Composite Hydrogel Modulates Intrinsic Immune-Cascade Neovascularization for Ocular Surface Reconstruction after Corneal Chemical Injury
Abstract
1. Introduction
2. Results and Discussion
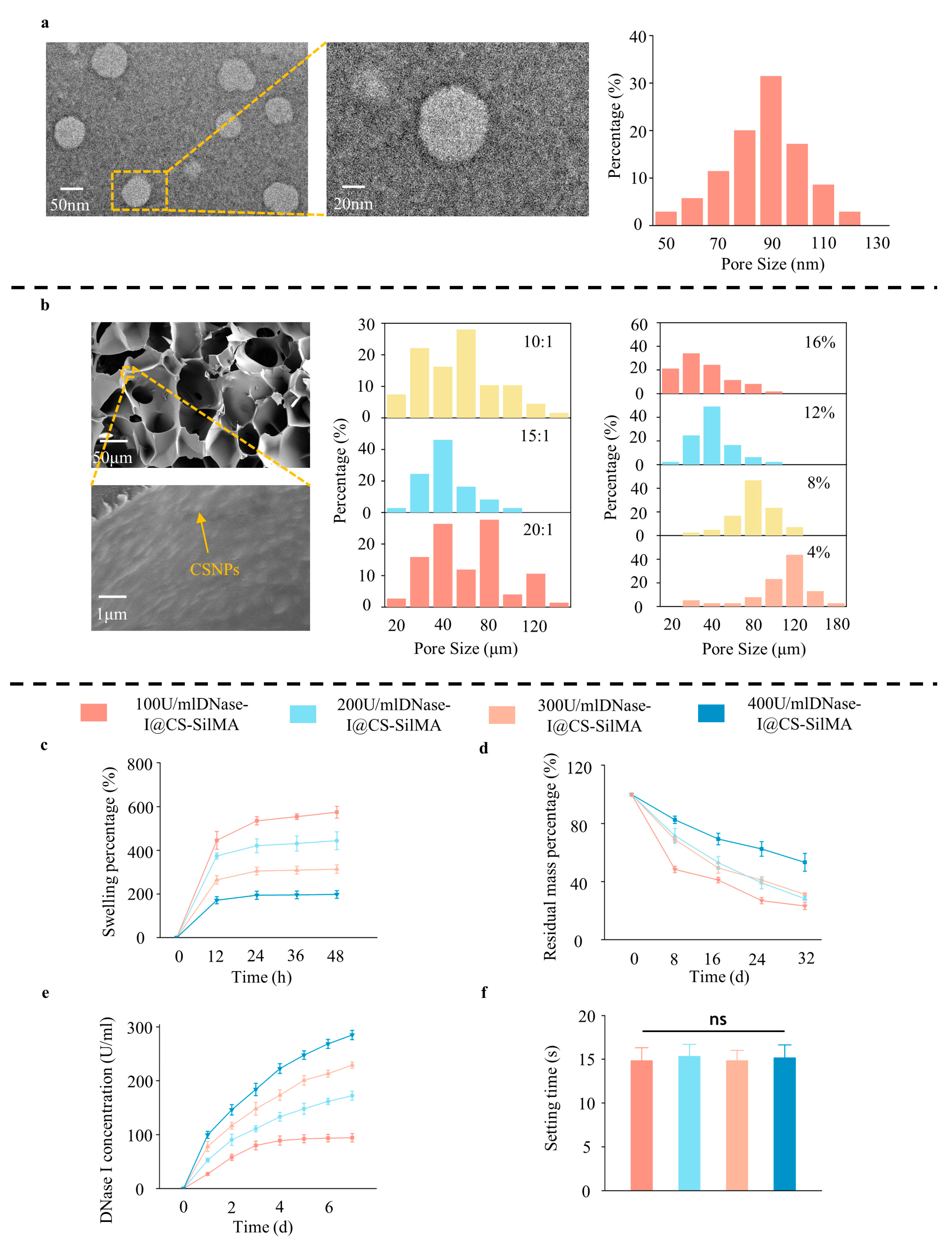
2.1. Preparation and Characterization of DNase I@CS-SilMA
2.2. SCD Biocompatibility Test
2.3. In Vitro Tests of SCD Inhibition of NETs
2.4. In Vivo Test of SCD Inhibition of NETs
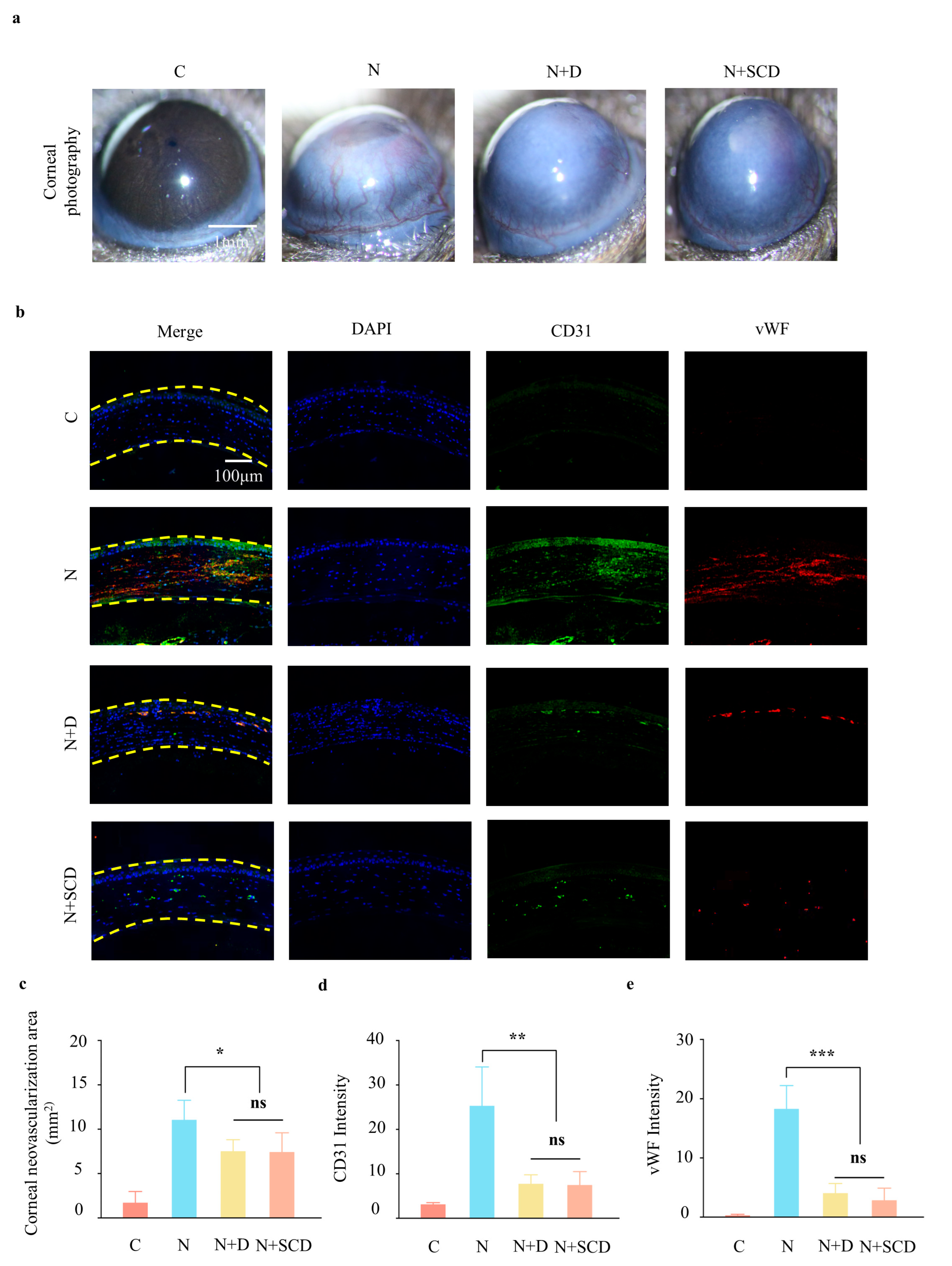
2.5. In Vivo Test of Neovascularization Inhibition by SCD
3. Conclusions
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Culture
4.3. Immunofluorescence Detection of cit-H3 and MPO
4.4. Construction of Corneal Alkali Burns Animal Models
4.5. Preparation of Composite Hydrogels with DNase I-Loaded Silk Chitosan Nanoparticles (SCD Ratios for 400 U/mL DNase-I@0.53%CS-8%SilMA as an Example)
4.6. SCD Composite Hydrogel Characterization
4.7. SCD Composite Hydrogel Biocompatibility Testing
4.8. Anterior Segment Photography
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dohlman, T.H.; Yin, J. Methods for Assessing Corneal Opacity. Semin. Ophthalmol. 2019, 34, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Clare, G.; Bunce, C.; Tuft, S. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst. Rev. 2022, 9, Cd009379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, W.; Bi, M.; Wu, J. The molecular mechanisms of action of PPAR-γ agonists in the treatment of corneal alkali burns (Review). Int. J. Mol. Med. 2016, 38, 1003–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, P.; Jiang, M.; Li, K.; Li, H.; Zhou, Y.; Xiao, X.; Xu, Y.; Krishfield, S.; Lipsky, P.E.; Tsokos, G.C.; et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 2021, 22, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Jarrot, P.A.; Kaplanski, G. Pathogenesis of ANCA-associated vasculitis: An update. Autoimmun. Rev. 2016, 15, 704–713. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Yuan, K.; Zheng, J.; Huang, X.; Zhang, Y.; Han, Y.; Hu, R.; Jin, X. Neutrophil extracellular traps promote corneal neovascularization-induced by alkali burn. Int. Immunopharmacol. 2020, 88, 106902. [Google Scholar] [CrossRef]
