Protective Effect of Alginate Microcapsules with Different Rheological Behavior on Lactiplantibacillus plantarum 299v
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Characterization of the Aqueous Solutions of Sodium Alginate
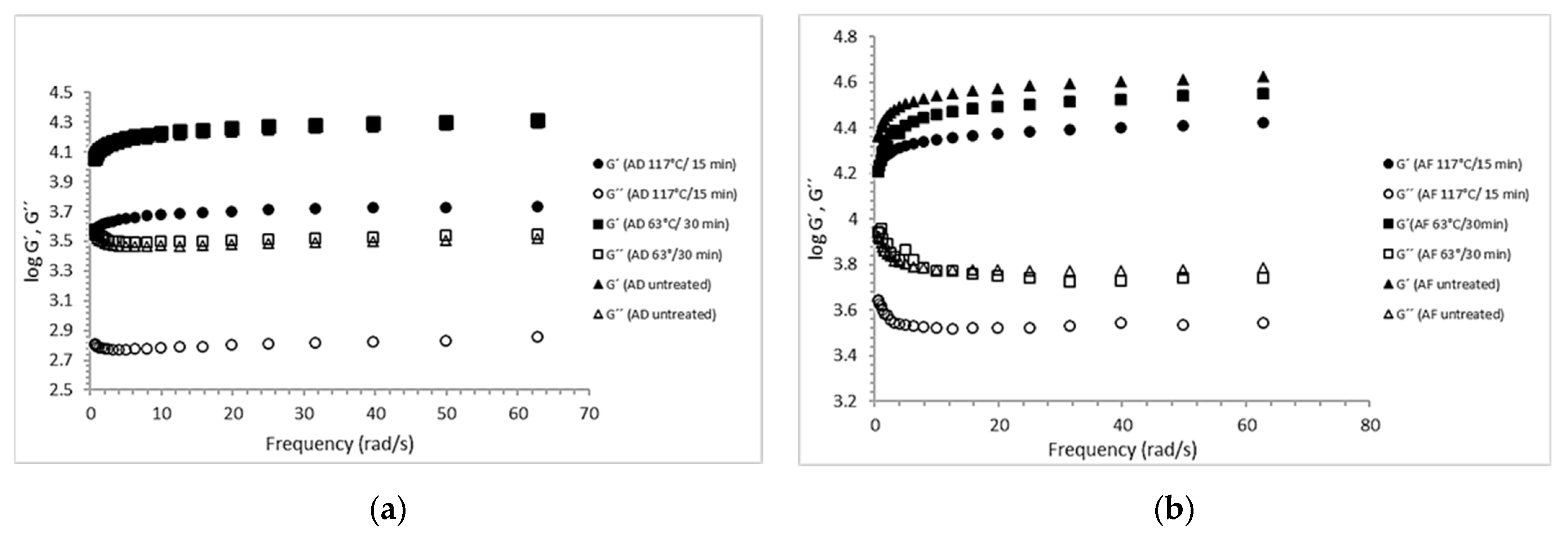
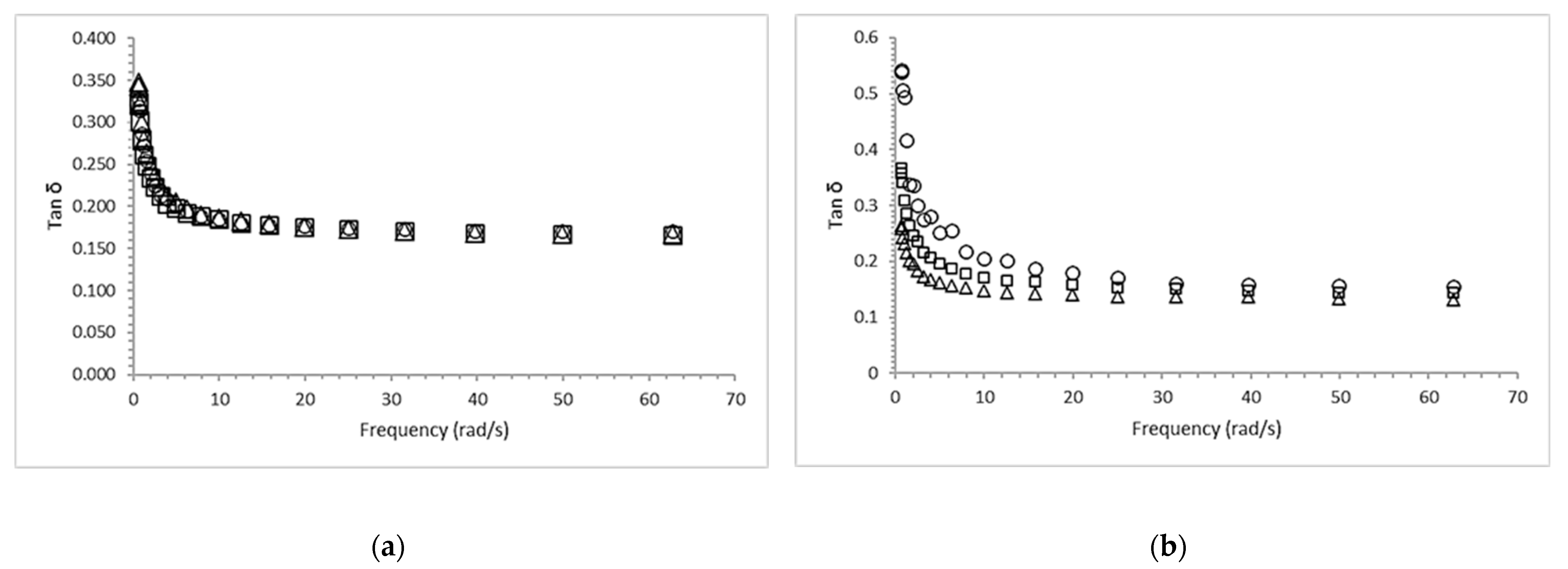
2.2. Dynamic Viscoelastic Properties of Calcium Alginate Gels
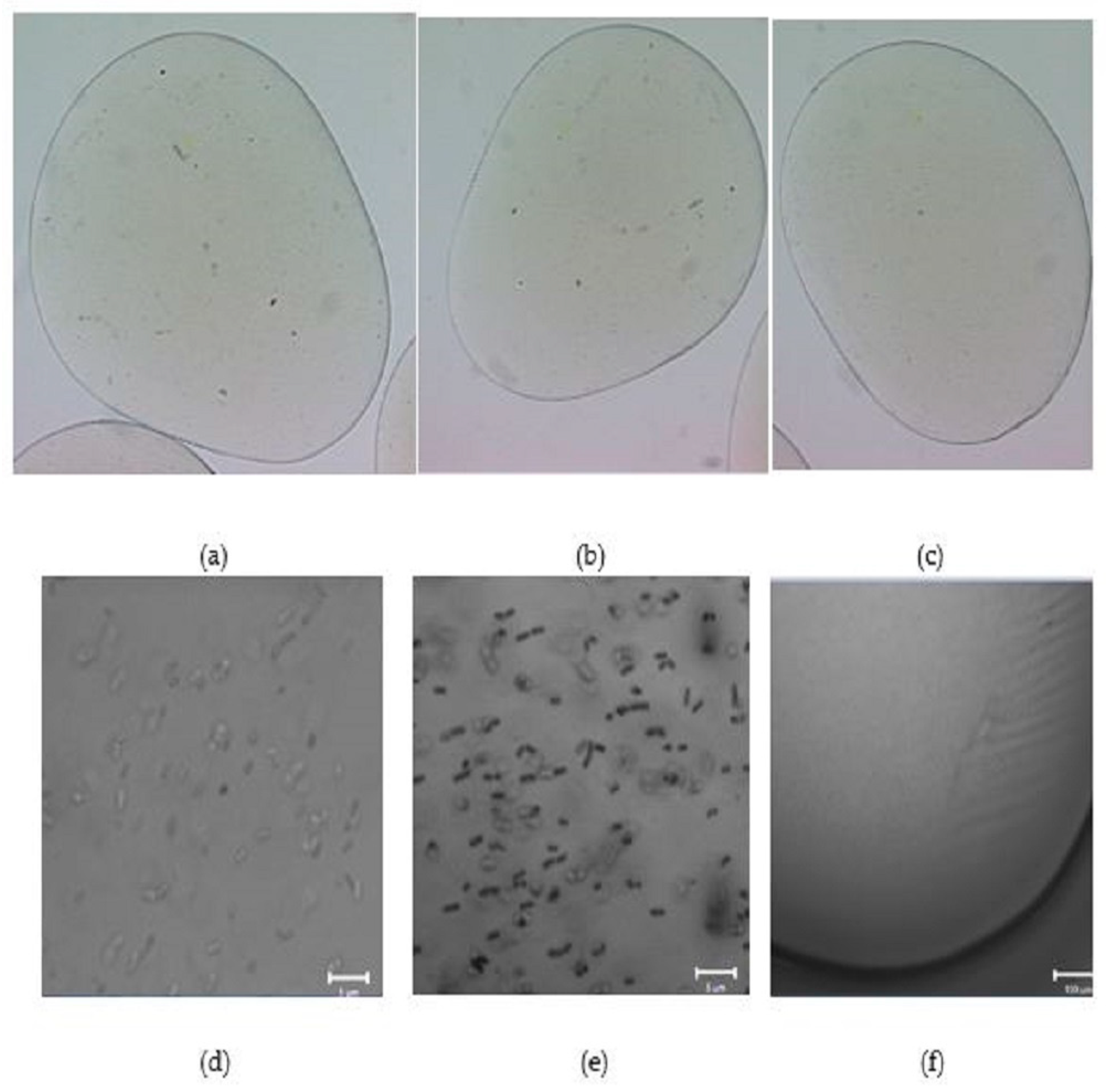
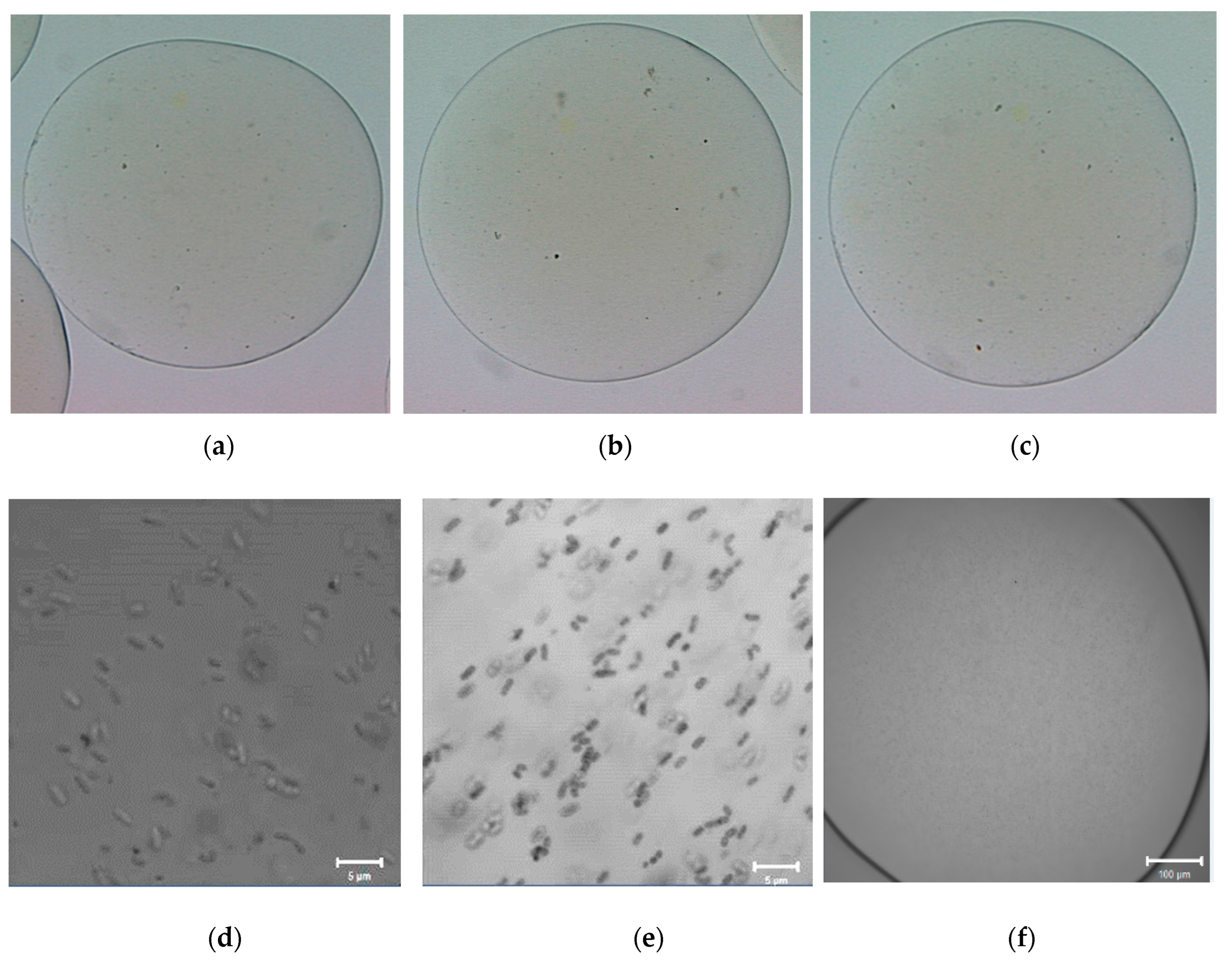
