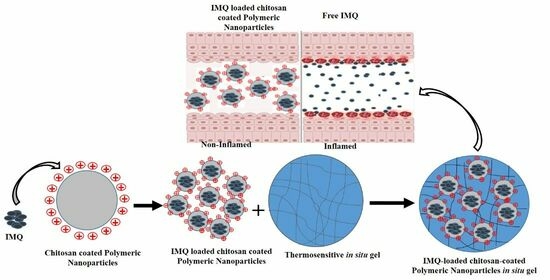
Imiquimod-Loaded Chitosan-Decorated Di-Block and Tri-Block Polymeric Nanoparticles Loaded In Situ Gel for the Management of Cervical Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size and Zeta Potential
2.2. Encapsulation Efficiency and Loading Capacity
2.3. In Vitro Release Studies
2.4. Surface Morphology
2.5. Characterization of the In Situ Hydrogels
2.5.1. Determination of the Sol–Gel Transition Temperature
2.5.2. Viscosity Measurement
2.5.3. Ex Vivo Permeability Study
2.6. In Vitro Cytotoxicity Studies
2.7. Detection of the Inflammatory Potential of the IMQ NPs
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of IMQ NPs and CS-IMQ NPs
4.3. Particle Size Distribution and Zeta Potential Measurement
4.4. Encapsulation Efficiency and Loading Capacity Determination
4.5. In Vitro Release Study
4.6. The Particle Morphology
4.7. Preparation of Thermosensitive In Situ Hydrogels
4.8. Characterization of Thermosensitive In Situ Hydrogels
4.9. Determination of the Sol–Gel Transition Temperature
4.10. Viscosity Measurement
4.11. Ex Vivo Permeation of IMQ through the Vaginal Mucosa
4.12. In Vitro Cytotoxicity Studies
4.13. Detection of the Inflammatory Potential of the IMQ NPs
4.14. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Weinmann, S.; Naleway, A.; Swamy, G.; Krishnarajah, G.; Arondekar, B.; Fernandez, J.; Myers, E. Pregnancy Outcomes after Treatment for Cervical Cancer Precursor Lesions: An Observational Study. PLoS ONE 2017, 12, e0165276. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P. Efficacy of imiquimod 5% cream for persistent human papillomavirus in genital intraepithelial neoplasm. Taiwan J. Obstet. Gynecol. 2013, 52, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Polterauer, S.; Natter, C.; Rahhal, J.; Hefler, L.; Tempfer, C.B.; Heinze, G.; Stary, G.; Reinthaller, A.; Speiser, P. Treatment of cervical intraepithelial neoplasia with topical imiquimod: A randomized controlled trial. Obstet. Gynecol. 2012, 120, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Bianchi, P.; Sesti, F.; Sopracordevole, F.; Biamonti, A.; Scirpa, P.; Schimberni, M.; Cozza, G.; Marziani, R.; Di Martino, G.; et al. Adjuvant topical treatment with imiquimod 5% after excisional surgery for VIN 2/3. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2949–2952. [Google Scholar]
- Ramineni, S.K.; Cunningham, L.L., Jr.; Dziubla, T.D.; Puleo, D.A. Development of imiquimod-loaded mucoadhesive films for oral dysplasia. J. Pharm. Sci. 2013, 102, 593–603. [Google Scholar] [CrossRef]
- Nie, S.; Xing, Y.; Kim, G.J.; Simons, J.W. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef]
- Guimarães, P.P.G.; Oliveira, S.R.; de Castro Rodrigues, G.; Gontijo, S.M.L.; Lula, I.S.; Cortés, M.E.; Denadai, Â.M.L.; Sinisterra, R.D.J.M. Development of sulfadiazine-decorated PLGA nanoparticles loaded with 5-fluorouracil and cell viability. Molecules 2015, 20, 879–899. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, S.S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: Synthesis, formulation, characterization and in vitro drug release. Biomaterials 2006, 27, 262–270. [Google Scholar] [CrossRef]
- Sanna, V.; Roggio, A.M.; Posadino, A.M.; Cossu, A.; Marceddu, S.; Mariani, A.; Alzari, V.; Uzzau, S.; Pintus, G.; Sechi, M. Novel docetaxel-loaded nanoparticles based on poly(lactide-co-caprolactone) and poly(lactide-co-glycolide-co-caprolactone) for prostate cancer treatment: Formulation, characterization, and cytotoxicity studies. Nanoscale Res. Lett. 2011, 6, 260. [Google Scholar] [CrossRef]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Park, T.G. Synthesis and characterization of elastic PLGA/PCL/PLGA tri-block copolymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Srirangarajan, S.; Agnihotri, S.A.; Patil, S.A.; Ravindra, S.; Setty, S.B.; Aminabhavi, T.M. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(epsilon-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: In vitro and in vivo studies. J. Control. Release 2007, 119, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.; Patel, P.; Airao, V.; Bhatt, V.; Sheth, N.J.B. Novel silibinin loaded chitosan-coated PLGA/PCL nanoparticles based inhalation formulations with improved cytotoxicity and bioavailability for lung cancer. BioNanoScience 2021, 11, 67–83. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Evangelista, R.C. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int. J. Pharm. 2005, 290, 137–144. [Google Scholar] [CrossRef]