- Wan, T.; Zhang, Y.; Yuan, K.; Min, J.; Mou, Y.; Jin, X. Acetylsalicylic Acid Promotes Corneal Epithelium Migration by Regulating Neutrophil Extracellular Traps in Alkali Burn. Front. Immunol. 2020, 11, 551057. [Google Scholar] [CrossRef] [PubMed]
- Suck, D. DNA recognition by DNase I. J. Mol. Recognit. JMR 1994, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Liu, Z.; Chang, L.; Bai, X.; Kang, L. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood 2021, 138, 91–103. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Yazdani, H.O.; Morder, K.; Geller, D.A.; Simmons, R.L.; Tohme, S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021, 12, 785222. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y.; et al. pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. ACS Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef]
- Wang, S.; Ghezzi, C.E.; Gomes, R.; Pollard, R.E.; Funderburgh, J.L.; Kaplan, D.L. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 2017, 112, 1–9. [Google Scholar] [CrossRef]
- Koh, L.D.; Yeo, J.; Lee, Y.Y.; Ong, Q.; Han, M.; Tee, B.C. Advancing the frontiers of silk fibroin protein-based materials for futuristic electronics and clinical wound-healing (Invited review). Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 151–172. [Google Scholar] [CrossRef]
- Katas, H.; Raja, M.A.; Lam, K.L. Development of Chitosan Nanoparticles as a Stable Drug Delivery System for Protein/siRNA. Int. J. Biomater. 2013, 2013, 146320. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Li, Z.; Bratlie, K.M. The Influence of Polysaccharides-Based Material on Macrophage Phenotypes. Macromol. Biosci. 2021, 21, e2100031. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, F.; Zhang, X.; Peng, Z.; Jiang, B.; Liang, M.; Wang, Y. Silk fibroin hydrogel promote burn wound healing through regulating TLN1 expression and affecting cell adhesion and migration. J. Mater. Sci. Mater. Med. 2020, 31, 48. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.L.; Dana, R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin. Ophthalmol. 