2.3. Morphology of Empty and L. plantarum 299v-Loaded Alginate Capsules
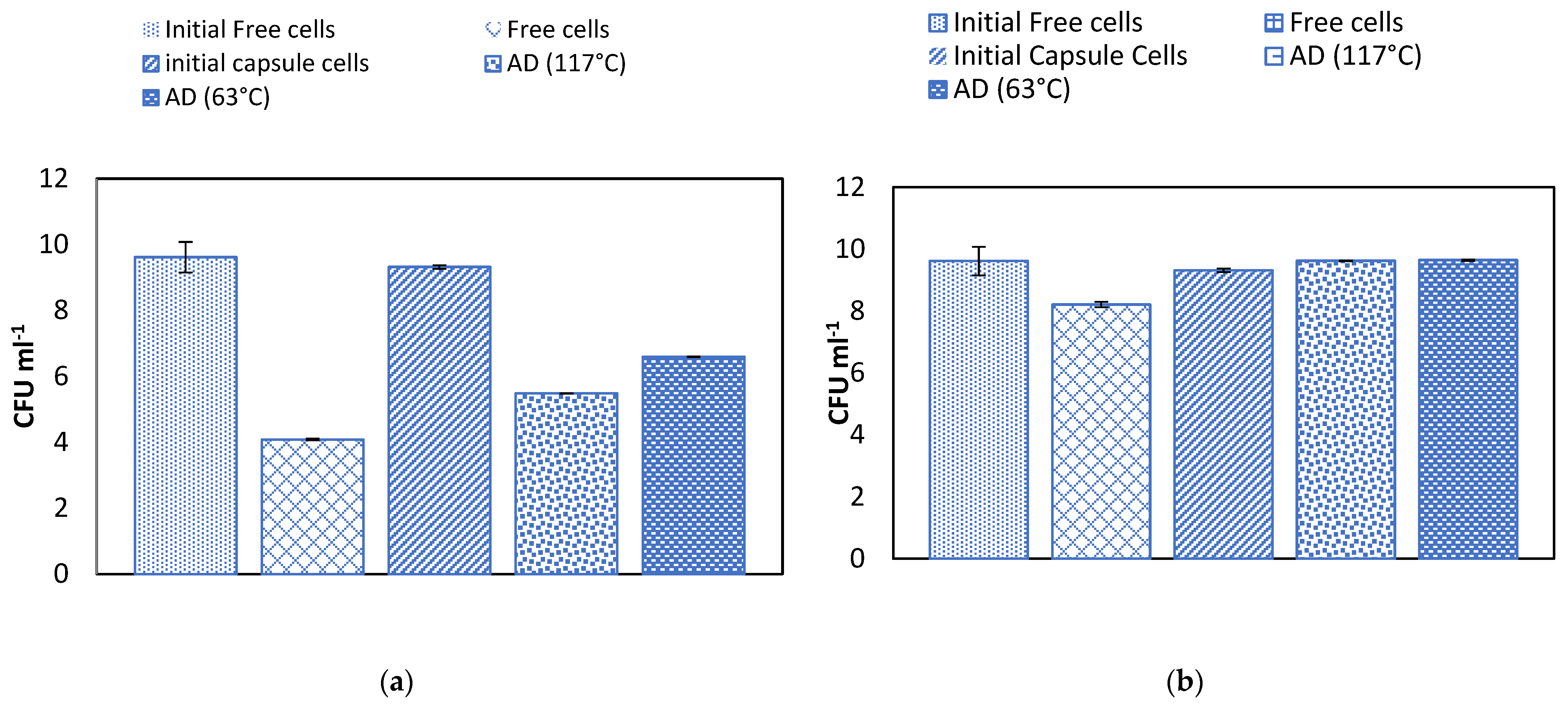
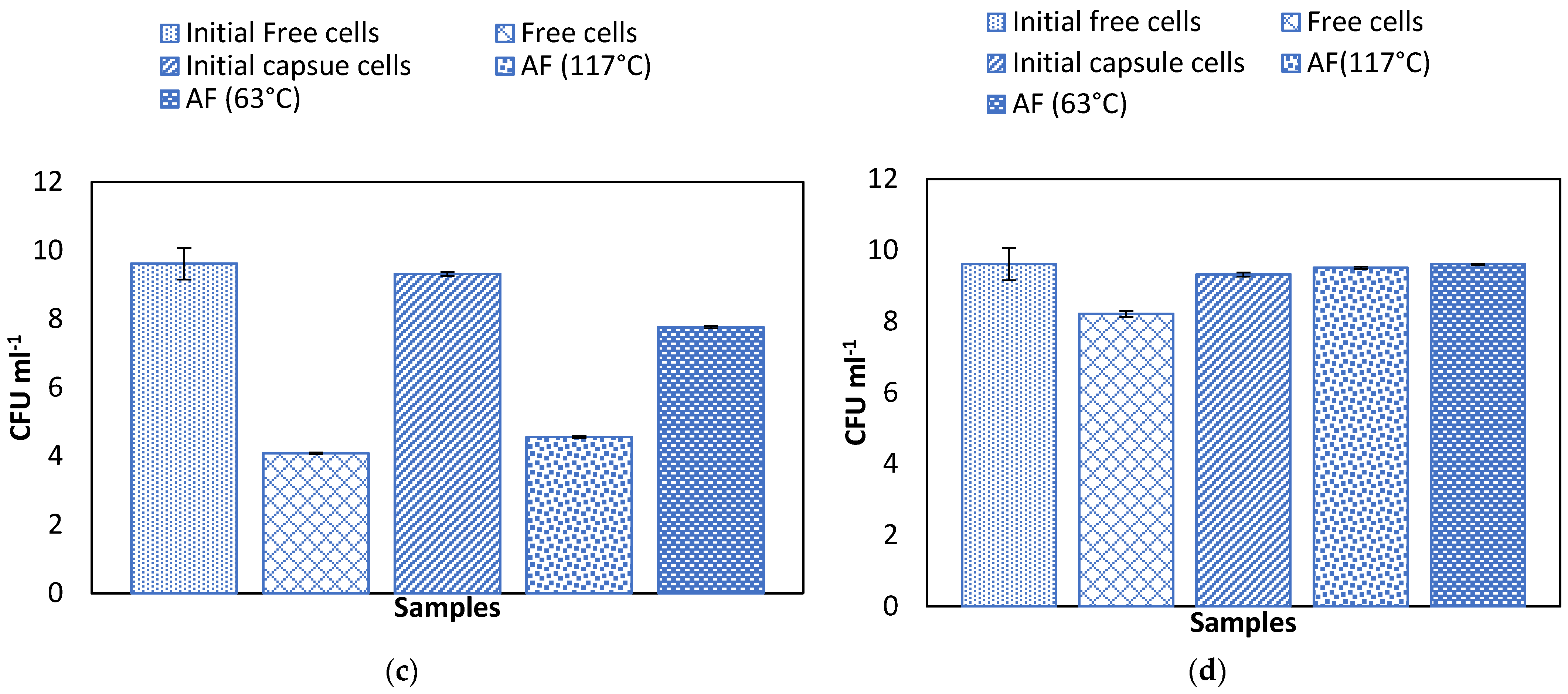
2.4. Effect of Thermal Treatment of the Alginate Solutions on Their Ability to Form Protective Capsules
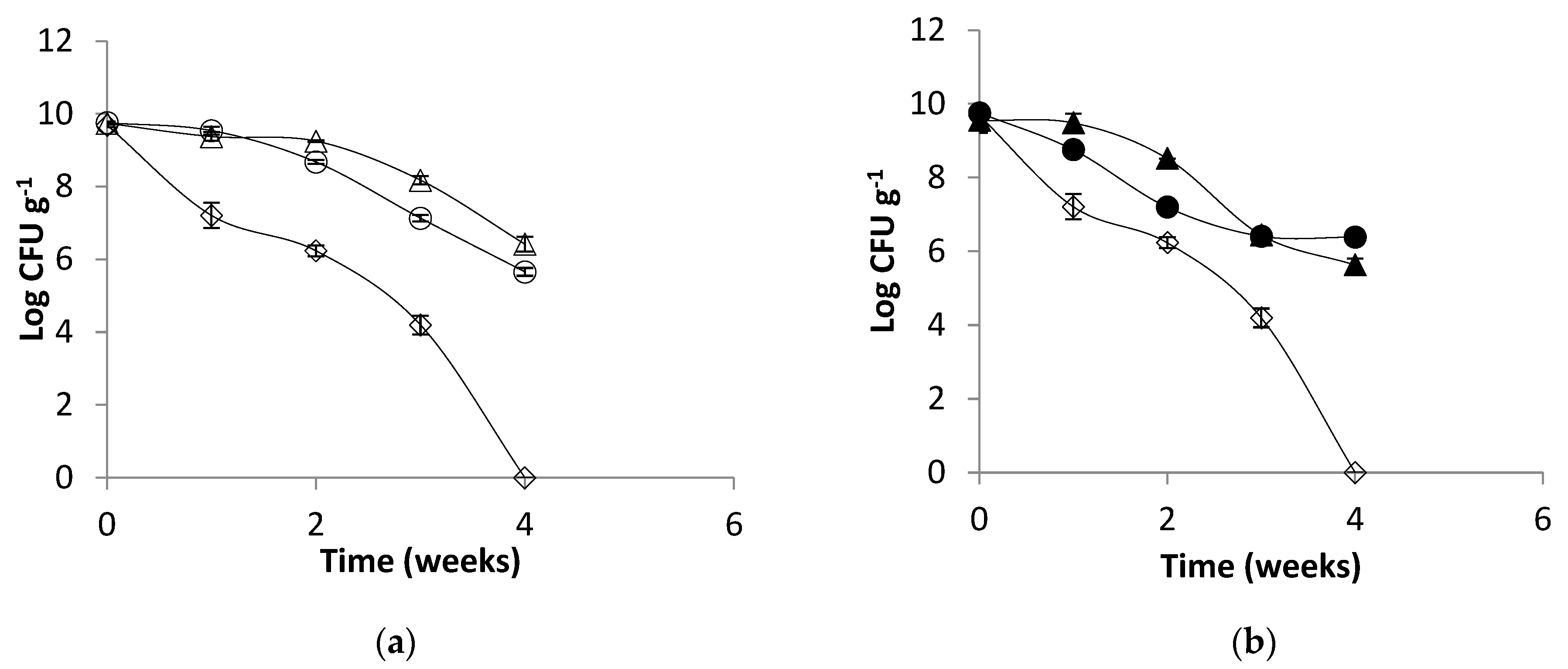
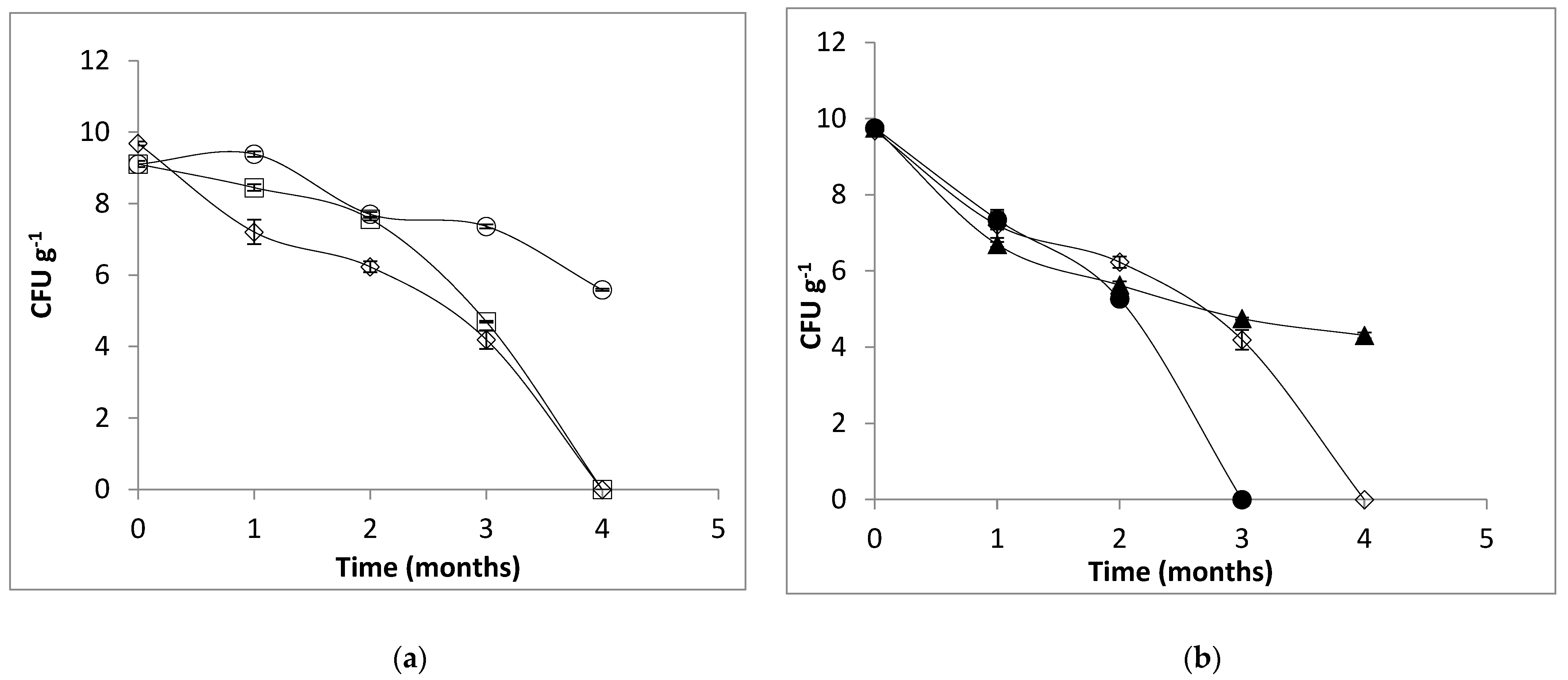
2.5. Effect of Temperature on Alginate Beads and Survival of Microencapsulated Cells in Simulated Gastric Juice
