Tissue Reaction to Low-Density Polyacrylamide Gel as a Carrier for Microimplants in the Adipose Fin of Rainbow Trout
Abstract
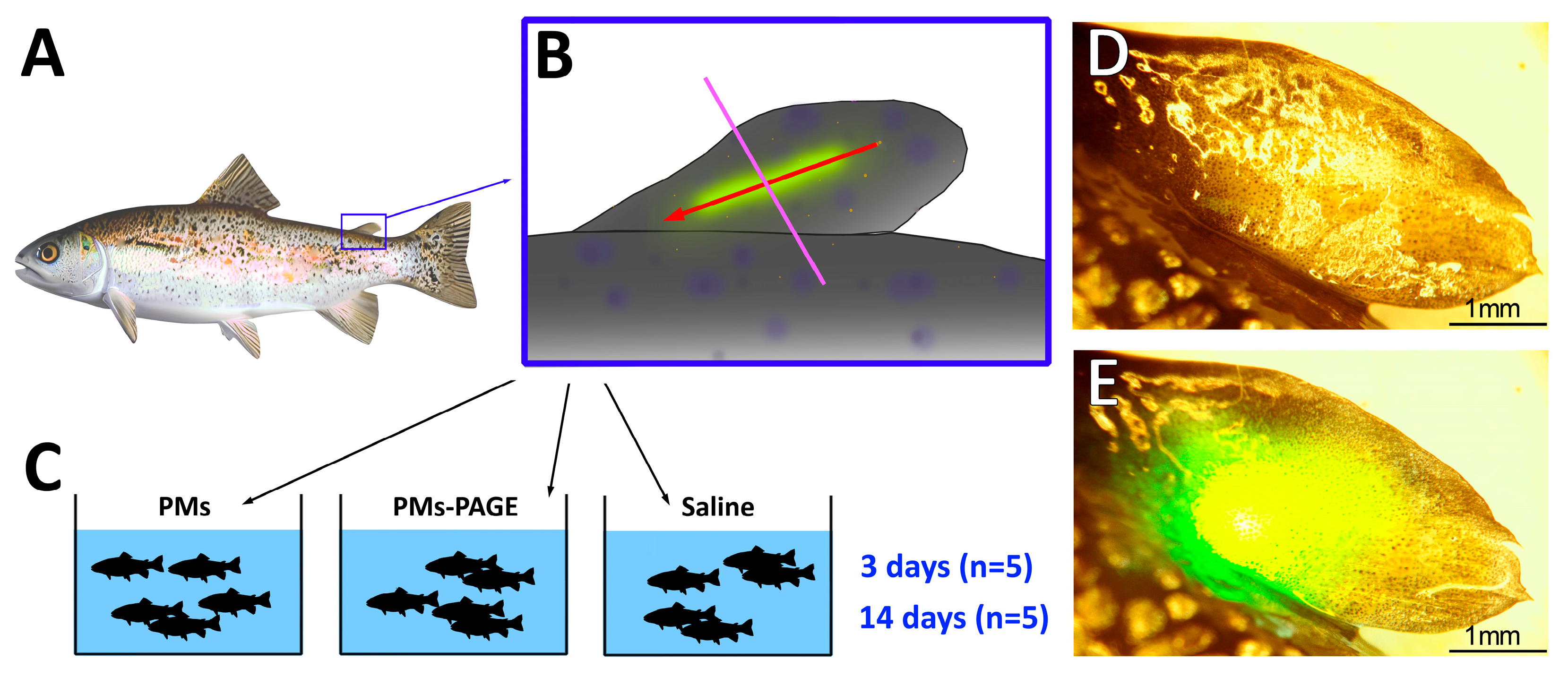
1. Introduction
2. Results
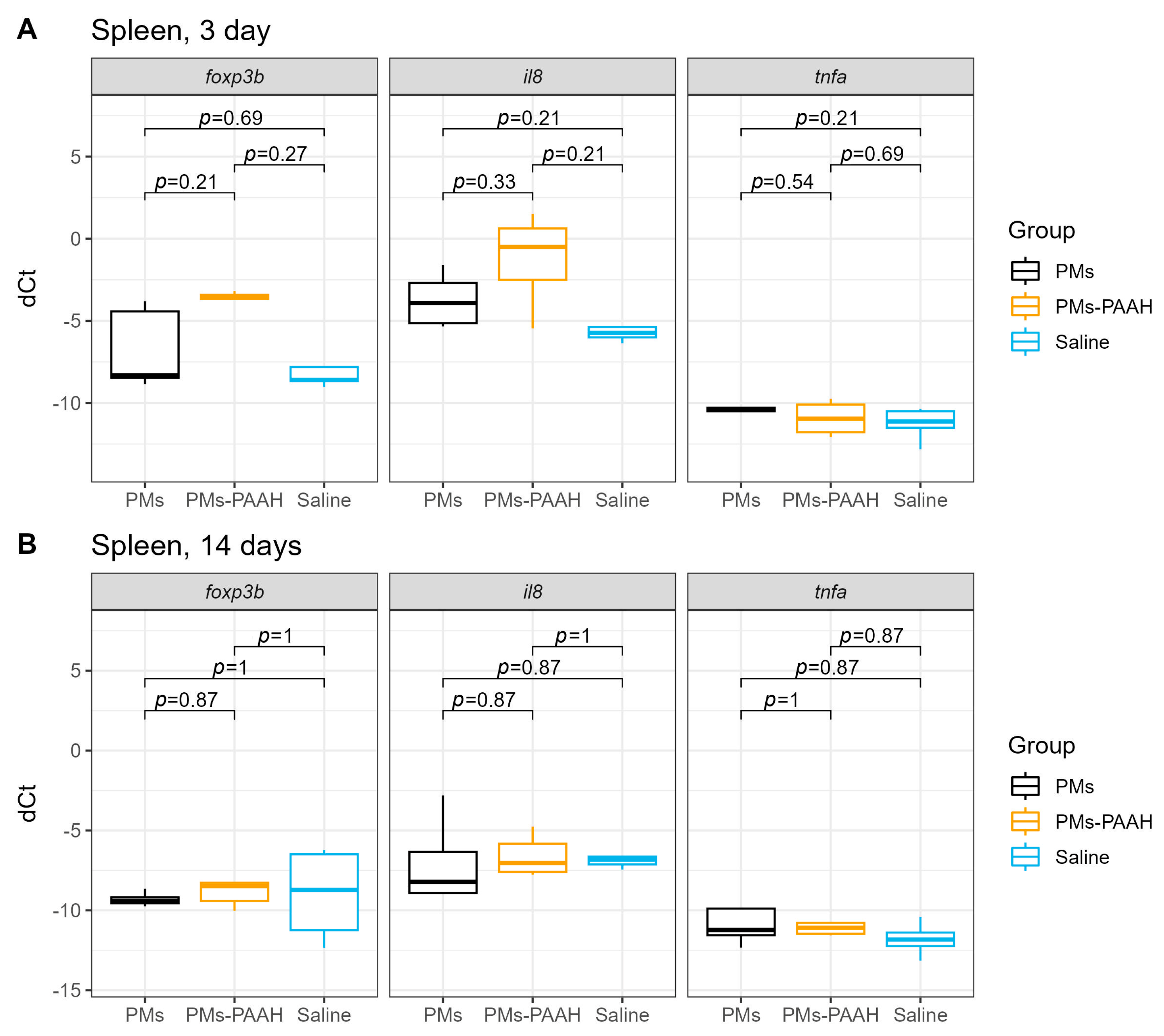
2.1. Immune Genes Expression in the Spleen
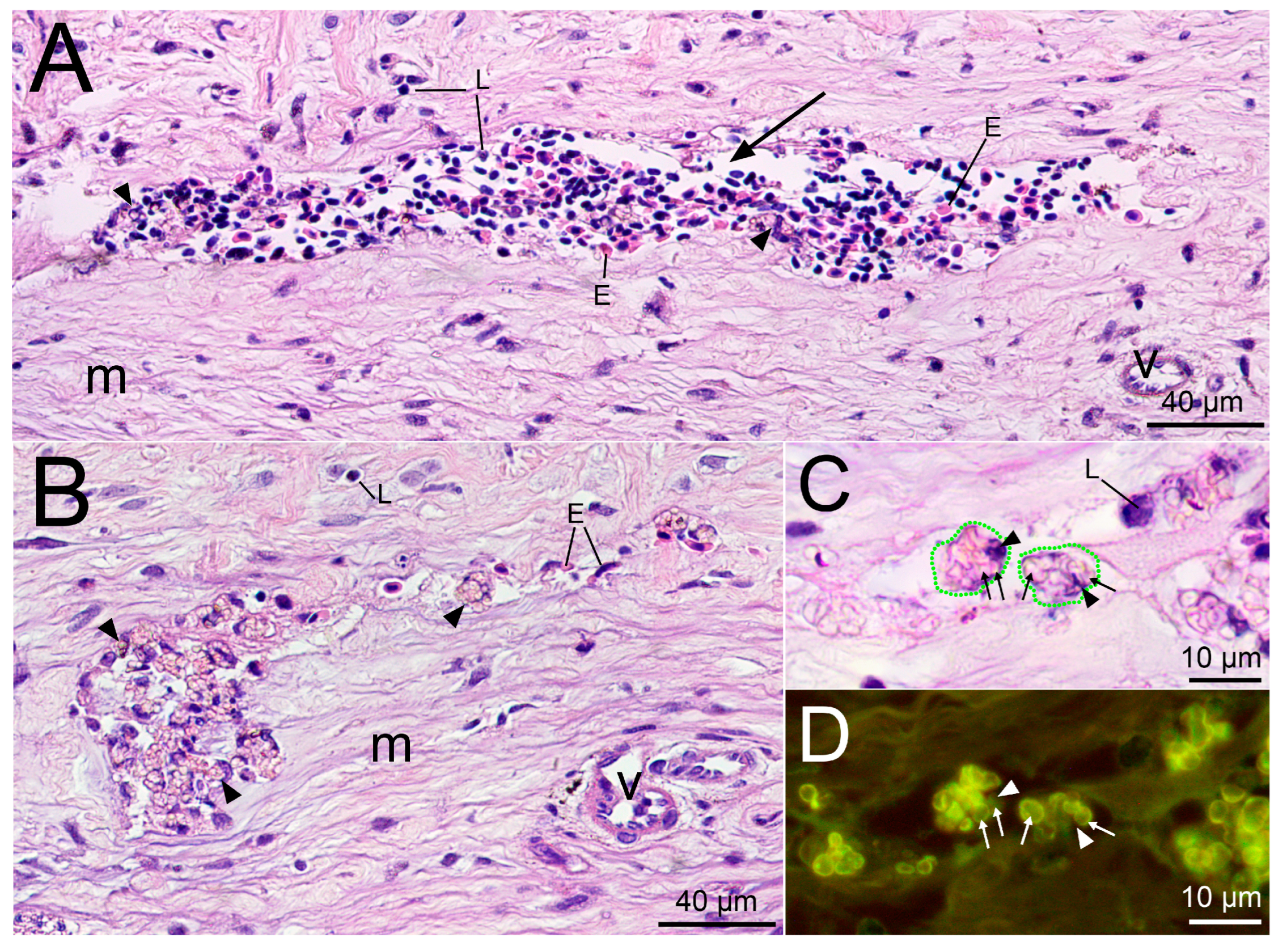
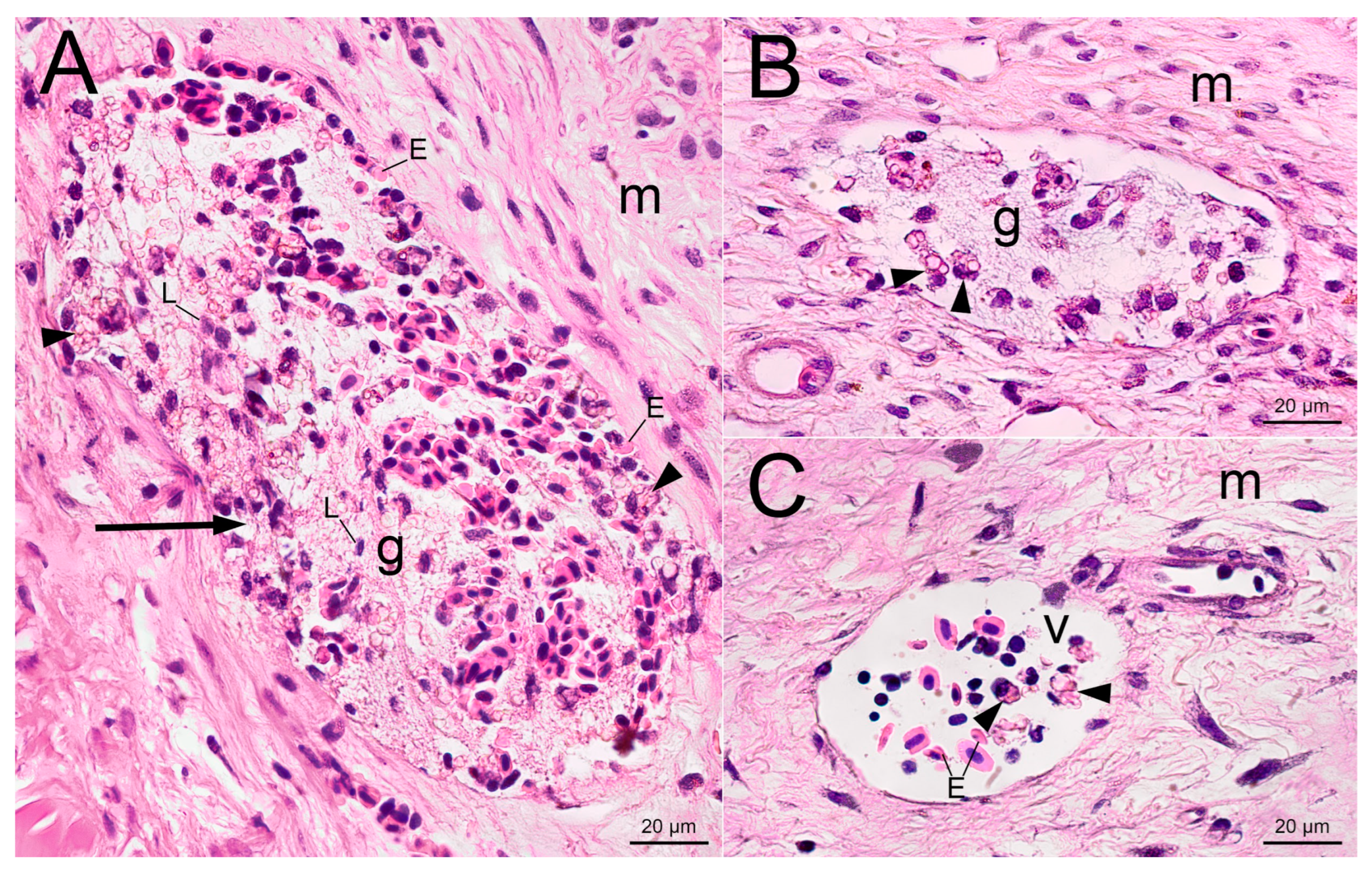
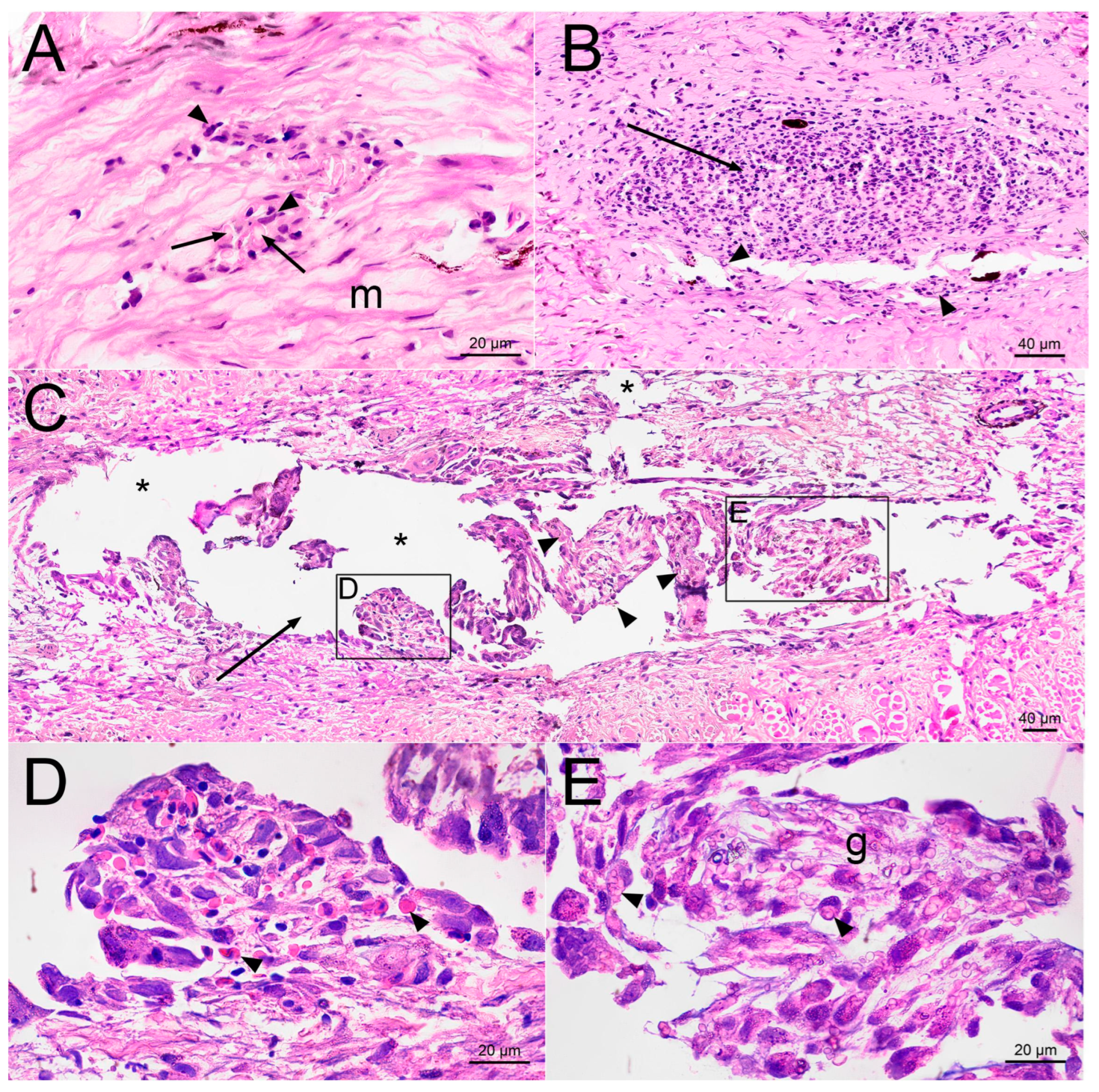
2.2. Histological Examination
3. Discussion
4. Materials and Methods
4.1. Preparation of Microcapsules
4.2. Preparation of Injection Mixtures
4.3. Animals and Housing
4.4. Injection into the Adipose Fin
4.5. RNA Extraction and Quantitative PCR (qPCR)
4.6. Histological and Histopathological Examination
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Bian, S.; Zhu, B.; Rong, G.; Sawan, M. Towards wearable and implantable continuous drug monitoring: A review. J. Pharm. Anal. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the Foreign−Body Response to Implanted Biomaterials: Strategies and Applications of New Materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Montero−Baker, M.F.; Au−Yeung, K.Y.; Wisniewski, N.A.; Gamsey, S.; Morelli−Alvarez, L.; Mills, J.L., Sr.; Campos, M.; Helton, K.L. The First−in−Man “Si Se Puede” Study for the use of micro−oxygen sensors (MOXYs) to determine dynamic relative oxygen indices in the feet of patients with limb−threatening ischemia during endovascular therapy. J. Vasc. Surg. 2015, 61, 1501–1510.E1. [Google Scholar] [CrossRef]
