Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications
Abstract
1. Introduction
2. Tissue Engineering Scaffolds for Vascular Regeneration
3. Technologies for the Preparation of Chitosan Hydrogel Scaffolds in Vascular Remodeling
3.1. Macrovascular Remodeling Scaffolds
3.1.1. Biological Coating Technology
3.1.2. Electrospinning Technology
3.1.3. 3D Printing Technology
3.2. Microvascular Remodeling Scaffolds
3.3. Other Common Technologies in Vascular Remodeling
4. Chitosan and Its Derivatives for Vascular Regeneration
| Modification Method | Material | Characteristic | Application | Producers’ Company | Specifications |
|---|---|---|---|---|---|
| Chitin deacetylated | Chitosan | Odorless and non-toxic solid, mostly white or off-white flake or powder, containing amino polysaccharide, soluble in aqueous acetic acid | Pharmaceutical active ingredient [127,128,129]; Drug dispersant [130,131]; Drug requests [132,133] | Shanghai Macklin’s Reagent Co. Shanghai Aladdin Biochemical Technology Co. etc. | Degree of deacetylation: 85%, 90%, 95%, 98% Viscosity (mPa·s): High (>400), Medium (200–400), Low (<200) |
| Chitosanase degradation | Chitosan oligosaccharide | Oligosaccharide polymerized from less than 10 glucosamine. | Enhanced cartilage [134,135,136]; Antioxidant [137,138]; Strengthens the immune system [139,140,141] | Adamas Reagents Ltd. Shanghai Macklin’s Reagent Co. etc. | Purity: 3000 Molecular weight Chitosan oligosaccharide lactate (<2000 Molecular weight) |
| Chemical modification of chitosan | Carboxymethyl chitosan | Introduction of carboxymethyl, amphoteric polyelectrolyte, according to the substitution of carboxymethyl, three products can be obtained. | Hemostatic materials [142,143,144]; Wound healing dressing [145,146,147]; Tissue repair gel [148,149,150,151] | Tianjin Heowns Biochem Technology Co. Leanlong Bohua (Tianjin) Pharmaceutical Chemicals Co. Dalian Meilun Biotechnology Co. Shanghai Yuanye Biotechnology Co. etc. | Degree of deacetylation: 85% Viscosity (mPa·s): 10–80 Carboxylation: ≥80% |
| Hydroxypropyl chitosan | Hydroxypropyl group weakens the intermolecular and intramolecular hydrogen bonds of chitosan, destroys the spatial structure of chitosan, and enhances its water solubility and reactivity. | Increased healing tension [152] and moisturizing [153,154] | Shanghai Macklin’s Reagent Co. Shanghai Meryer Biochemical Technology Co. etc. | Substitution: ≥80% | |
| Quaternized chitosan | Introducing quaternary ammonium group or directly grafted small molecular quaternary ammonium salt to chitosan-NH3 active group. | Antiseptic bacteriostasis [155]; DNA carrier [156,157,158]; Composite film material [159,160,161] | Shanghai Macklin’s Reagent Co. Shanghai Yuanye Biotechnology Co. etc. | Substitution: 90–98% | |
| Chitosan sulfate | a modified product of SO42− group introduced into chitosan after vulcanization treatment. It is a polyamphoteric electrolyte, similar in structure to heparin. | Heparin substitutes [162,163,164,165]; Nonspecific immune enhancers [166,167,168] | - | - |
4.1. Carboxymethyl Chitosan
4.2. Quaternary Ammonium Modified Chitosan
4.3. Chitosan Sulfate
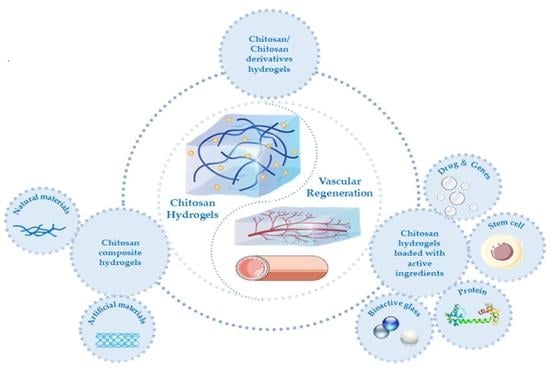
5. Chitosan Hydrogels and Their Applications in Vascular Remodeling
5.1. Chitosan and Its Derivative Hydrogels
5.2. Chitosan Composite Hydrogels
5.2.1. Chitosan/Natural Material Composite Hydrogels
5.2.2. Chitosan/Artificial Material Composite Hydrogel
5.3. Chitosan Hydrogels Loaded with Active Ingredients
5.3.1. Pharmaceutical Molecules and Genes
5.3.2. Stem Cells
5.3.3. Stem Cell Exosomes
5.3.4. Proteins
5.3.5. Bioactive Glass
5.3.6. Others
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavakol, D.N.; Fleischer, S.; Falcucci, T.; Graney, P.L.; Halligan, S.P.; Kaplan, D.L.; Vunjak-Novakovic, G. Emerging Trajectories for Next Generation Tissue Engineers. Acs Biomater. Sci. Eng. 2021, 8, 4598–4604. [Google Scholar] [CrossRef]
- Jenndahl, L.; Osterberg, K.; Bogestal, Y.; Simsa, R.; Gustafsson-Hedberg, T.; Stenlund, P.; Petronis, S.; Krona, A.; Fogelstrand, P.; Strehl, R.; et al. Personalized tissue-engineered arteries as vascular graft transplants: A safety study in sheep. Regen. Ther. 2022, 21, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situformation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, Q.; Lin, S.; Yuan, W.; Li, R.; Li, J.; Wei, K.; Chen, X.; Zhang, K.; Yang, Y.; et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 2019, 210, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Zhou, S.; Bei, Z.; Wei, J.; Yan, X.; Wen, H.; Cao, Y.; Li, H. Mussel-inspired injectable chitosan hydrogel modified with catechol for cell adhesion and cartilage defect repair. J. Mater. Chem. B 2022, 10, 1019–1030. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Z.; Zhang, Y.; Tian, J. Study on Chitosan-Based Nanocomposite Hydrogel in Soft Tissue Defect of Hand. Nanosci. Nanotechnol. Lett. 2020, 12, 1120–1126. [Google Scholar] [CrossRef]
- Mehrabi, A.; Karimi, A.; Mashayekhan, S.; Samadikuchaksaraei, A.; Milan, P.B. In-situ forming hydrogel based on thiolated chitosan/carboxymethyl cellulose (CMC) containing borate bioactive glass for wound healing. Int. J. Biol. Macromol. 2022, 222, 620–635. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801433. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv. Mater. 2022, 34, 2202044. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Ye, F.; Zhao, Y. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Huling, J.; Min, S.-i.; Kim, D.S.; Ko, I.K.; Atala, A.; Yoo, J.J. Kidney regeneration with biomimetic vascular scaffolds based on vascular corrosion casts. Acta Biomater. 2019, 95, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Duijvelshoff, R.; Cabrera, M.S.; Sanders, B.; Dekker, S.; Smits, A.I.P.M.; Baaijens, F.P.T.; Bouten, C.V.C. Transcatheter-Delivered Expandable Bioresorbable Polymeric Graft With Stenting Capacity Induces Vascular Regeneration. JACC-Basic Transl. Sci. 2020, 5, 1095–1110. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Yang, L.; Sun, L.; Mu, S.; Zong, H.; Li, Q.; Wang, F.; Song, S.; Yang, C.; et al. Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111441. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Wu, Y.; Sun, Z.; Li, B.; Jing, H.; Zhang, C.; Li, C.; Leng, X.; Wang, Z.; et al. The surrounding tissue contributes to smooth muscle cells’ regeneration and vascularization of small diameter vascular grafts. Biomater. Sci. 2019, 7, 914–925. [Google Scholar] [CrossRef]
- Peifen, M.; Mengyun, L.; Jinglong, H.; Danqian, L.; Yan, T.; Liwei, X.; Han, Z.; Jianlong, D.; Lingyan, L.; Guanghui, Z.; et al. New skin tissue engineering scaffold with sulfated silk fibroin/chitosan/hydroxyapatite and its application. Biochem. Biophys. Res. Commun. 2022, 640, 117–124. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M.S.; Bonakdar, S. Electrospun Skin Tissue Engineering Scaffolds Based on Polycaprolactone/Hyaluronic Acid/L-ascorbic Acid. Fibers Polym. 2021, 22, 19–29. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Togay, S.O.; Sengil, A.Z.; Ekren, N.; Haskoylu, M.E.; Oner, E.T.; Altuncu, N.A.; Ozturk, G.; Crabbe-Mann, M.; et al. Bioinspired scaffold induced regeneration of neural tissue. Eur. Polym. J. 2019, 114, 98–108. [Google Scholar] [CrossRef]
