CrossRef]
- Fan, Y.; Duan, W.; Xu, K.; Yan, C.; Zheng, C. Zr, N-Co-Doped Carbon Quantum Dot Crosslinking Agents for Use in Fracturing Fluids. ACS Appl. Nano Mater. 2023, 6, 7920–7930. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B.; Rodič, P.; Iskra, J. Hybrid sol–gel coating agents based on zirconium(IV) propoxide and epoxysilane. J. Sol-Gel Sci. Technol. 2015, 74, 447–459. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Zuo, Z.; Liao, M.; Peng’ao, P. Preparation and property evaluation of a temperature-resistant Zr-crosslinked fracturing fluid. J. Ind. Eng. Chem. 2021, 96, 121–129. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.; Feng, X.; Liu, H.; Li, F.; Wang, M.; Li, H. Room-temperature self-healing tough nanocomposite hydrogel crosslinked by zirconium hydroxide nanoparticles. Compos. Sci. Technol. 2017, 140, 54–62. [Google Scholar] [CrossRef]
- Ma, L.; Xie, J.; Yan, X.; Fan, Z.; Li, H.; Lu, L.; Chen, L.; Xin, Y.; Yin, P. Wearable membranes from zirconium-oxo clusters cross-linked polymer networks for ultrafast chemical warfare agents decontamination. Chin. Chem. Lett. 2022, 33, 3241–3244. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S.; Alkhowaildi, M. Rheological Study of Seawater-Based Fracturing Fluid Containing Polymer, Crosslinker, and Chelating Agent. ACS Omega 2022, 7, 31318–31326. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S.; Kalgaonkar, R. Individual Seawater Ions’ Impact on the Rheology of Crosslinked Polymers in the Presence of a Chelating Agent. Energy Fuels 2023, 37, 7328–7338. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Frounchi, M.; Dadbin, S. Synthesis, characterization and comparison of PAM, cationic PDMC and P(AM-co-DMC) based on solution polymerization. J. Ind. Eng. Chem. 2011, 17, 580–586. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Zheng, C.; Zhang, P.; Huang, B.; Guo, N.; Jin, L. Synthesis and properties of epoxy-polyurethane/silica nanocomposites by a novel sol method and in-situ solution polymerization route. Appl. Surf. Sci. 2014, 303, 67–75. [Google Scholar] [CrossRef]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1237–1242. [Google Scholar] [CrossRef] [PubMed]





| System | Number | Length/cm | Diameter/cm | Initial Permeability/mD | Damage Permeability/mD | Damage Rate/% | Average Damage Rate/% | RSD/% |
|---|---|---|---|---|---|---|---|---|
| polymer gel | 1 | 5.01 | 2.50 | 2.30 | 1.93 | 16.1 | 16.6 | 3.03 |
| 2 | 4.98 | 2.50 | 2.18 | 1.82 | 16.5 | |||
| 3 | 4.98 | 2.50 | 2.34 | 1.94 | 17.1 | |||
| guanidine gum | 1 | 4.99 | 2.50 | 2.26 | 1.57 | 30.5 | 30.9 | 4.49 |
| 2 | 5.02 | 2.50 | 2.53 | 1.71 | 32.4 | |||
| 3 | 4.95 | 2.50 | 2.59 | 1.82 | 29.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Zhao, M.; Yang, Z.; Wang, X.; Dai, C. Development and Gelation Mechanism of Ultra-High-Temperature-Resistant Polymer Gel. Gels 2023, 9, 726. https://doi.org/10.3390/gels9090726
Ma Z, Zhao M, Yang Z, Wang X, Dai C. Development and Gelation Mechanism of Ultra-High-Temperature-Resistant Polymer Gel. Gels. 2023; 9(9):726. https://doi.org/10.3390/gels9090726
Chicago/Turabian StyleMa, Zhenfeng, Mingwei Zhao, Ziteng Yang, Xiangyu Wang, and Caili Dai. 2023. "Development and Gelation Mechanism of Ultra-High-Temperature-Resistant Polymer Gel" Gels 9, no. 9: 726. https://doi.org/10.3390/gels9090726