- Lee, M.A.; Nguyen, F.T.; Scott, K.; Chan, N.Y.L.; Bakh, N.A.; Jones, K.K.; Pham, C.; Garcia−Salinas, P.; Garcia−Parraga, D.; Fahlman, A.; et al. Implanted Nanosensors in Marine Organisms for Physiological Biologging: Design, Feasibility, and Species Variability. ACS Sens. 2019, 4, 32–43. [Google Scholar] [CrossRef]
- Kaefer, K.; Krüger, K.; Schlapp, F.; Uzun, H.; Celiksoy, S.; Flietel, B.; Heimann, A.; Schroeder, T.; Kempski, O.; Sönnichsen, C. Implantable Sensors Based on Gold Nanoparticles for Continuous Long−Term Concentration Monitoring in the Body. Nano Lett. 2021, 21, 3325–3330. [Google Scholar] [CrossRef]
- Kanick, S.C.; Schneider, P.A.; Klitzman, B.; Wisniewski, N.A.; Rebrin, K. Continuous monitoring of interstitial tissue oxygen using subcutaneous oxygen microsensors: In vivo characterization in healthy volunteers. Microvasc. Res. 2019, 124, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Fahimipour, F.; Keith, A.N.; Vashahi, F.; Popryadukhin, P.; Vatankhah−Varnosfaderani, M.; Sheiko, S.S. Injectable non−leaching tissue−mimetic bottlebrush elastomers as an advanced platform for reconstructive surgery. Nat. Commun. 2021, 12, 3961. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Cheml. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Andrus, L.P.; Unruh, R.; Wisniewski, N.A.; McShane, M.J. Characterization of Lactate Sensors Based on Lactate Oxidase and Palladium Benzoporphyrin Immobilized in Hydrogels. Biosensors 2015, 5, 398–416. [Google Scholar] [CrossRef]