- Niu, Y.; Galluzzi, M. A biodegradable block polyurethane nerve-guidance scaffold enhancing rapid vascularization and promoting reconstruction of transected sciatic nerve in Sprague-Dawley rats. J. Mater. Chem. B 2020, 8, 11063–11073. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, Z.; Ma, J.; Ma, X. Modulation of angiogenic potential of tissue-engineered peripheral nerve by covalent incorporation of heparin and loading with vascular endothelial growth factor. Neurosci. Lett. 2019, 705, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, J.; Kador, K.E.; Lynch, A.P.; Ahearne, M. Characterization of extracellular matrix modified poly(epsilon-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 108, 110415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, S.; Choi, J.H.; Jung, B.S.; Kim, K.S.; Song, J.E.; Reis, R.L.; Khang, G. Enhanced Silk Fibroin-Based Film Scaffold Using Curcumin for Corneal Endothelial Cell Regeneration. Macromol. Res. 2021, 29, 713–719. [Google Scholar] [CrossRef]
- Xue, Y.; Kim, H.-J.; Lee, J.; Liu, Y.; Hoffman, T.; Chen, Y.; Zhou, X.; Sun, W.; Zhang, S.; Cho, H.-J.; et al. Co-Electrospun Silk Fibroin and Gelatin Methacryloyl Sheet Seeded with Mesenchymal Stem Cells for Tendon Regeneration. Small 2022, 18, 2107714. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yao, Z.; Wang, C. Study of a new nano-hydroxyapatite/basic fibroblast growth factor composite promoting periodontal tissue regeneration. Mater. Express 2020, 10, 1802–1807. [Google Scholar] [CrossRef]
- Sahbazoglu, K.B.; Demirbilek, M.; Bayari, S.H.; Buber, E.; Toklucu, S.; Turk, M.; Karabulut, E.; Akalin, F.A. In vitro comparison of nanofibrillar and macroporous-spongious composite tissue scaffolds for periodontal tissue engineering. Connect. Tissue Res. 2022, 63, 183–197. [Google Scholar] [CrossRef]
- Yao, Y.; Raymond, J.E.; Kauffmann, F.; Maekawa, S.; Sugai, J.V.; Lahann, J.; Giannobile, W.V. Multicompartmental Scaffolds for Coordinated Periodontal Tissue Engineering. J. Dent. Res. 2022, 101, 1457–1466. [Google Scholar] [CrossRef]
- Thottappillil, N.; Nair, P.D. Dual source co-electrospun tubular scaffold generated from gelatin-vinyl acetate and poly-epsilon-caprolactone for smooth muscle cell mediated blood vessel engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111030. [Google Scholar] [CrossRef]
- Dastagir, K.; Dastagir, N.; Limbourg, A.; Reimers, K.; Strauss, S.; Vogt, P.M. In vitro construction of artificial blood vessels using spider silk as a supporting matrix. J. Mech. Behav. Biomed. Mater. 2020, 101, 103436. [Google Scholar] [CrossRef]
- Altinova, H.; Hammes, S.; Palm, M.; Achenbach, P.; Gerardo-Nava, J.; Deumens, R.; Fuehrmann, T.; van Neerven, S.G.A.; Hermans, E.; Weis, J.; et al. Dense fibroadhesive scarring and poor blood vessel-maturation hamper the integration of implanted collagen scaffolds in an experimental model of spinal cord injury. Biomed. Mater. 2020, 15, 015012. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Brigo, L.; Tirinato, L.; Pagliari, F.; Gandin, A.; Contessotto, P.; Giugni, A.; Brusatin, G. Three-dimensionally two-photon lithography realized vascular grafts. Biomed. Mater. 2021, 16, 035013. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Shen, Z.; Li, M.; Zhang, W.; Liu, Y.; Zhang, Y.; Duan, J.; Ma, Z.; Sang, S. 3D printed GelMA/carboxymethyl chitosan composite scaffolds for vasculogenesis. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 524–536. [Google Scholar] [CrossRef]
- Ichanti, H.; Sladic, S.; Kalies, S.; Haverich, A.; Andree, B.; Hilfiker, A. Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels 2020, 6, 27. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Zhao, Y.; Ma, P.; Zha, S.; Chen, P.; Lu, H.; Jiang, X.; Wan, S.; Luo, J.; et al. Dual-Peptide-Functionalized Nanofibrous Scaffolds Recruit Host Endothelial Progenitor Cells for Vasculogenesis to Repair Calvarial Defects. Acs Appl. Mater. Interfaces 2020, 12, 3474–3493. [Google Scholar] [CrossRef]
- Pulat, G.O.; Gokmen, O.; Cevik, Z.B.Y.; Karaman, O. Role of functionalized self-assembled peptide hydrogels in in vitro vasculogenesis. Soft Matter 2021, 17, 6616–6626. [Google Scholar] [CrossRef]
- Ding, C.-F.; Lin, Y.-C.; Yang, C.-C.; Chang, C.-M.; Huang, K.-C.; Tseng, S.-F.; Lin, K.-M.; Hsiao, W.-T. Surface Modification of Addition Manufactured Ti-6Al-4V Alloys by Ultraviolet Pulsed Laser Scanning Technique: Morphologies, Roughness And Electrical Properties. J. Laser Micro Nanoeng. 2021, 16, 24–29. [Google Scholar] [CrossRef]
- Ahmadiyan, S.; Khalil-Allafi, J.; Etminanfar, M.R.; Safavi, M.S.; Hosseini, M. Antibacterial activity and biocompatibility of Ag-coated Ti implants: Importance of surface modification parameters. Trans. Inst. Met. Finish. 2022, 100, 93–102. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, J.; Song, J.; Xie, M.; Liu, Y.; Dong, Z.; Liu, X.; Li, X.; Zhang, M.; Chen, Y.; et al. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/ GPX4 signaling. Free. Radic. Biol. Med. 2022, 181, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Boodagh, P.; Selvakumar, P.P.; Keyser, S. Strategies to counteract adverse remodeling of vascular graft: A 3D view of current graft innovations. Front. Bioeng. Biotechnol. 2023, 10, 1097334. [Google Scholar] [CrossRef]
- van Haaften, E.E.; Wissing, T.B.; Kurniawan, N.A.; Smits, A.I.P.M.; Bouten, C.V.C. Human In Vitro Model Mimicking Material-Driven Vascular Regeneration Reveals How Cyclic Stretch and Shear Stress Differentially Modulate Inflammation and Matrix Deposition. Adv. Biosyst. 2020, 4, 1900249. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wu, Y.; Du, P.; Sun, L.; Yu, Z.; Song, S.; Yin, J.; Ma, X.; Jing, C.; et al. Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in vitro and in vivo. Int. J. Nanomed. 2020, 15, 8697–8715. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.-L.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater. 2019, 8, 1900065. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, P.C.; Isabel Rial-Hermida, M.; Montini-Ballarin, F.; Abraham, G.A.; Concheiro, A.; Alvarez-Lorenzo, C. Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 75, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 80, 213–221. [Google Scholar] [CrossRef]
- Mokhtari, N.; Kharazi, A.Z. Blood compatibility and cell response improvement of poly glycerol sebacate/poly lactic acid scaffold for vascular graft applications. J. Biomed. Mater. Res. Part A 2021, 109, 2673–2684. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, J.; Du, J.; Pan, Y.; Shi, J.; Peng, Y.; Chen, W.; Yuan, L.; Ye, S.-H.; Wagner, W.R.; et al. Orthogonally Functionalizable Polyurethane with Subsequent Modification with Heparin and Endothelium-Inducing Peptide Aiming for Vascular Reconstruction. Acs Appl. Mater. Interfaces 2016, 8, 14442–14452. [Google Scholar] [CrossRef]
- Hashi, C.K.; Zhu, Y.; Yang, G.-Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, K.L.; Gupta, P.; Shehabeldin, M.S.; Cunnane, E.M.; Ramaswamy, A.K.; Verdelis, K.; Dileo, M.V.; Little, S.R.; Weinbaum, J.S.; Sfeir, C.S.; et al. CCL2 loaded microparticles promote acute patency in silk-based vascular grafts implanted in rat aortae. Acta Biomater. 2021, 135, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B.S. Layer-by-Layer Assembly: Recent Progress from Layered Assemblies to Layered Nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, V.K.; Sabino, R.M.; Kantam, P.; Popat, K.C. Surface modification strategies to improve titanium hemocompatibility: A comprehensive review. Mater. Adv. 2021, 2, 5824–5842. [Google Scholar] [CrossRef]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, J.; Wang, M.; Feng, Y.; Ding, J. Matrix-Metalloproteinase-Responsive Gene Delivery Surface for Enhanced in Situ Endothelialization. Acs Appl. Mater. Interfaces 2020, 12, 40121–40132. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, Y. Surface Engineering of Cardiovascular Devices for Improved Hemocompatibility and Rapid Endothelialization. Adv. Healthc. Mater. 2020, 9, 2000920. [Google Scholar] [CrossRef]
- Wang, B.; Hua, J.; You, R.; Yan, K.; Ma, L. Electrochemically deposition of catechol-chitosan hydrogel coating on coronary stent with robust copper ions immobilization capability and improved interfacial biological activity. Int. J. Biol. Macromol. 2021, 181, 435–443. [Google Scholar] [CrossRef]