- Mittal, G.; Sahana, D.; Bhardwaj, V.; Kumar, M.R. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 2007, 119, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Dilnawaz, F.; Sahoo, S.K.J.N. Sustained antibacterial activity of doxycycline-loaded poly (D, L-lactide-co-glycolide) and poly (ε-caprolactone) nanoparticles. Nanomedicine 2009, 4, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Advanced materials interfaces 2009, 6, 1900572. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L.J.A.P. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Sowmya, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Alkholief, M.; Kalam, M.A.; Anwer, M.K.; Alshamsan, A.J.P. Effect of solvents, stabilizers and the concentration of stabilizers on the physical properties of poly (D,L-lactide-co-glycolide) nanoparticles: Encapsulation, in vitro release of indomethacin and cytotoxicity against HepG2-cell. Pharmaceutics 2022, 14, 870. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; da Costa, T.G.; de Lima, R.; Fraceto, L.F.; de Araujo, D.R. Using Chitosan-Coated Polymeric Nanoparticles-Thermosensitive Hydrogels in association with Limonene as Skin Drug Delivery Strategy. BioMed Res. Int. 2022, 2022, 9165443. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Alomrani, A.H.; Harisa, G.I.; Ashour, A.E.; Kumar, A.; Yassin, A.E. Novel docetaxel chitosan-coated PLGA/PCL nanoparticles with magnified cytotoxicity and bioavailability. BioMedicine 2018, 106, 1461–1468. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Cai, Q.; Bei, J. Degradation and 5-fluorouracil release behavior in vitro of polycaprolactone/poly (ethylene oxide)/polylactide tri-component copolymer. Polym. Adv. Technol. 2001, 12, 253–258. [Google Scholar] [CrossRef]
- Miyajima, M.; Koshika, A.; Okada, J.i.; Ikeda, M.; Nishimura, K. Effect of polymer crystallinity on papaverine release from poly (L-lactic acid) matrix. J. Control. Release 1997, 49, 207–215. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Liu, L.; Sun, Q.; Shuai, X.; Zhu, W.; Chen, Y.J.B. Amphiphilic toothbrushlike copolymers based on poly (ethylene glycol) and poly (ε-caprolactone) as drug carriers with enhanced properties. Biomacromolecules 2010, 11, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Panchai, P.; Julin, K.; Basnet, P.; Nystad, M.; Johannessen, M.; Škalko-Basnet, N. Chitosan-based delivery system enhances antimicrobial activity of chlorhexidine. Front. Microbiol. 2022, 13, 1023083. [Google Scholar] [CrossRef] [PubMed]
- Ramyadevi, D.; Rajan, K.; Vedhahari, B.; Ruckmani, K.; Subramanian, N.J.C.; Biointerfaces, S.B. Heterogeneous polymer composite nanoparticles loaded in situ gel for controlled release intra-vaginal therapy of genital herpes. Colloids Surf. B Biointerfaces 2016, 146, 260–270. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Alexandris, N.; Konstantinou, E.; Mesiakaris, K.; Zanidis, C.; Farsalinos, K.; Poulas, K. Imiquimod—A toll like receptor 7 agonist—Is an ideal option for management of COVID 19. Environ. Res. 2020, 188, 109858. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.L.; Gerster, J.F.; Imbertson, L.M.; Reiter, M.J.; Miller, R.L.; Gibson, S.J.; Wagner, T.L.; Tomai, M.A. Cytokine induction by the immunomodulators imiquimod and S-27609. J. Leukoc. Biol. 1995, 58, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef]