- Rong, G.; Tuttle, E.E.; Neal Reilly, A.; Clark, H.A. Recent Developments in Nanosensors for Imaging Applications in Biological Systems. Annu. Rev. Anal. Chem. 2019, 12, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Jin, X.; Muthupalani, S.; Bakh, N.A.; Gong, X.; Strano, M.S. In−Vivo fluorescent nanosensor implants based on hydrogel−encapsulation: Investigating the inflammation and the foreign−body response. J. Nanobiotechnol. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Pollard, S.; Anderson, J.; Bah, F.; Mateus, M.; Sidhu, M.; Simmons, D. Non−Lethal Blood Sampling of Fish in the lab and Field with Methods for Dried Blood Plasma Spot Omic Analyses. Front. Genet 2022, 13, 795348. [Google Scholar] [CrossRef] [PubMed]
- Sihoka, C.; Wagenaar, I. Model−based serial blood sampling protocol for minimal mortality and better recovery in small to medium sized tilapia. Biol. Open 2018, 7, bio037978. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Alfaro, A.C.; Young, T. Metabolomics in salmonid aquaculture research: Applications and future perspectives. Aquac. Res. 2022, 14, 547–577. [Google Scholar] [CrossRef]
- Rzhechitskiy, Y.; Gurkov, A.; Bolbat, N.; Shchapova, E.; Nazarova, A.; Timofeyev, M.; Borvinskaya, E. Adipose Fin as a Natural “Optical Window” for Implantation of Fluorescent Sensors into Salmonid Fish. Animals 2022, 12, 3042. [Google Scholar] [CrossRef] [PubMed]