- Yan, K.; Yang, C.; Zhong, W.; Lu, Z.; Li, X.; Shi, X.; Wang, D. Wire templated electrodeposition of vessel-like structured chitosan hydrogel by using a pulsed electrical signal. Soft Matter 2020, 16, 9471–9478. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Z.; Shen, L.; Guo, Z.; Chou, L.L.; Zhong, W.; Du, Q.; Ge, J. The effect of a layer-by-layer chitosan-heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials 2009, 30, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Zhao, H.; Xiao, R.; Lu, J.; Zhang, L.; Guo, J. Electrospun hemocompatible PU/gelatin-heparin nanofibrous bilayer scaffolds as potential artificial blood vessels. Macromol. Res. 2012, 20, 347–350. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Fang, Z.; Xiao, R.; Yuan, W.; Khan, M. Fabrication and characterization of electrospun gelatin-heparin nanofibers as vascular tissue engineering. Macromol. Res. 2013, 21, 860–869. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, Y.; Wang, H.; Yang, D.; An, B.; Zhang, W.; Khan, M.; Guo, J. Hemocompatible surface of electrospun nanofibrous scaffolds by ATRP modification. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 3644–3651. [Google Scholar] [CrossRef]
- Maji, K.; Pramanik, K. Electrospun scaffold for bone regeneration. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 842–857. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Textile-based sandwich scaffold using wet electrospun yarns for skin tissue engineering. J. Mech. Behav. Biomed. Mater. 2021, 119, 104499. [Google Scholar] [CrossRef]
- Zhou, W.; Feng, Y.; Yang, J.; Fan, J.; Lv, J.; Zhang, L.; Guo, J.; Ren, X.; Zhang, W. Electrospun scaffolds of silk fibroin and poly(lactide-co-glycolide) for endothelial cell growth. J. Mater. Sci.-Mater. Med. 2015, 26, 56. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, W.; Khan, M.; Li, Q.; Feng, Y.; Yao, F.; Zhang, W. Hydrophilic PCU scaffolds prepared by grafting PEGMA and immobilizing gelatin to enhance cell adhesion and proliferation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 50, 201–209. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Z.; Gao, L.; Zheng, L. Preparation and properties of thermal bonded fibrous artificial blood vessels. J. Text. Res. 2021, 42, 46–50. [Google Scholar]
- Hu, J.; Jian, Z.; Lu, C.; Liu, N.; Yue, T.; Lan, W.; Liu, Y. New Method for Preparing Small-Caliber Artificial Blood Vessel with Controllable Microstructure on the Inner Wall Based on Additive Material Composite Molding. Micromachines 2021, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Feng, Y.; Li, Q.; Hao, X.; Liu, W.; Zhou, W.; Shi, C.; Ren, X.; Zhang, W. PLGA/SF blend scaffolds modified with plasmid complexes for enhancing proliferation of endothelial cells. React. Funct. Polym. 2015, 91, 19–27. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, W.; Ren, X.; Liu, W.; Guo, M.; Ullah, I.; Zhang, W. Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering. Polymers 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, W.; Ren, X.; Lu, W.; Guo, M.; Behl, M.; Lendlein, A.; Zhang, W. Evaluation of Electrospun PCL-PIBMD Meshes Modified with Plasmid Complexes in Vitro and in Vivo. Polymers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, Q.; Duo, X.; Hao, X.; Zhang, W.; Shi, C.; Guo, J.; Ren, X.; Feng, Y. Electrospun PCL-PIBMD/SF blend scaffolds with plasmid complexes for endothelial cell proliferation. RSC Adv. 2017, 7, 39452–39464. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Huang, J.; Hao, M.; Hu, X.; Qian, X.; Fan, J.; Yang, H.; Yang, B. Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds. Polymers 2022, 14, 3404. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, L.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Co-immobilization of ACH(11) antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization. Acta Biomater. 2019, 97, 344–359. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Wei, T.; Bai, L.; Zhao, J.; Wang, K.; Feng, Y. Endothelial Cell-Mediated Gene Delivery for In Situ Accelerated Endothelialization of a Vascular Graft. Acs Appl. Mater. Interfaces 2021, 13, 16097–16105. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, J.; Li, Q.; Guo, J.; Ren, X.; Xia, S.; Zhang, W.; Feng, Y. Biofunctionalized Electrospun PCL-PIBMD/SF Vascular Grafts with PEG and Cell-Adhesive Peptides for Endothelialization. Macromol. Biosci. 2019, 19, 1800386. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 105, 110083. [Google Scholar] [CrossRef]
- Wang, M.; Gu, J.; Hao, Y.; Qin, X.; Yu, Y.; Zhang, H. Adhesive, sustained-release, antibacterial, cytocompatible hydrogel-based nanofiber membrane assembled from polysaccharide hydrogels and functionalized nanofibers. Cellulose 2022, 30, 323–337. [Google Scholar] [CrossRef]
- Miller, R.J.; Chan, C.Y.; Rastogi, A.; Grant, A.M.; White, C.M.; Bette, N.; Schaub, N.J.; Corey, J.M. Combining electrospun nanofibers with cell-encapsulating hydrogel fibers for neural tissue engineering. J. Biomater. Sci.-Polym. Ed. 2018, 29, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Kalkandelen, C.; Oktar, F.N.; Uzun, M.; Sahin, Y.M.; Karademir, B.; Arslan, S.; Ozbolat, I.T.; Mahirogullari, M.; Gunduz, O. 3D Printing Artificial Blood Vessel Constructs Using PCL/Chitosan/Hydrogel Biocomposites. Chemistryselect 2019, 4, 2387–2391. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Chen, G.; Boehm, M.; Esworthy, T.; Zhang, L.G. 3D printed biomimetic flexible blood vessels with iPS cell-laden hierarchical multilayers. Biomed. Eng. Adv. 2022, 4, 100065. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Chen, K.; Wang, F.; Feng, C.; Xu, L.; Zhang, D. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int. J. Biol. Macromol. 2021, 182, 669–679. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Deng, Y.; Xie, X.; Xu, L.; Xu, X.; Yuan, W.; Yang, B.; Yang, X.; Xia, X.; et al. Ultrafast Self-Gelling and Wet Adhesive Powder for Acute Hemostasis and Wound Healing. Adv. Funct. Mater. 2021, 31, 2102583. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, L.; Farhadi, F.; Xia, W.; Chang, J.; He, Y.; Li, H. An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 2019, 192, 149–158. [Google Scholar] [CrossRef]
- Malektaj, H.; Imani, R.; Siadati, M.H. Study of injectable PNIPAAm hydrogels containing niosomal angiogenetic drug delivery system for potential cardiac tissue regeneration. Biomed. Mater. 2021, 16, 045031. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable hydrogels from enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone repair and regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 841–849. [Google Scholar] [CrossRef]
- Ge, J.; Li, Y.; Wang, M.; Gao, C.; Yang, S.; Lei, B. Engineering conductive antioxidative antibacterial nanocomposite hydrogel scaffolds with oriented channels promotes structure-functional skeletal muscle regeneration. Chem. Eng. J. 2021, 425, 130333. [Google Scholar] [CrossRef]
- Im, S.B.; Tripathi, G.; Thi Thao Thanh, L.; Lee, B.T. Early-stage bone regeneration of hyaluronic acid supplemented with porous 45s5 bioglass-derived granules: An injectable system. Biomed. Mater. 2021, 16, 045034. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Wang, Z.; Hou, J.; Liu, S.; Yang, Q.; Yu, L.; Guo, W.; Wang, Y.; Guo, B.; et al. Injectable remote magnetic nanofiber/hydrogel multiscale scaffold for functional anisotropic skeletal muscle regeneration. Biomaterials 2022, 285, 121537. [Google Scholar] [CrossRef] [PubMed]