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Rheology of Aqueous Solutions of Sodium Alginate
4.3. Dynamic Shear Rheology of Calcium Alginate Gels
4.4. Bacterial, Growth Conditions and Preparation of Cell Suspensions
4.5. Microencapsulation
4.6. Morphology of Empty and L. plantarum 299v-Loaded Alginate Capsules
4.7. Protective Ability of the Alginate Capsules
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Rokka, S.; Rantamäki, P. Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Rodriguez-Huezo, M.E.; Lobato-Calleros, C.; Reyes-Ocampo, J.G.; Sandoval-Castilla, O.; Perez-Alonso, C.; Pimentel-Gonzales, D.J. Survivability of entrapped Lactobacillus rhamnosus in liquid-and gel-core alginate beads during storge and simulated gastrointestinal conditions. Rev. Mex. Ing. Quim. 2011, 10, 353–361. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT Food Sci. Technol. 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Pitino, I.; Licciardello, F.; Muratore, G.; Caggia, C. Survival of Lactobacillus rhamnosus probiotic strains in peach jam during storage at different temperatures. Food Sci. Technol. 2013, 33, 652–659. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Pinto, S.S.; Muñoz, I.B.; Amboni, R.D.M.C. Effect of microencapsulation on survival of Bifidobacterium BB-12 exposed to simulated gastrointestinal conditions and heat treatments. LWT-Food Sci. Technol. 2013, 50, 39–44. [Google Scholar] [CrossRef]
- Nag, A.; Das, S. Effect of trehalose and lactose as cryoprotectant during freeze-drying, in vitro gastro-intestinal transit and survival of microencapsulated freeze-dried Lactobacillus casei 431 cells. Int. J. Dairy Technol. 2013, 66, 162–169. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr. Issues Intest. Microbiol. 2002, 3, 39–48. Available online: https://pubmed.ncbi.nlm.nih.gov/12400637/ (accessed on 22 August 2023).