- Shchapova, E.; Titov, E.; Gurkov, A.; Nazarova, A.; Borvinskaya, E.; Timofeyev, M. Durability of Implanted Low−Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish. Polymers 2022, 14, 3956. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long−term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef]
- Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int. J. Toxicol. 2005, 24, 21–50. [CrossRef]
- von Buelow, S.; Norbert, P. Efficacy and Safety of Polyacrylamide Hydrogel for Facial Soft−Tissue Augmentation in a 2−Year Follow−Up: A Prospective Multicenter Study for Evaluation of Safety and Aesthetic Results in 101 Patients. Plast Reconstr. Surg. 2006, 118, 85S–91S. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Weisel, G.F. The salmonoid adipose fin. Copeia 1968, 3, 626–627. [Google Scholar] [CrossRef]
- Buckland−Nicks, J.A.; Gillis, M.; Reimchen, T.E. Neural network detected in a presumed vestigial trait: Ultrastructure of the salmonid adipose fin. Proc. R. Soc. B Biol. Sci. 2012, 279, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.A.; Smith, W.L.; Coates, M.I. The origins of adipose fins: An analysis of homoplasy and the serial homology of vertebrate appendages. Proc. Biol. Sci. 2014, 281, 20133120. [Google Scholar] [CrossRef]