- Sarviya, N.; Basu, S.M.; Induvahi, V.; Giri, J. Laponite-Gelatin Nanofibrous Microsphere Promoting Human Dental Follicle Stem Cells Attachment and Osteogenic Differentiation for Noninvasive Stem Cell Transplantation. Macromol. Biosci. 2022, 23, 2200347. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Mansour, A. Viscoelasticity, mechanical properties, and in vitro biodegradation of injectable chitosan-poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/nanohydroxyapatite composite hydrogel. Bull. Mater. Sci. 2018, 41, 141. [Google Scholar] [CrossRef]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef] [PubMed]
- Baysan, G.; Gunes, O.C.; Akokay, P.; Husemoglu, R.B.; Ertugruloglu, P.; Albayrak, A.Z.; Cecen, B.; Havitcioglu, H. Loofah-chitosan and poly (-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) based hydrogel scaffolds for meniscus tissue engineering applications. Int. J. Biol. Macromol. 2022, 221, 1171–1183. [Google Scholar] [CrossRef]
- Kuang, W.; Liu, C.; Xu, H. The application of decellularized nucleus pulposus matrix/chitosan with transforming growth factor beta 3 for nucleus pulposus tissue engineering. Cytotechnology 2021, 73, 447–456. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef]
- Castro, A.P.G.; Ruben, R.B.; Goncalves, S.B.; Pinheiro, J.; Guedes, J.M.; Fernandes, P.R. Numerical and experimental evaluation of TPMS Gyroid scaffolds for bone tissue engineering. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 567–573. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Ilegbusi, O.; Foroosh, H. Segmentation and Pore Structure Estimation in SEM Images of Tissue Engineering Scaffolds Using Genetic Algorithm. Ann. Biomed. Eng. 2021, 49, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F. Computer-Aided Wet-Spinning. Methods Mol. Biol. 2021, 2147, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Calore, A.R.; Sinha, R.; Harings, J.; Bernaerts, K.V.; Mota, C.; Moroni, L. Additive Manufacturing Using Melt Extruded Thermoplastics for Tissue Engineering. Methods Mol. Biol. 2021, 2147, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Rojas, D.; Waibel, M.; Pencko, D.; Gunenthiran, S.; Ninan, N.; Loudovaris, T.; Drogemuller, C.; Coates, P.T.; Voelcker, N.H.; et al. Facile preparation of tissue engineering scaffolds with pore size gradients using the muesli effect and their application to cell spheroid encapsulation. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2020, 108, 2495–2504. [Google Scholar] [CrossRef]
- Li, G.; Qin, S.; Liu, X.; Zhang, D.; He, M. Structure and properties of nano-hydroxyapatite/poly(butylene succinate) porous scaffold for bone tissue engineering prepared by using ethanol as porogen. J. Biomater. Appl. 2019, 33, 776–791. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, K.; Mack, A.; Sachok, B.; Zhu, X.; Barber, W.E.; Wang, X. Synthesis and optimization of wide pore superficially porous particles by a one-step coating process for separation of proteins and monoclonal antibodies. J. Chromatogr. A 2015, 1414, 147–157. [Google Scholar] [CrossRef]
- Allijn, I.; du Preez, N.; Tasior, M.; Bansal, R.; Stamatialis, D. One-Step Fabrication of Porous Membrane-Based Scaffolds by Air-Water Interfacial Phase Separation: Opportunities for Engineered Tissues. Membranes 2022, 12, 453. [Google Scholar] [CrossRef]
- Lopresti, F.; Liga, A.; Capuana, E.; Gulfi, D.; Zanca, C.; Inguanta, R.; Brucato, V.; La Carrubba, V.; Pavia, F.C. Effect of Polyhydroxyalkanoate (PHA) Concentration on Polymeric Scaffolds Based on Blends of Poly-L-Lactic Acid (PLLA) and PHA Prepared via Thermally Induced Phase Separation (TIPS). Polymers 2022, 14, 2494. [Google Scholar] [CrossRef]
- Salehi, M.; Farzamfar, S.; Bozorgzadeh, S.; Bastami, F. Fabrication of Poly(L-Lactic Acid)/Chitosan Scaffolds by Solid-Liquid Phase Separation Method for Nerve Tissue Engineering: An In Vitro Study on Human Neuroblasts. J. Craniofacial Surg. 2019, 30, 784–789. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Z.; Shafiq, M.; Xie, X.; Xiao, X.; Castro, R.; Rodrigues, J.; Wu, J.; Zhou, G.; Mo, X. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Colloids Surf. B-Biointerfaces 2021, 201, 111637. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Ma, C. A novel protocol for injectable artificial cartilage constructs based on programmed shape-morphing hydrogels for cartilage regeneration. Chem. Eng. J. 2022, 446, 137109. [Google Scholar] [CrossRef]
- Valente, S.A.; Silva, L.M.; Lopes, G.R.; Sarmento, B.; Coimbra, M.A.; Passos, C.P. Polysaccharide-based formulations as potential carriers for pulmonary delivery—A review of their properties and fates. Carbohydr. Polym. 2022, 277, 118784. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Leite, A.J.; Costa, R.R.; Costa, A.M.S.; Maciel, J.S.; Costa, J.F.G.; de Paula, R.C.M.; Mano, J.F. The potential of cashew gum functionalization as building blocks for layer-by-layer films. Carbohydr. Polym. 2017, 174, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Bingjun, Q.; Jung, J.; Zhao, Y. Impact of Acidity and Metal Ion on the Antibacterial Activity and Mechanisms of beta- and alpha-Chitosan. Appl. Biochem. Biotechnol. 2015, 175, 2972–2985. [Google Scholar] [CrossRef]
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. Acs Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, E.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofre, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers 2020, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Yu, W. Bioinspired Anisotropic Chitosan Hybrid Hydrogel. Acs Appl. Bio Mater. 2020, 3, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Fan, C.; Xu, Z.; Liu, B.; Liu, W. A smart indwelling needle with on-demand switchable anticoagulant and hemostatic activities. Mater. Horiz. 2020, 7, 1091–1100. [Google Scholar] [CrossRef]
- do Nascimento, E.G.; de Caland, L.B.; de Medeiros, A.S.A.; Fernandes-Pedrosa, M.F.; Soares-Sobrinho, J.L.; dos Santos, K.S.C.R.; da Silva-Junior, A.A. Tailoring Drug Release Properties by Gradual Changes in the Particle Engineering of Polysaccharide Chitosan Based Powders. Polymers 2017, 9, 253. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, P.; Hong, F.F.; Cao, Z.; Ji, Y.; Chen, L. A novel approach for efficient fabrication of chitosan nanoparticles-embedded bacterial nanocellulose conduits. Carbohydr. Polym. 2021, 264, 118002. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrieres, J.; Peptu, C.A. Modulated release from liposomes entrapped in chitosan/gelatin hydrogels. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 43, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.-Q.; Liu, W.; Wang, D.-Y.; Meng, F.-Q.; Wang, H.-Y.; Qi, H.-Y. Development of local anesthetic drug delivery system by administration of organo-silica nanoformulations under ultrasound stimuli: In vitro and in vivo investigations. Drug Deliv. 2021, 28, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yu, H.; Long, H.; Zhang, H.; Hao, C.; Shi, L.; Du, Y.; Jiao, S.; Guo, A.; Ma, L.; et al. Low deacetylation degree chitosan oligosaccharide protects against IL-1 beta induced inflammation and enhances autophagy activity in human chondrocytes. J. Biomater. Sci.-Polym. Ed. 2022, 33, 517–531. [Google Scholar] [CrossRef]
- Kang, M.-L.; Kim, J.-E.; Im, G.-I. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016, 39, 65–78. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J. Nanobiotechnology 2021, 19, 343. [Google Scholar] [CrossRef]
- Wang, W.-D.; Chen, C.; Fu, X. Glycation mechanism of lactoferrin-chitosan oligosaccharide conjugates with improved antioxidant activity revealed by high-resolution mass spectroscopy. Food Funct. 2020, 11, 10886–10895. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Tan, L.; Sun, C.; Dong, J. Synthesis of N-furoyl chitosan and chito-oligosaccharides and evaluation of their antioxidant activity in vitro. Int. J. Biol. Macromol. 2013, 59, 391–395. [Google Scholar] [CrossRef]