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.C.; Liberal, R.D.C.O.; Fávaro-Trindade, C.S. Co-encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Li, D.T.; Xu, M.; Chen, H.Y.; Zhang, Z.L.; Tang, Z.X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Hashmi, S.A.S.; Deshpande, H.W.; Farooqui, A.S.; Syed, H.M.; Sontakke, M.D. Microencapsulation of Probiotic Culture Beads by Using Modified Psyllium Husk. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 392–400. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Orive, G.; Pedraz, J.L.; Santos-Vizcaino, E.; Hernandez, R.M. Chapater 3-Alginate Microcapsules for Drug Delivery. In Alginates and Their Biomedical Applications; Rehm, B., Moradali, M., Eds.; Springer: Singapore, 2018; Volume 11, pp. 67–100. [Google Scholar] [CrossRef]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, X.; Wu, Y.; Wang, Z.; Bai, Y.; Liu, X.; Han, D.; Zhao, J. Alginate Alleviates Dextran Sulfate Sodium-Induced Colitis by Promoting Bifidobacterium animalis and Intestinal Hyodeoxycholic Acid Synthesis in Mice. Microbiol. Spectr. 2022, 10, e0297922. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, G.; Lystad, K.Q.; Levine, D.W.; Dyrset, N. pH-controlled cell release and biomass distribution of alginate-immobilized Lactococcus lactis subsp. lactis. J. Appl. Microbiol. 2001, 91, 705–714. [Google Scholar] [CrossRef]
- Kawaguti, H.Y.; Sato, H.H. Production of isomaltulose obtained by Erwinia sp. cells submitted to different treatments and immobilized in calcium alginate. Food Sci. Technol. 2011, 31, 257–263. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Munarin, F.; Bozzini, S.; Visai, L.; Tanzi, M.C.; Petrini, P. Sterilization treatments on polysaccharides: Effects and side effects on pectin. Food Hydrocoll. 2013, 31, 74–84. [Google Scholar] [CrossRef]
- Leo, W.J.; McLoughlin, A.J.; Malone, D.M. Effects of sterilization treatments on some properties of alginate solutions and gels. Biotechnol. Prog. 1990, 6, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Serp, D.; Mueller, M.; Von Stockar, U.; Marison, I.W. Low-temperature electron microscopy for the study of polysaccharide ultrastructures in hydrogels. II. Effect of temperature on the structure of Ca2+-alginate beads. Biotechnol. Bioeng. 2002, 79, 253–259. [Google Scholar] [CrossRef]
- Funami, T.; Fang, Y.; Noda, S.; Ishihara, S.; Nakauma, M.; Draget, K.I.; Nishinari, K.; Phillips, G.O. Rheological properties of sodium alginate in an aqueous system during gelation in relation to supermolecular structures and Ca2+ binding. Food Hydrocoll. 2009, 23, 1746–1755. [Google Scholar] [CrossRef]
- Mano, J.F. Propiedades térmicas de los polímeros en la enseñanza de la ciencia de materiales e ingeniería. Estudios DSC sobre Poli (Tereftalato de Etileno). J. Mater. Educ. 2003, 25, 155–170. [Google Scholar]
- Hu, T.; Yang, Y.; Tan, L.; Yin, T.; Wang, Y.; Wang, G. Effects of gamma irradiation and moist heat for sterilization on sodium alginate. Biomed. Mater. Eng. 2014, 24, 1837–1849. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- McMaster, L.D.; Kokott, S.A. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods. World J. Microbiol. Biotechnol. 2005, 21, 723–728. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Yu, H.; Cauchois, G.; Schmitt, J.-F.; Louvet, N.; Six, J.-L.; Chen, Y.; Rahouadj, R.; Huselstein, C. Is there a cause-and-effect relationship between physicochemical properties and cell behavior of alginate-based hydrogel obtained after sterilization. J. Mech. Behav. Biomed. Mater. 2017, 68, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chansoria, P.; Narayanan, L.K.; Wood, M.; Alvarado, C.; Lin, A.; Shirwaiker, R.A. Effects of autoclaving, etoh, and uv sterilization on the chemical, mechanical, printability, and biocompatibility characteristics of alginate. ACS Biomater. Sci. Eng. 2020, 6, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- Moresi, M.; Bruno, M.; Parente, E. Viscoelastic properties of microbial alginate gels by oscillatory dynamic tests. J. Food Eng. 2004, 64, 179–186. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Holme, H.K.; Davidsen, L.; Kristiansen, A.; Smidsrød, O. Kinetics and mechanisms of depolymerization of alginate and chitosan in aqueous solution. Carbohydr. Polym. 2008, 73, 656–664. [Google Scholar] [CrossRef]