- Stewart, T.A.; Bonilla, M.M.; Ho, R.K.; Hale, M.E. Adipose fin development and its relation to the evolutionary origins of median fins. Sci. Rep. 2019, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Buckland−Nicks, J.A. New details of the neural architecture of the salmonid adipose fin. J. Fish. Biol. 2016, 89, 1991–2003. [Google Scholar] [CrossRef]
- Wisniewski, N.A.; Nichols, S.P.; Gamsey, S.J.; Pullins, S.; Au−Yeung, K.Y.; Klitzman, B.; Helton, K.L. Tissue−Integrating Oxygen Sensors: Continuous Tracking of Tissue Hypoxia. Adv. Exp. Med. Biol. 2017, 977, 377–383. [Google Scholar]
- Chen, L.; Sha, L.; Huang, S.P.; Li, S.R.; Wang, Z.X. Treatment for displacement of PAG mixture after injection augmentation mammoplasty. Int. J. Clin. Exp. Med. 2015, 8, 3360–3370. [Google Scholar]
- Peng, H.L.; Cheng, Y.H.; Lin, Y.H.; Ko, C.H. Unusual presentation of a late complication in a polyacrylamide gel−injected breast. Formos. J. Surg. 2017, 50, 77–80. [Google Scholar] [CrossRef]
- Zhou, Z.; Ru, K. −H. Observation on biologic characterization of polyacrylamide gel and silicon gel infused into mammary gland of domestic musk swine. Chin. J. Pract. Aesthet Plast Sur. 2001, 12, 69–71. (In Chinese) [Google Scholar]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Meglinski, I.; Timofeyev, M. Distribution of PEG−coated hollow polyelectrolyte microcapsules after introduction into the circulatory system and muscles of zebrafish. Biol. Open 2018, 7, bio030015. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Mutin, A.; Timofeyev, M. Histopathological analysis of zebrafish after introduction of non−biodegradable polyelectrolyte microcapsules into the circulatory system. PeerJ 2021, 9, e11337. [Google Scholar] [CrossRef] [PubMed]
- Gurkov, A.; Shchapova, E.; Bedulina, D.; Baduev, B.; Borvinskaya, E.; Meglinski, I.; Timofeyev, M. Remote in vivo stress assessment of aquatic animals with microencapsulated biomarkers for environmental monitoring. Sci. Rep. 2016, 6, 36427. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Shatilina, Z.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Parallel in vivo monitoring of pH in gill capillaries and muscles of fishes using microencapsulated biomarkers. Biol. Open 2017, 6, 673–677. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Karnaukhov, D.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Simple and Effective Administration and Visualization of Microparticles in the Circulatory System of Small Fishes Using Kidney Injection. J. Vis. Exp. 2018, 136, 57491. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda−Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open−source platform for biological−image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Wahli, T.; Verlhac, V.; Girling, P.; Gabaudan, J.; Aebischer, C. Influence of dietary vitamin C on the wound healing process in rainbow trout (Oncorhynchus mykiss). Aquaculture 2003, 225, 371–386. [Google Scholar] [CrossRef]
- Ang, J.; Pierezan, F.; Kim, S.; Heckman, T.I.; Sebastiao, F.A.; Yazdi, Z.; Abdelrazek, S.M.R.; Soto, E. Use of topical treatments and effects of water temperature on wound healing in common carp (Cyprinus carpio). J. Zoo Wildl. Med. 2021, 52, 103–116. [Google Scholar] [CrossRef]
- Ceballos−Francisco, D.; García−Carrillo, N.; Cuesta, A.; Esteban, M.Á. Ultrasonography study of the skin wound healing process in gilthead seabream (Sparus aurata). J. Fish Dis. 2021, 44, 1091–1100. [Google Scholar] [CrossRef]
- Gorgoglione, B.; Wang, T.; Secombes, C.J.; Holland, J.W. Immune gene expression profiling of Proliferative Kidney Disease in rainbow trout Oncorhynchus mykiss reveals a dominance of anti−inflammatory, antibody and T helper cell−like activities. Vet. Res. 2013, 44, 55. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.S.; Chen, C.C.; Wood, C.M.; Kelly, S.P. Effects of copper on a reconstructed freshwater rainbow trout gill epithelium: Paracellular and intracellular aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108705. [Google Scholar] [CrossRef] [PubMed]
- Tongsri, P.; Meng, K.; Liu, X.; Wu, Z.; Yin, G.; Wang, Q.; Liu, M.; Xu, Z. The predominant role of mucosal immunoglobulin IgT in the gills of rainbow trout (Oncorhynchus mykiss) after infection with Flavobacterium columnare. Fish Shellfish Immunol. 