- Bilal, M.; Nunes, L.V.; Saviatto Duarte, M.T.; Romanholo Ferreira, L.F.; Soriano, R.N.; Iqbal, H.M.N. Exploitation of Marine-Derived Robust Biological Molecules to Manage Inflammatory Bowel Disease. Mar. Drugs 2021, 19, 196. [Google Scholar] [CrossRef]
- Rajendran, S.R.C.K.; Okolie, C.L.; Udenigwe, C.C.; Mason, B. Structural features underlying prebiotic activity of conventional and potential prebiotic oligosaccharides in food and health. J. Food Biochem. 2017, 41, e12389. [Google Scholar] [CrossRef]
- Sutthasupha, P.; Promsan, S.; Thongnak, L.; Pengrattanachot, N.; Phengpol, N.; Jaruan, O.; Jaikumkao, K.; Muanprasat, C.; Pichyangkura, R.; Chatsudthipong, V.; et al. Chitosan oligosaccharide mitigates kidney injury in prediabetic rats by improving intestinal barrier and renal autophagy. Carbohydr. Polym. 2022, 288, 119405. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, X.; Chen, S.; Zhang, C.; Wang, Y.; Lin, H.; Zhao, L. Study of double-bonded carboxymethyl chitosan/cysteamine-modified chondroitin sulfate composite dressing for hemostatic application. Eur. Polym. J. 2022, 162, 110875. [Google Scholar] [CrossRef]
- Xia, L.; Wang, S.; Jiang, Z.; Chi, J.; Yu, S.; Li, H.; Zhang, Y.; Li, L.; Zhou, C.; Liu, W.; et al. Hemostatic performance of chitosan-based hydrogel and its study on biodistribution and biodegradability in rats. Carbohydr. Polym. 2021, 264, 117965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xin, Y.; Yin, B.; Ye, G.L.; Wang, J.X.; Shen, J.F.; Li, L.; Yang, Q.H. Synthesis and properties of crosslinked carboxymethyl chitosan and its hemostatic and wound healing effects on liver injury of rats. J. Biomater. Appl. 2019, 34, 442–450. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Jin, X. Preparation and Properties of Minocycline-Loaded Carboxymethyl Chitosan Gel/Alginate Nonwovens Composite Wound Dressings. Mar. Drugs 2019, 17, 575. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Balagangadharan, K.; Selvamurugan, N.; Seeli, D.S.; Prabaharan, M. Preparation and characterization of chitosan/carboxymethyl pullulan/bioglass composite films for wound healing. J. Biomater. Appl. 2022, 36, 1151–1163. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, W.; Feng, L.; Bao, J.; Chen, Q.; Zhao, W.; Zhao, C. Facile and green approach towards biomass-derived hydrogel powders with hierarchical micro-nanostructures for ultrafast hemostasis. J. Mater. Chem. B 2021, 9, 6678–6690. [Google Scholar] [CrossRef]
- Chien, Y.; Liao, Y.-W.; Liu, D.-M.; Lin, H.-L.; Chen, S.-J.; Chen, H.-L.; Peng, C.-H.; Liang, C.-M.; Mou, C.-Y.; Chiou, S.-H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef]
- Malik, M.H.; Shahzadi, L.; Batool, R.; Safi, S.Z.; Khan, A.S.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Thyroxine-loaded chitosan/carboxymethyl cellulose/hydroxyapatite hydrogels enhance angiogenesis in in-ovo experiments. Int. J. Biol. Macromol. 2020, 145, 1162–1170. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L.; de Souza, A.J.M.; Paiva, A.E. Ecofriendly multifunctional thiolated carboxymethyl chitosan-based 3D scaffolds with luminescent properties for skin repair and theragnostic of tissue regeneration. Int. J. Biol. Macromol. 2020, 165, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Mendez, S.; Tamano Maciavello, M.N.; Gomez Ribelles, J.L.; Sentanin, F.; Pawlicka, A.; Silva, M.M. Thermal-mechanical behaviour of chitosan-cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef]
- Berardesca, E.; Iorizzo, M.; Abril, E.; Guglielmini, G.; Caserini, M.; Palmieri, R.; Pierard, G.E. Clinical and instrumental assessment of the effects of a new product based on hydroxypropyl chitosan and potassium azeloyl diglycinate in the management of rosacea. J. Cosmet. Dermatol. 2012, 11, 37–41. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.; Park, H.-S.; Jang, A.; Min, J.; Kim, Y.-H. Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Ding, F.; You, J.; Weng, X.; Zhou, J.; Zhang, X.; Zhou, X.; Zhang, L. Exploring Quaternized Hydroxyethylcellulose as Potential Gene Carriers. Chin. J. Chem. 2012, 30, 2212–2218. [Google Scholar] [CrossRef]
- Faizuloev, E.; Marova, A.; Nikonova, A.; Volkova, I.; Gorshkova, M.; Izumrudov, V. Water-soluble N- (2-hydroxy-3-trimethylammonium)propyl chitosan chloride as a nucleic acids vector for cell transfection. Carbohydr. Polym. 2012, 89, 1088–1094. [Google Scholar] [CrossRef]
- Yu, S.; Hao, S.; Sun, B.; Zhao, D.; Yan, X.; Jin, Z.; Zhao, K. Quaternized Chitosan Nanoparticles in Vaccine Applications. Curr. Med. Chem. 2020, 27, 4932–4944. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, S.; Shi, S.Q.; Cai, L. Synthesis, Characterization, and Antibacterial Activity of N-substituted Quaternized Chitosan and Its Cellulose-based Composite Film. Bioresources 2020, 15, 415–428. [Google Scholar] [CrossRef]
- Jang, S.-C.; Chuang, F.-S.; Tsen, W.-C.; Kuo, T.-W. Quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. J. Appl. Polym. Sci. 2019, 136, 47778. [Google Scholar] [CrossRef]
- Rahimi, M.; Ahmadi, R.; Kafil, H.S.; Shafiei-Irannejad, V. A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Drozd, N.N.; Logvinova, Y.S.; Shagdarova, B.T.; Il’ina, A.V.; Varlamov, V.P. Analysis of the Action of Quaternized Chitosans with Different Molecular Weight on Anticoagulant Activity of Heparins In Vitro. Bull. Exp. Biol. Med. 2019, 167, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xia, X.; Pan, Z.; Lin, Y.; Li, L.; Jiao, Y.; Zhou, C.; Ding, S. Different influence of sulfated chitosan with different sulfonic acid group sites on HUVECs behaviors. J. Biomater. Sci.-Polym. Ed. 2020, 31, 1237–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.; Xie, W.; Chen, L.; Zheng, H.; Fan, L. Preparation and anticoagulant activity of N-succinyl chitosan sulfates. Int. J. Biol. Macromol. 2012, 51, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Yu, Y.; Dai, K.; Wang, J.; Liu, C. Accelerated Bone Regenerative Efficiency by Regulating Sequential Release of BMP-2 and VEGF and Synergism with Sulfated Chitosan. Acs Biomater. Sci. Eng. 2019, 5, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Salomon, S.K.; Makrakis, A.; Li, X.W.; Brocchini, S.; Alpar, H.O. Development of chitosan-pullulan composite nanoparticles for nasal delivery of vaccines: Optimisation and cellular studies. J. Microencapsul. 2015, 32, 755–768. [Google Scholar] [CrossRef]
- Zaki, N.M.; Mortada, N.D.; Awad, G.A.S.; ElHady, S.S.A. Rapid-onset intranasal delivery of metoclopramide hydrochloride—Part II: Safety of various absorption enhancers and pharmacokinetic evaluation. Int. J. Pharm. 2006, 327, 97–103. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, S.; Lu, L.; Song, X.; Li, P.; Wang, F. Curdlan sulfate-O-linked quaternized chitosan nanoparticles: Potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int. J. Nanomed. 2018, 13, 2377–2394. [Google Scholar] [CrossRef]
- Dong, C.; Chen, W.; Liu, C. Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour. Technol. 2014, 170, 239–247. [Google Scholar] [CrossRef]
- Rasad, M.S.B.A.; Halim, A.S.; Hashim, K.; Rashid, A.H.A.; Yusof, N.; Shamsuddin, S. In vitro evaluation of novel chitosan derivatives sheet and paste cytocompatibility on human dermal fibroblasts. Carbohydr. Polym. 2010, 79, 1094–1100. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, Z.-Q.; Shi, X.-Q.; Wang, N.; Bo, Y.-Y.; Guo, H.-E. A novel silkworm pupae carboxymethyl chitosan inhibits mouse L929 fibroblast proliferation. Scienceasia 2020, 46, 30–36. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Subhapradha, N.; Ramasamy, P.; Srinivasan, A.; Madeswaran, P.; Shanmugam, V.; Shanmugam, A. Sulfation of beta-chitosan and evaluation of biological activity from gladius of Sepioteuthis lessoniana. Int. J. Biol. Macromol. 2013, 62, 336–340. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Skorik, Y.A.; Kritchenkov, A.S.; Moskalenko, Y.E.; Golyshev, A.A.; Raik, S.V.; Whaley, A.K.; Vasina, L.V.; Sonin, D.L. Synthesis of N-succinyl- and N-glutaryl-chitosan derivatives and their antioxidant, antiplatelet, and anticoagulant activity. Carbohydr. Polym. 2017, 166, 166–172. [Google Scholar] [CrossRef]