- Matthew, J.E.; Nazario, Y.L.; Roberts, S.C. Effect of mammalian cell culture medium on the gelation properties of Pluronic PF127. Biomaterials 2002, 23, 4615–4619. [Google Scholar] [CrossRef]
- Holt, J.D.; Cameron, D.; Dias, N.; Holding, J.; Muntendam, A.; Oostebring, F.; Dreier, P.; Rohan, L.; Nuttall, J. The sheep as a model of preclinical safety and pharmacokinetic evaluations of candidate microbicides. Antimicrob. Agents Chemother. 2015, 59, 3761–3770. [Google Scholar] [CrossRef]
- Almomen, A.; Cho, S.; Yang, C.H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janat-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [PubMed]





| Polymeric Nanoparticles | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| PLGA | 216.4 ± 8.1 | 0.207 ± 0.017 | −20.13 ± 2.27 |
| PCL | 189.1 ± 3.9 | 0.243 ± 0.015 | −13.63 ± 1.62 |
| PLA-PCL | 237.3 ± 4.7 | 0.201 ± 0.021 | −18.67 ± 1.45 |
| PLGA-PCL | 206.7 ± 8.7 | 0.195 ± 0.007 | −16.51 ± 1.37 |
| CS-PLGA | 225.9 ± 7.5 | 0.277 ± 0.061 | 23.23 ± 3.11 |
| CS-PCL | 232.4 ± 3.9 | 0.286 ± 0.048 | 17.03 ± 1.51 |
| CS-PLA-PCL | 278.2 ± 5.4 | 0.229 ± 0.023 | 20.46 ± 2.08 |
| CS-PLGA-PCL | 218.5 ± 9.6 | 0.322 ± 0.037 | 18.01 ± 1.08 |
| Polymeric Nanoparticles | Yield% | EE% | LC% |
|---|---|---|---|
| PLGA | 76.43 | 51.09 ± 4.61 | 8.14 ± 0.29 |
| PCL | 73.21 | 46.81 ± 1.78 | 7.18 ± 0.43 |
| PLA-PCL | 87.22 | 39.86 ± 2.46 | 6.37 ± 0.62 |
| PLGA-PCL | 85.76 | 61.48 ± 5.19 | 10.32 ± 0.85 |
| CS-PLGA | 81.15 | 45.71 ± 2.84 | 6.15 ± 0.35 |
| CS-PCL | 88.62 | 38.74 ± 1.65 | 5.41 ± 0.79 |
| CS-PLA-PCL | 84.63 | 37.73 ± 2.88 | 5.81 ± 0.65 |
| CS-PLGA-PCL | 86.32 | 49.26 ± 2.73 | 7.67 ± 0.24 |
| Correlation Coefficient (R2) | |||||
|---|---|---|---|---|---|
| Formulations | Zero-Order | First-Order | Higuchi’s Model | Korsmeyer–Peppas Model | |
| R2 | n | ||||
| F1 | 0.768 | 0.972 | 0.995 | 0.997 | 0.532 |
| F2 | 0.611 | 0.739 | 0.976 | 0.993 | 0.426 |
| F3 | 0533 | 0.674 | 0.941 | 0.989 | 0.431 |
| F4 | 0.905 | 0.987 | 0.982 | 0.993 | 0.360 |
| CS-F1 | 0.718 | 0.934 | 0.951 | 0.996 | 0.382 |
| CS-F2 | 0.814 | 0.944 | 0.973 | 0.991 | 0.422 |
| CS-F3 | 0.901 | 0.953 | 0.974 | 0.998 | 0.361 |
| CS-F4 | 0.934 | 0.902 | 0.983 | 0.993 | 0.387 |
| Formulations | Js (μg/cm2·h) | P (cm/h × 10−3) | ER |
|---|---|---|---|
| Control | 0.198 ± 0.054 | 0.397 ± 0.006 | - |
| F4 | 4.263 ± 0.872 * | 8.526 ± 0.756 * | 21.5 |
| CF4 | 5.905 ± 0.711 * | 11.810 ± 2.006 * | 29.8 |
| Codes Ingredients | PLGA (mg) | PCL (mg) | PLA-PCL (mg) | PLGA-PCL (mg) | CS (mg/mL) | IMQ (mg) |
|---|---|---|---|---|---|---|
| F1 | 50 | - | - | - | - | 10 |
| F2 | - | 50 | - | - | - | 10 |
| F3 | - | - | 50 | - | - | 10 |
| F4 | - | - | - | 50 | - | 10 |
| CS-F1 | 50 | - | - | - | 0.5 | 10 |
| CS-F2 | - | 50 | - | - | 0.5 | 10 |
| CS-F3 | - | - | 50 | - | 0.5 | 10 |
| CS-F4 | - | - | - | 50 | 0.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomen, A.; Badran, M.; Alhowyan, A.A.; Alkholief, M.; Alshamsan, A. Imiquimod-Loaded Chitosan-Decorated Di-Block and Tri-Block Polymeric Nanoparticles Loaded In Situ Gel for the Management of Cervical Cancer. Gels 2023, 9, 713. https://doi.org/10.3390/gels9090713
Almomen A, Badran M, Alhowyan AA, Alkholief M, Alshamsan A. Imiquimod-Loaded Chitosan-Decorated Di-Block and Tri-Block Polymeric Nanoparticles Loaded In Situ Gel for the Management of Cervical Cancer. Gels. 2023; 9(9):713. https://doi.org/10.3390/gels9090713
Chicago/Turabian StyleAlmomen, Aliyah, Mohamed Badran, Adel Ali Alhowyan, Musaed Alkholief, and Aws Alshamsan. 2023. "Imiquimod-Loaded Chitosan-Decorated Di-Block and Tri-Block Polymeric Nanoparticles Loaded In Situ Gel for the Management of Cervical Cancer" Gels 9, no. 9: 713. https://doi.org/10.3390/gels9090713
APA StyleAlmomen, A., Badran, M., Alhowyan, A. A., Alkholief, M., & Alshamsan, A. (2023). Imiquimod-Loaded Chitosan-Decorated Di-Block and Tri-Block Polymeric Nanoparticles Loaded In Situ Gel for the Management of Cervical Cancer. Gels, 9(9), 713. https://doi.org/10.3390/gels9090713