2020, 99, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a Class of Permutation Tests: The coin Package. J. Stat Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R−project.org/ (accessed on 15 June 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borvinskaya, E.; Matrosova, S.; Sukhovskaya, I.; Drozdova, P.; Titov, E.; Anikienko, I.; Lubyaga, Y.; Gurkov, A.; Timofeyev, M. Tissue Reaction to Low-Density Polyacrylamide Gel as a Carrier for Microimplants in the Adipose Fin of Rainbow Trout. Gels 2023, 9, 629. https://doi.org/10.3390/gels9080629
Borvinskaya E, Matrosova S, Sukhovskaya I, Drozdova P, Titov E, Anikienko I, Lubyaga Y, Gurkov A, Timofeyev M. Tissue Reaction to Low-Density Polyacrylamide Gel as a Carrier for Microimplants in the Adipose Fin of Rainbow Trout. Gels. 2023; 9(8):629. https://doi.org/10.3390/gels9080629
Chicago/Turabian StyleBorvinskaya, Ekaterina, Svetlana Matrosova, Irina Sukhovskaya, Polina Drozdova, Evgeniy Titov, Inna Anikienko, Yulia Lubyaga, Anton Gurkov, and Maxim Timofeyev. 2023. "Tissue Reaction to Low-Density Polyacrylamide Gel as a Carrier for Microimplants in the Adipose Fin of Rainbow Trout" Gels 9, no. 8: 629. https://doi.org/10.3390/gels9080629
APA StyleBorvinskaya, E., Matrosova, S., Sukhovskaya, I., Drozdova, P., Titov, E., Anikienko, I., Lubyaga, Y., Gurkov, A., & Timofeyev, M. (2023). Tissue Reaction to Low-Density Polyacrylamide Gel as a Carrier for Microimplants in the Adipose Fin of Rainbow Trout. Gels, 9(8), 629. https://doi.org/10.3390/gels9080629