- Xing, R.E.; Liu, S.; Guo, Z.Y.; Yu, H.H.; Wang, P.B.; Li, C.P.; Li, Z.; Li, P.C. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorganic Med. Chem. 2005, 13, 1573–1577. [Google Scholar] [CrossRef]
- Aguanell, A.; del Pozo, M.L.; Perez-Martin, C.; Pontes, G.; Bastida, A.; Fernandez-Mayoralas, A.; Garcia-Junceda, E.; Revuelta, J. Chitosan sulfate-lysozyme hybrid hydrogels as platforms with fine-tuned and sustained inherent antibiotic and antioxidant activities. Carbohydr. Polym. 2022, 291, 119611. [Google Scholar] [CrossRef]
- Luo, P.; Nie, M.; Wen, H.; Xu, W.; Fan, L.; Cao, Q. Preparation and characterization of carboxymethyl chitosan sulfate/oxidized konjac glucomannan hydrogels. Int. J. Biol. Macromol. 2018, 113, 1024–1031. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar] [CrossRef]
- Afgan, S.; Yadav, P.; Jaiswal, S.; Pal, K.; Kumar, R.; Singh, V.; Koch, B. Development of chitosan towards the self-healing and mechanically stronger biocompatible hydrogel. J. Indian Chem. Soc. 2022, 99, 100704. [Google Scholar] [CrossRef]
- Wang, Y.; Garcia, C.R.; Ding, Z.; Gabrilska, R.; Rumbaugh, K.P.; Wu, J.; Liu, Q.; Li, W. Adhesive, Self-Healing, and Antibacterial Chitosan Hydrogels with Tunable Two-Layer Structures. ACS Sustain. Chem. Eng. 2020, 8, 18006–18014. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Xu, J.; Liu, M.; Li, P.; Li, X.; Fan, Y. A biomimetic hierarchical small intestinal submucosa-chitosan sponge/chitosan hydrogel scaffold with a micro/nano structure for dural repair. J. Mater. Chem. B 2021, 9, 7821–7834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, Y.; Cheng, J.; Cao, J.; Li, M.; Yu, H.; Wei, D.; Li, B.; Wang, Y.; Zhou, Y. Surface configuration of microarc oxidized Ti with regionally loaded chitosan hydrogel containing ciprofloxacin for improving biological performance. Mater. Today Bio 2022, 16, 100380. [Google Scholar] [CrossRef] [PubMed]
- Rahmi; Lelifajri; Nurfatimah, R. Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr. Polym. 2018, 199, 499–505. [Google Scholar] [CrossRef]
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kim, H.J.; Choi, J.W.; Kimura, S.; Wada, M. Cellulose-chitosan beads crosslinked by dialdehyde cellulose. Cellulose 2017, 24, 5517–5528. [Google Scholar] [CrossRef]
- Song, X.; Wang, K.; Tang, C.-Q.; Yang, W.-W.; Zhao, W.-F.; Zhao, C.-S. Design of Carrageenan-Based Heparin-Mimetic Gel Beads as Self-Anticoagulant Hemoperfusion Adsorbents. Biomacromolecules 2018, 19, 1966–1978. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Ciric, A.; Medarevic, D.; Calija, B.; Dobricic, V.; Rmandic, M.; Barudzija, T.; Malenovic, A.; Djekic, L. Effect of ibuprofen entrapment procedure on physicochemical and controlled drug release performances of chitosan/xanthan gum polyelectrolyte complexes. Int. J. Biol. Macromol. 2021, 167, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; Wang, Y.; Ji, F.; Wang, A.; Yang, M.; He, X.; Li, L. Bivalent Regulation and Related Mechanisms of H3K4/27/9me3 in Stem Cells. Stem Cell Rev. Rep. 2022, 18, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-x.; Yang, J.-w.; Wu, Y.-y.; Hu, X.-q.; Zhang, H.-b. The stability improvement of dextransucrase by artificial extension modification of the V domain of the enzyme. Enzym. Microb. Technol. 2021, 151, 109919. [Google Scholar] [CrossRef]
- Li, Q.; Cui, J.; Huang, H.; Yue, Z.; Chang, Y.; Li, N.; Han, Z.; Han, Z.-c.; Guo, Z.; Li, Z. IGF-1C domain-modified chitosan hydrogel accelerates cutaneous wound healing by promoting angiogenesis. Future Med. Chem. 2020, 12, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ramezani, H.; Sun, M.; Xie, M.; Nie, J.; Lv, S.; Cai, J.; Fu, J.; He, Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020, 8, 5020–5028. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Cheng, X.; Zhao, G.; Zheng, G.; Li, X.; Dong, R. Theoretical and Experimental Research on Multi-Layer Vessel-like Structure Printing Based on 3D Bio-Printing Technology. Micromachines 2021, 12, 1517. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, H.-L.; Wu, D.; Weng, Y.-T.; Wang, Z.-Y.; Yu, S.-H.; Wang, Z. Biomimetic Lamellar Chitosan Scaffold for Soft Gingival Tissue Regeneration. Adv. Funct. Mater. 2021, 31, 2105348. [Google Scholar] [CrossRef]
- Tong, J.; Yang, C.; Qi, L.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Tubular chitosan hydrogels with a tuneable lamellar structure programmed by electrical signals. Chem. Commun. 2022, 58, 5781–5784. [Google Scholar] [CrossRef]
- Maleki, S.; Shamloo, A.; Kalantarnia, F. Tubular TPU/SF nanofibers covered with chitosan-based hydrogels as small-diameter vascular grafts with enhanced mechanical properties. Sci. Rep. 2022, 12, 6179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ji, N.; Xiong, L.; Sun, Q. Rapid gelling, self-healing, and fluorescence-responsive chitosan hydrogels formed by dynamic covalent crosslinking. Carbohydr. Polym. 2020, 246, 116586. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, N.; Rodriguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or kappa-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wu, D. Chitosan-Based High-Mechanical Double-Network Hydrogels: Construction, Modulation and Applications. Acta Chim. Sin. 2021, 79, 1–9. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, L.; Yang, C.; Wang, L.; Yi, S.; Tong, X.; Xiao, B.; Chen, J. Comparison of Sericins from Different Sources as Natural Therapeutics against Ulcerative Colitis. Acs Biomater. Sci. Eng. 2021, 7, 4626–4636. [Google Scholar] [CrossRef]
- Adali, T.; Kalkan, R.; Karimizarandi, L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int. J. Biol. Macromol. 2019, 124, 541–547. [Google Scholar] [CrossRef]
- Herron, C.; Hastings, C.L.; Herron-Rice, C.; Kelly, H.M.; O’Dwyer, J.; Duffy, G.P. A Thermoresponsive Chitosan/beta-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia. Polymers 2021, 13, 3568. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Tao, L.; Wei, Y.; Hsu, S.-h. A novel biodegradable self-healing hydrogel to induce blood capillary formation. Npg Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef]
- Cui, L.; Li, J.; Guan, S.; Zhang, K.; Zhang, K.; Li, J. Injectable multifunctional CMC/HA-DA hydrogel for repairing skin injury. Mater. Today Bio 2022, 14, 100257. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Shi, Z.; Wang, Y.; Song, X.; Wang, L.; Han, M.; Du, H.; He, C.; Zhao, W.; et al. Anticoagulant chitosan-kappa-carrageenan composite hydrogel sorbent for simultaneous endotoxin and bacteria cleansing in septic blood. Carbohydr. Polym. 2020, 243, 116470. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, T.; Brown, Z.; Gu, Y.; Teng, K.; Ye, W.; Ming, W. Preparation of Ascidian-Inspired Hydrogel Thin Films to Selectively Induce Vascular Endothelial Cell and Smooth Muscle Cell Growth. Acs Appl. Bio Mater. 2020, 3, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Liang, X.; Chen, F.; Chen, Y.; Zhou, J. Novel multifunctional dual-dynamic-bonds crosslinked hydrogels for multi-strategy therapy of MRSA-infected wounds. Appl. Mater. Today 2022, 26, 101362. [Google Scholar] [CrossRef]
- Yu, F.; Khan, A.u.R.; Li, Y.; Zhao, B.; Xie, X.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; Li, J.; Pan, J.; et al. A multifunctional nanofiber reinforced photo-crosslinking hydrogel for skin wound healing. Compos. Part B-Eng. 2022, 247, 110294. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Song, T.; Zhang, B.; Li, D.; Xiao, Y.; Zhang, X. A chitosan-based multifunctional hydrogel containing in situ rapidly bioreduced silver nanoparticles for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2135–2147. [Google Scholar] [CrossRef]