- Dartois, A.; Singh, J.; Kaur, L.; Singh, H. Influence of Guar Gum on the In Vitro Starch Digestibility—Rheological and Microstructural Characteristics. Food Biophys. 2010, 5, 149–160. [Google Scholar] [CrossRef]
- Gargallo, L.; Radic’, D. Chapter 2: Viscoelastic Behaviour of Polymers. In Physicochemical Behavior and Supramolecular Organization of Polymers; Springer: Dordrecht, The Netherlands, 1980; pp. 266–315. [Google Scholar] [CrossRef]
- Fuongfuchat, A.; Jamieson, A.M.; Blackwell, J.; Gerken, T.A. Rheological studies of the interaction of mucins with alginate and polyacrylate. Carbohydr. Res. 1996, 284, 85–99. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Hu, J.; Liu, H. Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method. Foods 2023, 12, 2256. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival during Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Brinques, G.B.; Ayub, M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J. Food Eng. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Zhao, M.; Qu, F.; Wu, Z.; Nishinari, K.; Phillips, G.O.; Fang, Y. Protection mechanism of alginate microcapsules with different mechanical strength for Lactobacillus plantarum ST-III. Food Hydrocoll. 2017, 66, 396–402. [Google Scholar] [CrossRef]
- Tsen, J.-H.; Huang, H.-Y.; King, V.A.-E. Enhancement of freezing-resistance of Lactobacillus rhamnosus by the application of cell immobilization. J. Gen. Appl. Microbiol. 2007, 53, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Bai, Y.; Wang, S.B.; Chen, A.Z.; Wu, W.G. Effect of the Storage Temperatures on BRL-encapsulated-alginate-PLO-alginate (APornA) Microcapsules. J. Biomim. Biomater. Tissue Eng. 2013, 18, 110. [Google Scholar] [CrossRef]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2015, 52, 4029–4041. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Heo, T.-R. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Appl. Environ. Microbiol. 2000, 66, 869–873. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.A.; Bártolo, P.J.; Mendes, A.; Filho, R.M. Rheological behavior of alginate solutions for biomanufacturing. J. Appl. Polym. 2009, 113, 3866–3871. [Google Scholar] [CrossRef]
- Andriamanantoanina, H.; Rinaudo, M. Characterization of the alginates from five madagascan brown algae. Carbohydr. Polym. 2010, 82, 555–560. [Google Scholar] [CrossRef]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- González-Sánchez, F.; Azaola, A.; Gutiérrez-López, G.F.; Hernández-Sánchez, H. Viability of microencapsulated Bifidobacterium animalis ssp. lactis BB12 in kefir during refrigerated storage. Int. J. Dairy Technol. 2010, 63, 431–436. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef] [PubMed]

| Sodium Alginate | Power-Law Model Fitting Parameters | ||
|---|---|---|---|
| n | k | R | |
| AD untreated | 0.514 | 7.627 | 0.998 |
| AD pasteurized | 0.513 | 7.551 | 0.998 |
| AD sterilized | 0.849 | 0.434 | 0.999 |
| AF untreated | 0.754 | 1.001 | 0.999 |
| AF pasteurized | 0.756 | 0.961 | 0.999 |
| AF sterilized | 0.986 | 0.044 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Gallegos, M.A.; Solorza-Feria, J.; Cornejo-Mazón, M.; Velázquez-Martínez, J.R.; Rodríguez-Huezo, M.E.; Gutiérrez-López, G.F.; Hernández-Sánchez, H. Protective Effect of Alginate Microcapsules with Different Rheological Behavior on Lactiplantibacillus plantarum 299v. Gels 2023, 9, 682. https://doi.org/10.3390/gels9090682
Hernández-Gallegos MA, Solorza-Feria J, Cornejo-Mazón M, Velázquez-Martínez JR, Rodríguez-Huezo ME, Gutiérrez-López GF, Hernández-Sánchez H. Protective Effect of Alginate Microcapsules with Different Rheological Behavior on Lactiplantibacillus plantarum 299v. Gels. 2023; 9(9):682. https://doi.org/10.3390/gels9090682
Chicago/Turabian StyleHernández-Gallegos, Minerva Aurora, Javier Solorza-Feria, Maribel Cornejo-Mazón, José Rodolfo Velázquez-Martínez, María Eva Rodríguez-Huezo, Gustavo F. Gutiérrez-López, and Humberto Hernández-Sánchez. 2023. "Protective Effect of Alginate Microcapsules with Different Rheological Behavior on Lactiplantibacillus plantarum 299v" Gels 9, no. 9: 682. https://doi.org/10.3390/gels9090682
APA StyleHernández-Gallegos, M. A., Solorza-Feria, J., Cornejo-Mazón, M., Velázquez-Martínez, J. R., Rodríguez-Huezo, M. E., Gutiérrez-López, G. F., & Hernández-Sánchez, H. (2023). Protective Effect of Alginate Microcapsules with Different Rheological Behavior on Lactiplantibacillus plantarum 299v. Gels, 9(9), 682. https://doi.org/10.3390/gels9090682