2011, 26, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Kobata, S.; Hashizume, N.; Okada, Y.; Yamanaka, O. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea 1993, 12, 383–390. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Friedman, M.; Azrad-Lebovitz, T.; Morzaev, D.; Zahavi, A.; Marianayagam, N.J.; Nicholson, J.D.; Brookman, M.; Michowiz, S.; Hochhauser, E.; Goldenberg-Cohen, N. Protective Effect of TLR4 Ablation against Corneal Neovascularization following Chemical Burn in a Mouse Model. Curr. Eye Res. 2019, 44, 505–513. [Google Scholar] [CrossRef]
- Benayoun, Y.; Casse, G.; Forte, R.; Dallaudière, B.; Adenis, J.P.; Robert, P.Y. Corneal neovascularization: Epidemiological, physiopathological, and clinical features. J. Fr. d’Ophtalmol. 2013, 36, 627–639. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 385–395. [Google Scholar] [CrossRef]
- Gu, X.J.; Liu, X.; Chen, Y.Y.; Zhao, Y.; Xu, M.; Han, X.J.; Liu, Q.P.; Yi, J.L.; Li, J.M. Involvement of NADPH oxidases in alkali burn-induced corneal injury. Int. J. Mol. Med. 2016, 38, 75–82. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xi, K.; Deng, G.; Zou, X.; Lu, P. Composite Hydrogel Modulates Intrinsic Immune-Cascade Neovascularization for Ocular Surface Reconstruction after Corneal Chemical Injury. Gels 2023, 9, 676. https://doi.org/10.3390/gels9090676
Zhang J, Xi K, Deng G, Zou X, Lu P. Composite Hydrogel Modulates Intrinsic Immune-Cascade Neovascularization for Ocular Surface Reconstruction after Corneal Chemical Injury. Gels. 2023; 9(9):676. https://doi.org/10.3390/gels9090676
Chicago/Turabian StyleZhang, Jun, Kun Xi, Guohua Deng, Xi Zou, and Peirong Lu. 2023. "Composite Hydrogel Modulates Intrinsic Immune-Cascade Neovascularization for Ocular Surface Reconstruction after Corneal Chemical Injury" Gels 9, no. 9: 676. https://doi.org/10.3390/gels9090676
APA StyleZhang, J., Xi, K., Deng, G., Zou, X., & Lu, P. (2023). Composite Hydrogel Modulates Intrinsic Immune-Cascade Neovascularization for Ocular Surface Reconstruction after Corneal Chemical Injury. Gels, 9(9), 676. https://doi.org/10.3390/gels9090676