- Qu, J.; Li, J.; Zhu, W.; Xu, Y.; Zhou, S.; Yang, Y.; Qian, X. Hybrid nanocomposite multinetwork hydrogel containing magnesium hydroxide nanoparticles with enhanced antibacterial activity for wound dressing applications. Polymer 2022, 251, 124902. [Google Scholar] [CrossRef]
- Fonseca, J.d.M.; Medeiros, S.d.F.; Alves, G.M.; dos Santos, D.M.; Campana-Filho, S.P.; dos Santos, A.M. Chitosan microparticles embedded with multi-responsive poly(N-vinylcaprolactam-co-itaconic acid-co-ethylene-glycol dimethacrylate)-based hydrogel nanoparticles as a new carrier for delivery of hydrophobic drugs. Colloids Surf. B-Biointerfaces 2019, 175, 73–83. [Google Scholar] [CrossRef]
- Yang, C.; Huang, H.; Fan, S.; Yang, C.; Chen, Y.; Yu, B.; Li, W.; Liao, J. A Novel Dual-Crosslinked Functional Hydrogel Activated by POSS for Accelerating Wound Healing. Adv. Mater. Technol. 2021, 6, 2001012. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial poly (ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Ara, C.; Jabeen, S.; Afshan, G.; Farooq, A.; Akram, M.S.; Asmatullah; Islam, A.; Ziafat, S.; Nawaz, B.; Khan, R.U. Angiogenic potential and wound healing efficacy of chitosan derived hydrogels at varied concentrations of APTES in chick and mouse models. Int. J. Biol. Macromol. 2022, 202, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhang, X.; Shen, J.; Li, C.; Zhang, J.; Feng, X. Freezing-triggered gelation of quaternized chitosan reinforced with microfibrillated cellulose for highly efficient removal of bilirubin. J. Mater. Chem. B 2022, 10, 8650–8663. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Gao, B.; Zhou, J.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Cascaded bio-responsive delivery of eNOS gene and ZNF(580) gene to collaboratively treat hindlimb ischemia via pro-angiogenesis and anti-inflammation. Biomater. Sci. 2020, 8, 6545–6560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, B.; Muhammad, K.; Zhang, X.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Multifunctional gene delivery systems with targeting ligand CAGW and charge reversal function for enhanced angiogenesis. J. Mater. Chem. B 2019, 7, 1906–1919. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Zaidi, S.S.A.; Guo, J.; Ren, X.; Shi, C.; Zhang, W.; Feng, Y. Oligohistidine and targeting peptide functionalized TAT-NLS for enhancing cellular uptake and promoting angiogenesis in vivo. J. Nanobiotechnology 2018, 16, 29. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, Q.; Muhammad, K.; Ren, X.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. A progressively targeted gene delivery system with a pH triggered surface charge-switching ability to drive angiogenesis in vivo. Biomater. Sci. 2019, 7, 2061–2075. [Google Scholar] [CrossRef]
- Wang, J.; Zaidi, S.S.A.; Hasnain, A.; Guo, J.; Ren, X.; Xia, S.; Zhang, W.; Feng, Y. Multitargeting Peptide-Functionalized Star-Shaped Copolymers with Comblike Structure and a POSS-Core To Effectively Transfect Endothelial Cells. Acs Biomater. Sci. Eng. 2018, 4, 2155–2168. [Google Scholar] [CrossRef]
- Yang, J.; Hao, X.; Li, Q.; Akpanyung, M.; Nejjari, A.; Neve, A.L.; Ren, X.; Guo, J.; Feng, Y.; Shi, C.; et al. CAGW Peptide- and PEG-Modified Gene Carrier for Selective Gene Delivery and Promotion of Angiogenesis in HUVECs in Vivo. Acs Appl. Mater. Interfaces 2017, 9, 4485–4497. [Google Scholar] [CrossRef]
- Gao, B.; Wang, X.; Wang, M.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. “Green process” inspires gene delivery: Establishing positive feedback between CO2-enhanced bioactive carrier and gene expression to maximize ECs outputs for multi-pathways CLI therapy. Chem. Eng. J. 2021, 421, 127808. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Suleiman, G.S.A.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. A “controlled CO release” and “pro-angiogenic gene” dually engineered stimulus-responsive nanoplatform for collaborative ischemia therapy. Chem. Eng. J. 2021, 424, 130430. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Yang, C.; Feng, Y. A two-pronged approach to regulate the behaviors of ECs and SMCs by the dual targeting-nanoparticles. Colloids Surf. B-Biointerfaces 2021, 208, 112068. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, B.; Wang, M.; Wang, Q.; Xia, S.; Zhang, W.; Meng, X.; Feng, Y. CO delivery nanosystem based on regenerative bioactive zinc MOFs highlights intercellular crosstalk for enhanced vascular remodeling in CLI therapy. Chem. Eng. J. 2023, 452, 139670. [Google Scholar] [CrossRef]
- Gao, B.; Wang, X.; Wang, M.; You, K.; Suleiman, G.S.A.; Ren, X.-K.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Superlow Dosage of Intrinsically Bioactive Zinc Metal-Organic Frameworks to Modulate Endothelial Cell Morphogenesis and Significantly Rescue Ischemic Disease. ACS Nano 2022, 16, 1395–1408. [Google Scholar] [CrossRef]
- Hao, X.; Gai, W.; Ji, F.; Wang, L.; Zhao, J.; Yang, F.; Jiang, H.; Feng, Y. Biomimetic and responsive nanoparticles loading JQ1 for dual-targeting treatment of vascular restenosis via multiple actions. Chem. Eng. J. 2022, 431, 133452. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Wang, M.; Chen, X.; Kong, D.; Wang, K.; Feng, Y. Oligoglycine and fluoropolymer functionalized enzyme-responsive gene delivery surface for rapid in situ endothelialization of vascular grafts. Appl. Mater. Today 2022, 27, 101476. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Feng, Y. Recent advances in inhibiting atherosclerosis and restenosis: From pathogenic factors, therapeutic molecules to nano-delivery strategies. J. Mater. Chem. B 2022, 10, 1685–1708. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Zhou, J.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Unexpected Amplification of Synergistic Gene Expression to Boom Vascular Flow in Advantageous Dual-Gene Co-expression Plasmid Delivery Systems over Physically Mixed Strategy. Acs Appl. Bio Mater. 2020, 3, 7228–7235. [Google Scholar] [CrossRef]
- Gao, B.; Wang, X.; Wang, M.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. From single to a dual-gene delivery nanosystem: Coordinated expression matters for boosting the neovascularization in vivo. Biomater. Sci. 2020, 8, 2318–2328. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Wang, H.; Guo, J.; Ren, X.-k.; Xia, S.; Zhang, W.; Feng, Y. Multifunctional REDV-G-TAT-G-NLS-Cys peptide sequence conjugated gene carriers to enhance gene transfection efficiency in endothelial cells. Colloids Surf. B-Biointerfaces 2019, 184, 110510. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, Q.; Wang, X.; Wang, M.; Ren, X.-k.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. A “self-accelerating endosomal escape” siRNA delivery nanosystem for significantly suppressing hyperplasia via blocking the ERK2 pathway. Biomater. Sci. 2019, 7, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, Q.; Wang, H.; Muhammad, K.; Guo, J.; Ren, X.; Shi, C.; Xia, S.; Zhang, W.; Feng, Y. CAGW Modified Polymeric Micelles with Different Hydrophobic Cores for Efficient Gene Delivery and Capillary-like Tube Formation. Acs Biomater. Sci. Eng. 2018, 4, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tong, C.; Yang, J.; Cong, P.; Liu, Y.; Shi, X.; Liu, X.; Zhang, J.; Zou, R.; Xiao, K.; et al. Injectable melatonin-loaded carboxymethyl chitosan (CMCS)-based hydrogel accelerates wound healing by reducing inflammation and promoting angiogenesis and collagen deposition. J. Mater. Sci. Technol. 2021, 63, 236–245. [Google Scholar] [CrossRef]
- Sun, X.; Jia, P.; Zhang, H.; Dong, M.; Wang, J.; Li, L.; Bu, T.; Wang, X.; Wang, L.; Lu, Q.; et al. Green Regenerative Hydrogel Wound Dressing Functionalized by Natural Drug-Food Homologous Small Molecule Self-Assembled Nanospheres. Adv. Funct. Mater. 2022, 32, 2106572. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef]
- Zhang, Q.; Pei, Q.; Yang, J.; Guo, S.; Yang, A.; Qian, Y.; Li, C.; Feng, Q.; Lv, H.; Zhou, X.; et al. Vascularized nanocomposite hydrogel mechanically reinforced by polyelectrolyte-modified nanoparticles. J. Mater. Chem. B 2022, 10, 5439–5453. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Zheng, L.; Wu, J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Res. 2022, 16, 905–916. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Li, H.; Feng, G.; Nie, Y.; Wei, Y.; Li, N.; Han, Z.; Han, Z.-c.; Kong, D.; et al. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater. 2020, 113, 289–304. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef]
- Datta, S.; Rameshbabu, A.P.; Bankoti, K.; Roy, M.; Gupta, C.; Jana, S.; Das, A.K.; Sen, R.; Dhara, S. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 119, 111604. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-W.; Hou, Y.-T.; Hsu, S.-h. Angiogenic potential of co-spheroids of neural stem cells and endothelial cells in injectable gelatin-based hydrogel. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yue, Z.; Cui, J.; Yao, Y.; Song, X.; Cui, B.; Qi, X.; Han, Z.; Han, Z.-C.; Guo, Z.; et al. IGF-1C domain-modified hydrogel enhances therapeutic potential of mesenchymal stem cells for hindlimb ischemia. Stem Cell Res. Ther. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, A.; Wang, N.; Yao, Y.; Chen, X.; Liu, Y. Hydroxybutyl chitosan/oxidized glucomannan self-healing hydrogels as BMSCs-derived exosomes carriers for advanced stretchable wounds. Appl. Mater. Today 2022, 26, 101342. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. Acs Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21. Front. Bioeng. Biotechnol. 2022, 9, 1484. [Google Scholar] [CrossRef]
- Jiang, M.; Pan, Y.; Liu, Y.; Dai, K.; Zhang, Q.; Wang, J. Effect of sulfated chitosan hydrogel on vascularization and osteogenesis. Carbohydr. Polym. 2022, 281, 119059. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.L.; Han, J.; Si, R.J.; Liu, P.P.; Zhang, Z.R.; Wang, A.M.; Zhang, J. Chitosan hydrogel encapsulated with LL-37 peptide promotes deep tissue injury healing in a mouse model. Mil. Med. Res. 2020, 7, 409–417. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Liu, P.; Wang, K.; Fang, H.; Yin, J.; Zhu, D.; Yang, Q.; Gao, J.; Ke, Q.; et al. A multifunctional substance P-conjugated chitosan hydrochloride hydrogel accelerates full-thickness wound healing by enhancing synchronized vascularization, extracellular matrix deposition, and nerve regeneration. Biomater. Sci. 2021, 9, 4199–4210. [Google Scholar] [CrossRef]
- Aleem, A.R.; Shahzadi, L.; Tehseen, S.; Alvi, F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Amino acids loaded chitosan/collagen based new membranes stimulate angiogenesis in chorioallantoic membrane assay. Int. J. Biol. Macromol. 2019, 140, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, X.; Luo, Z.; Loh, X.J.; Ma, Y.; Ye, E.; Wu, Y.-L.; He, C.; Li, Z. Nanoenzyme-chitosan hydrogel complex with cascade catalytic and self-reinforced antibacterial performance for accelerated healing of diabetic wounds. Nanoscale 2022, 14, 14970–14983. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H. Incorporation of Bioglass Improved the Mechanical Stability and Bioactivity of Alginate/Carboxymethyl Chitosan Hydrogel Wound Dressing. ACS Appl. Bio Mater. 2021, 4, 1677–1692. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Zhang, Y.; Wang, E.; Ma, B.; Xu, Q.; Ma, L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 121, 111868. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, W.; Lin, J.; Fang, C.; Deng, J.; Zhang, P.; Zhou, M.; Liu, P.; Weng, J.; Yu, F.; et al. Magnesium Oxide Nanoparticle Coordinated Phosphate-Functionalized Chitosan Injectable Hydrogel for Osteogenesis and Angiogenesis in Bone Regeneration. Acs Appl. Mater. Interfaces 2022, 14, 7592–7608. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; You, S.; Gao, T.; Lan, Y.; Deng, H.; Li, Z.; Hu, R.; Shen, J. All-in-one: Harnessing multifunctional injectable natural hydrogels for ordered therapy of bacteria-infected diabetic wounds. Chem. Eng. J. 2022, 439, 135691. [Google Scholar] [CrossRef]
- Tehrani, F.D.; Shabani, I.; Shabani, A. A hybrid oxygen-generating wound dressing based on chitosan thermosensitive hydrogel and decellularized amniotic membrane. Carbohydr. Polym. 2022, 281, 119020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Liu, Z.; Ma, C.; Yan, H.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; Sun, X. Three-Dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application. Tissue Eng. Part B-Rev. 2019, 25, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. Acs Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W. Advanced electrospun hydrogel fibers for wound healing. Compos. Part B-Eng. 2021, 223, 109101. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Januario, A.P.; Felix, R.; Alves, N.; Lemos, M.F.L.; Dias, J.R. Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics 2021, 13, 2152. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.M.; Inamori, D.; Masai, H.; Tamaki, T.; Terao, J. Luminescent and mechanical enhancement of phosphorescent hydrogel through cyclic insulation of platinum-acetylide crosslinker. Polym. Chem. 2019, 10, 5280–5284. [Google Scholar] [CrossRef]
- Chang, A.; Babhadiashar, N.; Barrett-Catton, E.; Asuri, P. Role of Nanoparticle-Polymer Interactions on the Development of Double-Network Hydrogel Nanocomposites with High Mechanical Strength. Polymers 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Lei, T.; Bi, W.; Sun, S.; Deng, S.; Zhang, X.; Yang, Y.; Xiao, Z.; Du, H. Chitosan Hydrogel Supplemented with Metformin Promotes Neuron-like Cell Differentiation of Gingival Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 3276. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, C.; Shang, X. Decellularized Nucleus Pulposus Matrix/Chitosan Hybrid Hydrogels for Nucleus Pulposus Tissue Engineering. Glob. Spine J. 2022, 73, 21925682221135768. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Waris, T.S.; Shahzadi, L.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Boron for tissue regeneration-it’s loading into chitosan/collagen hydrogels and testing on chorioallantoic membrane to study the effect on angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 525–534. [Google Scholar] [CrossRef]
- Dieu Linh, T.; Phuong Le, T.; Thai Thanh Hoang, T.; Park, K.D. Novel enzymatically crosslinked chitosan hydrogels with free-radical-scavenging property and promoted cellular behaviors under hyperglycemia. Prog. Nat. Sci.-Mater. Int. 2020, 30, 661–668. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, C.-W.; Chang, S.-W.; Hsu, S.-h. An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater. 2021, 122, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, J.; Zhao, Y.-Y.; Jin, F.; Dong, X.-Z.; Zhao, Z.-S.; Duan, X.-M.; Zheng, M.-L. Biocompatible Three-Dimensional Hydrogel Cell Scaffold Fabricated by Sodium Hyaluronate and Chitosan Assisted Two-Photon Polymerization. ACS Appl. Bio Mater. 2019, 2, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
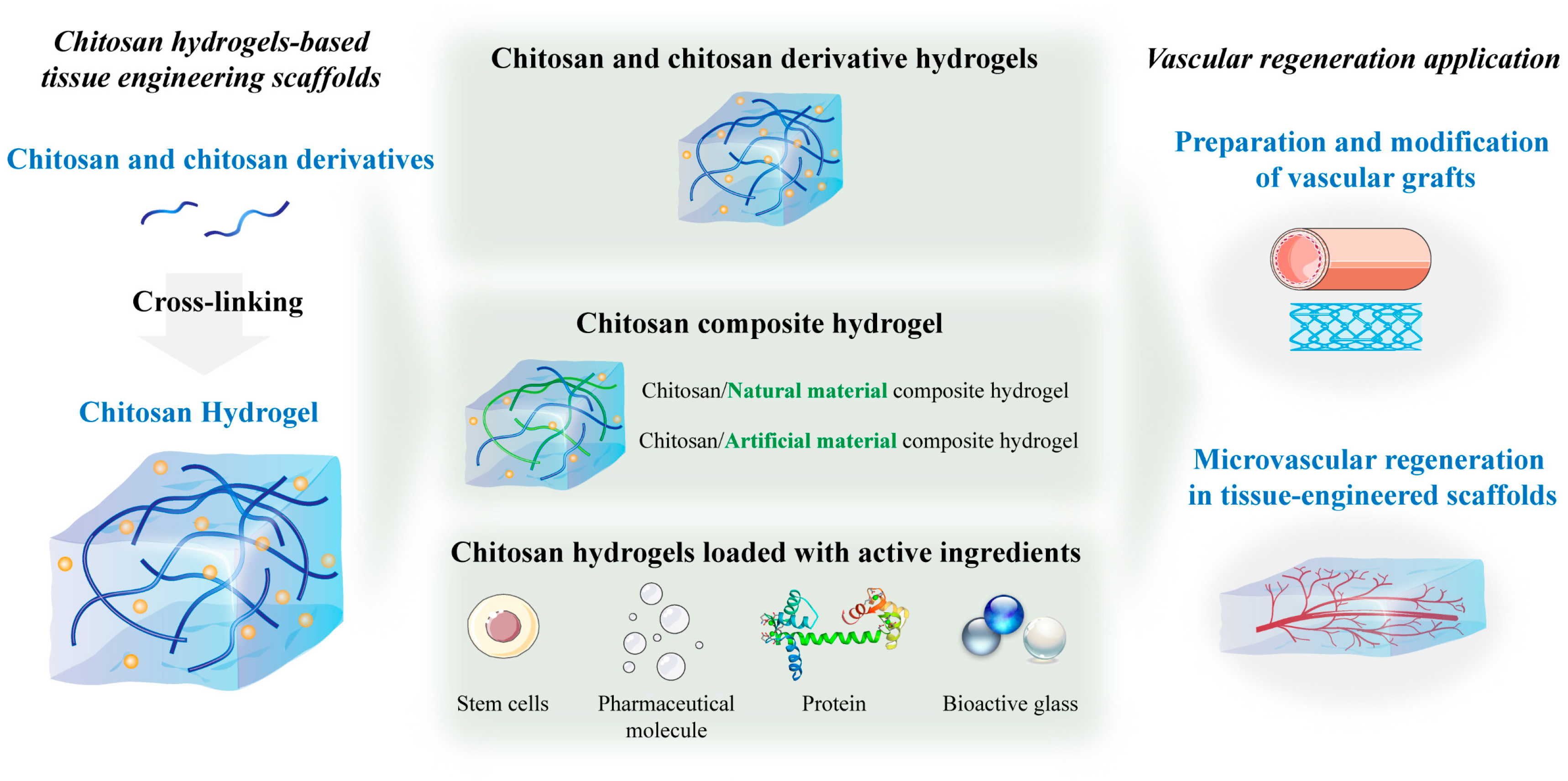
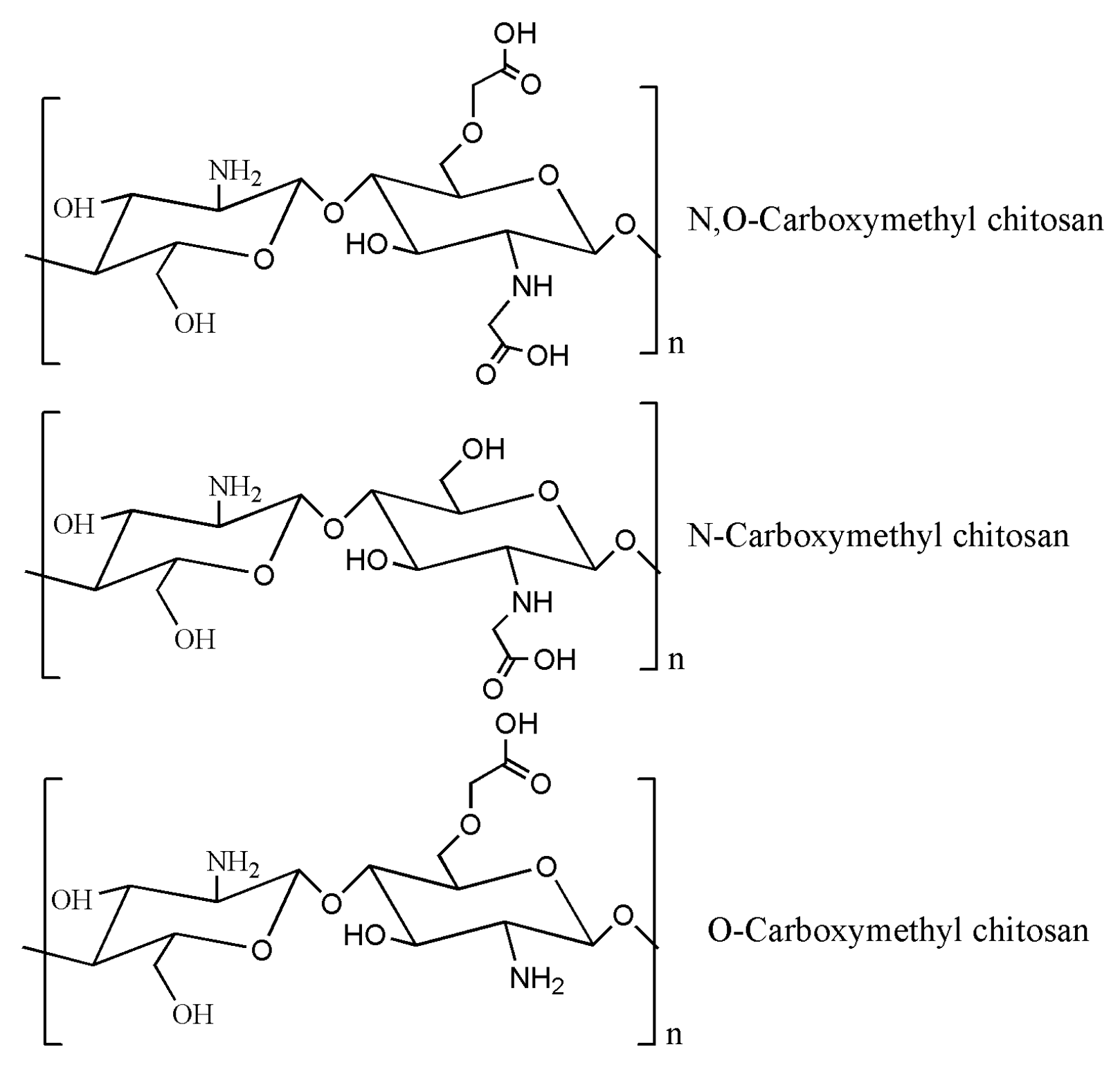
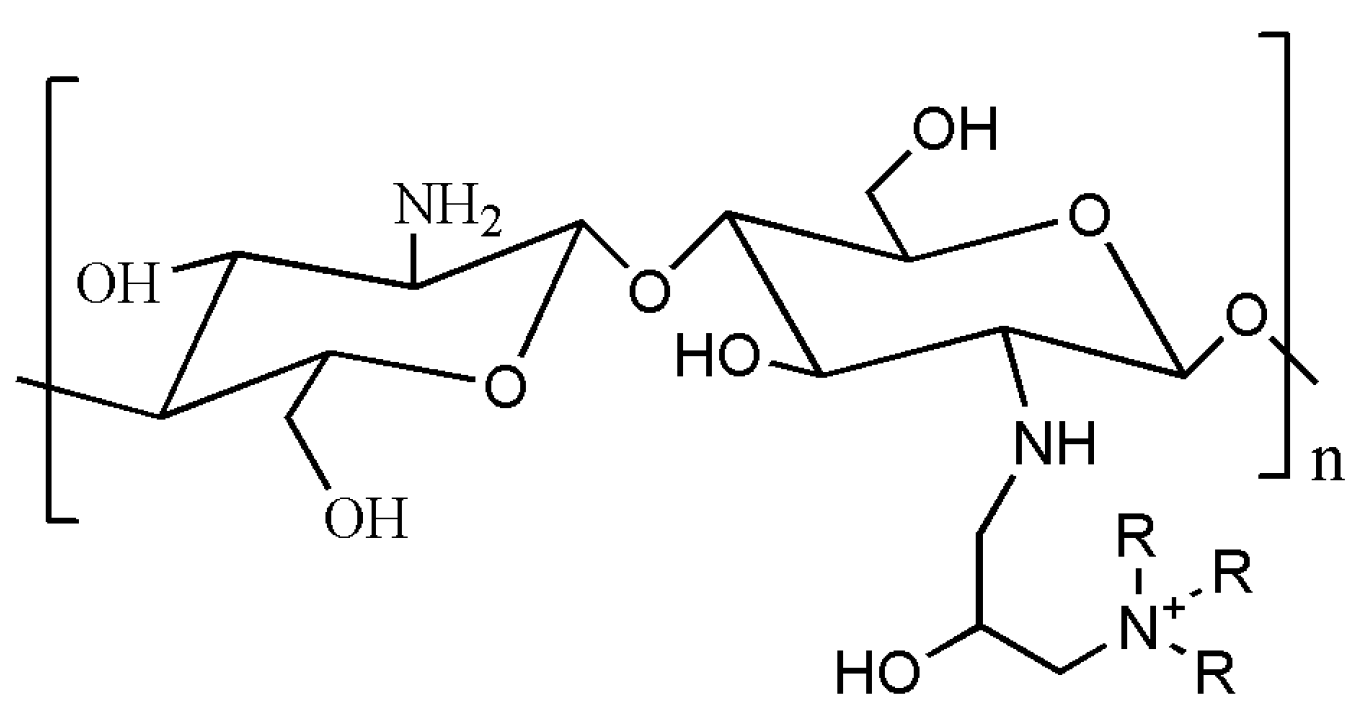
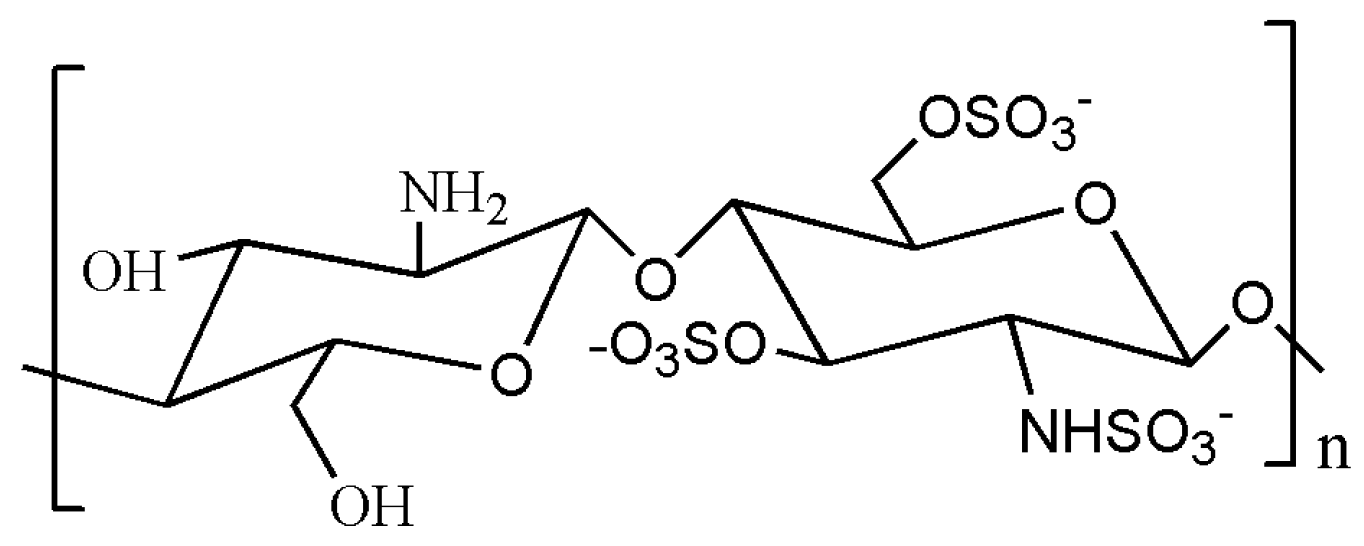
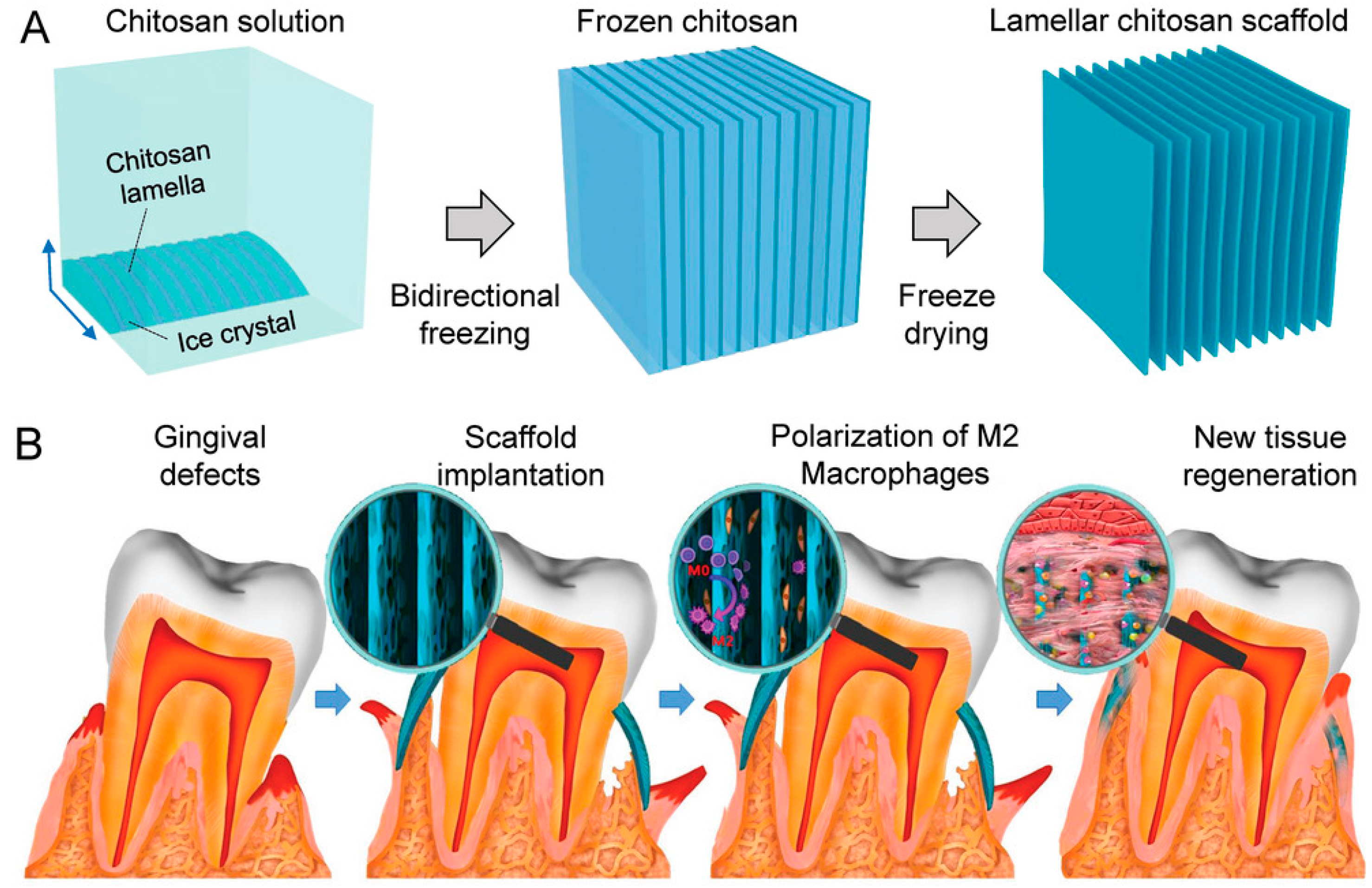
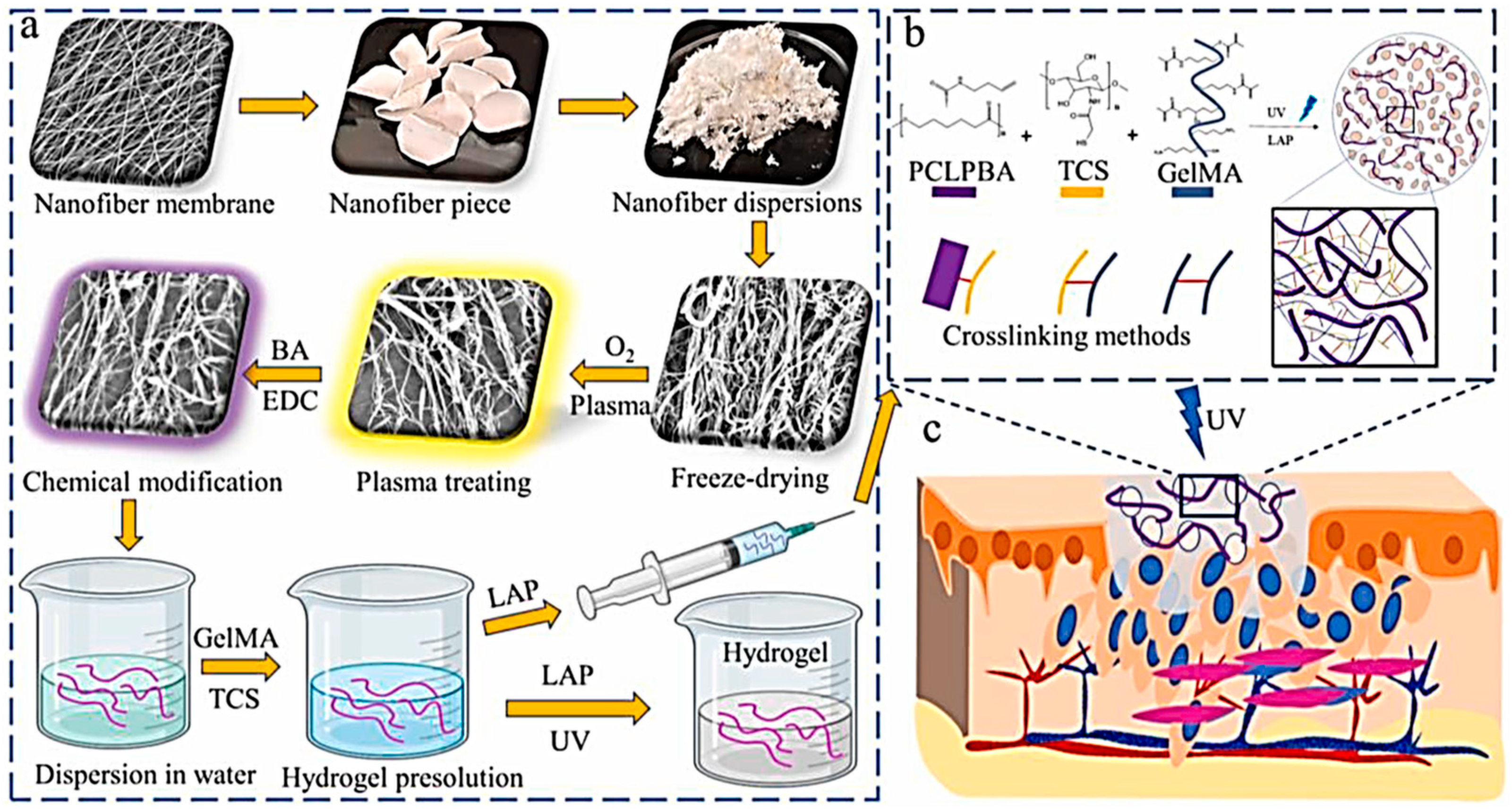
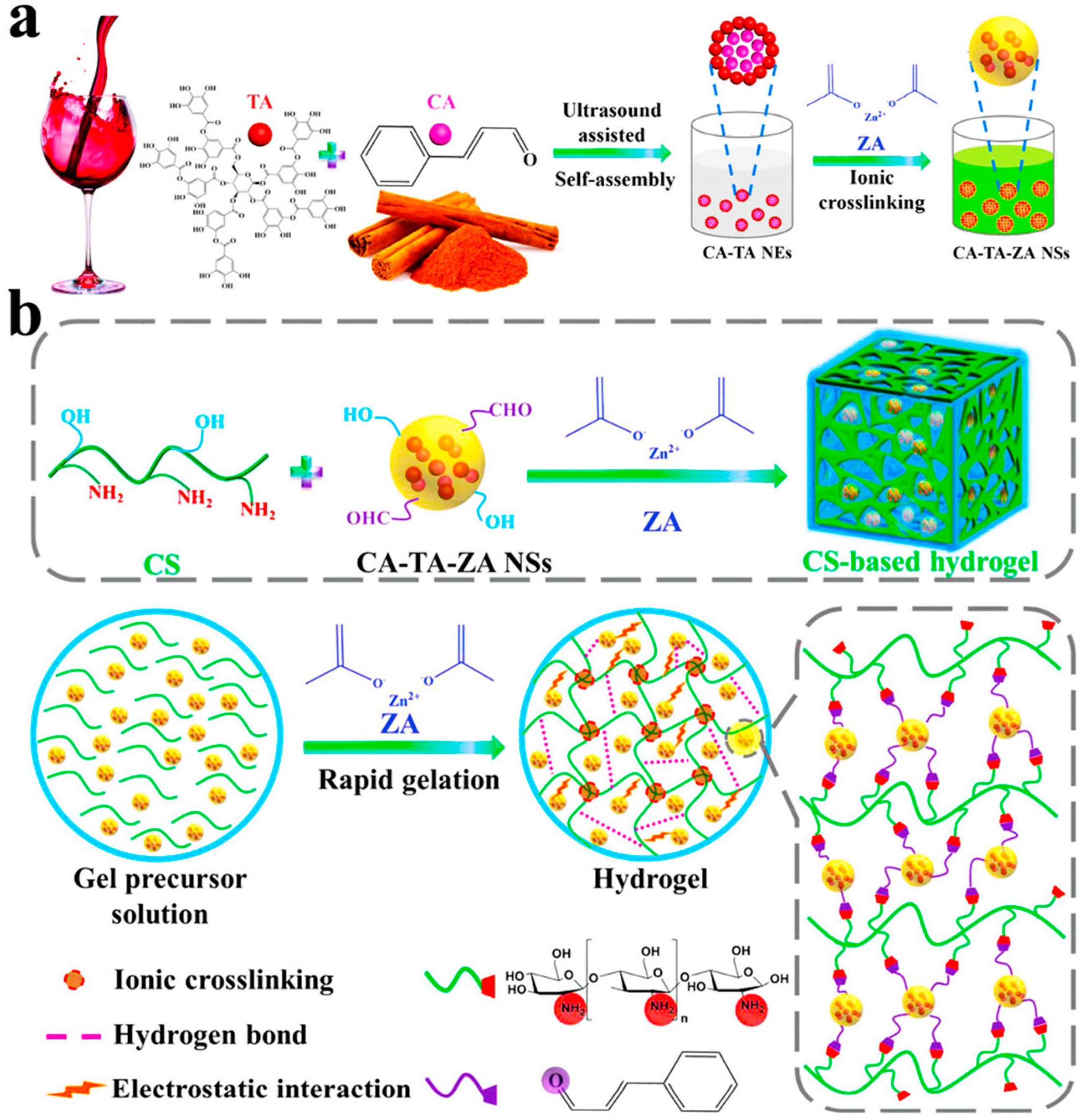
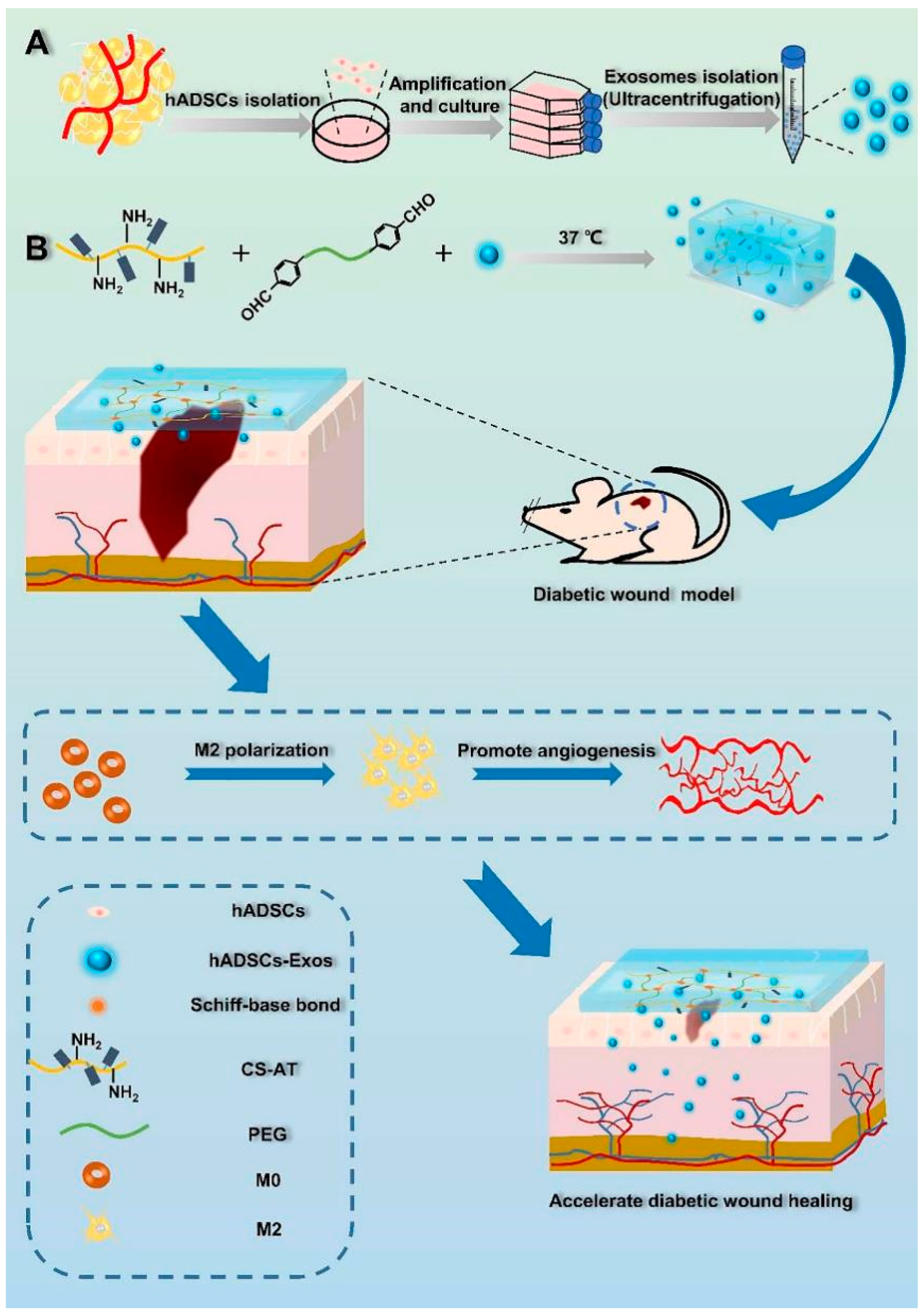
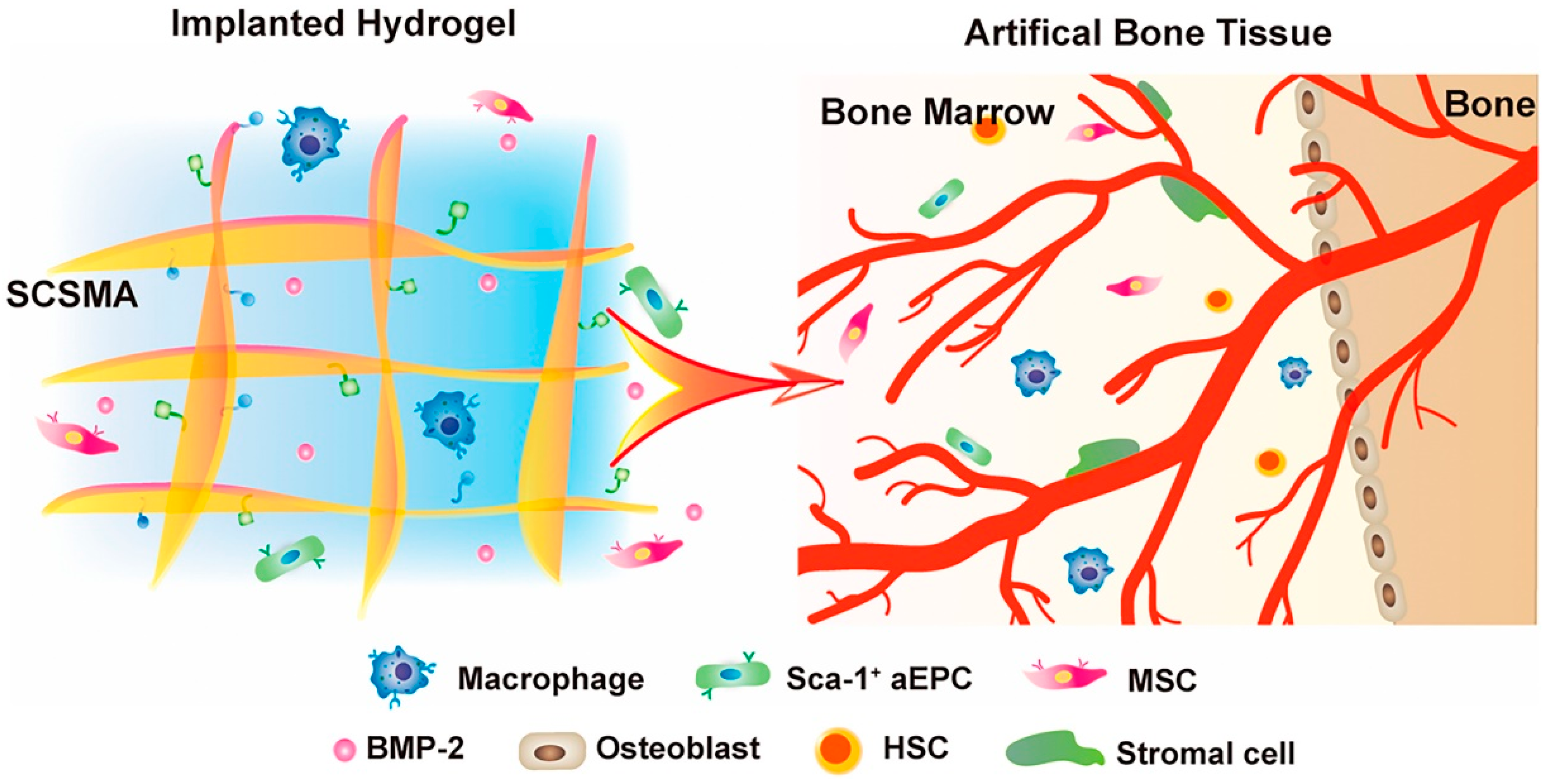
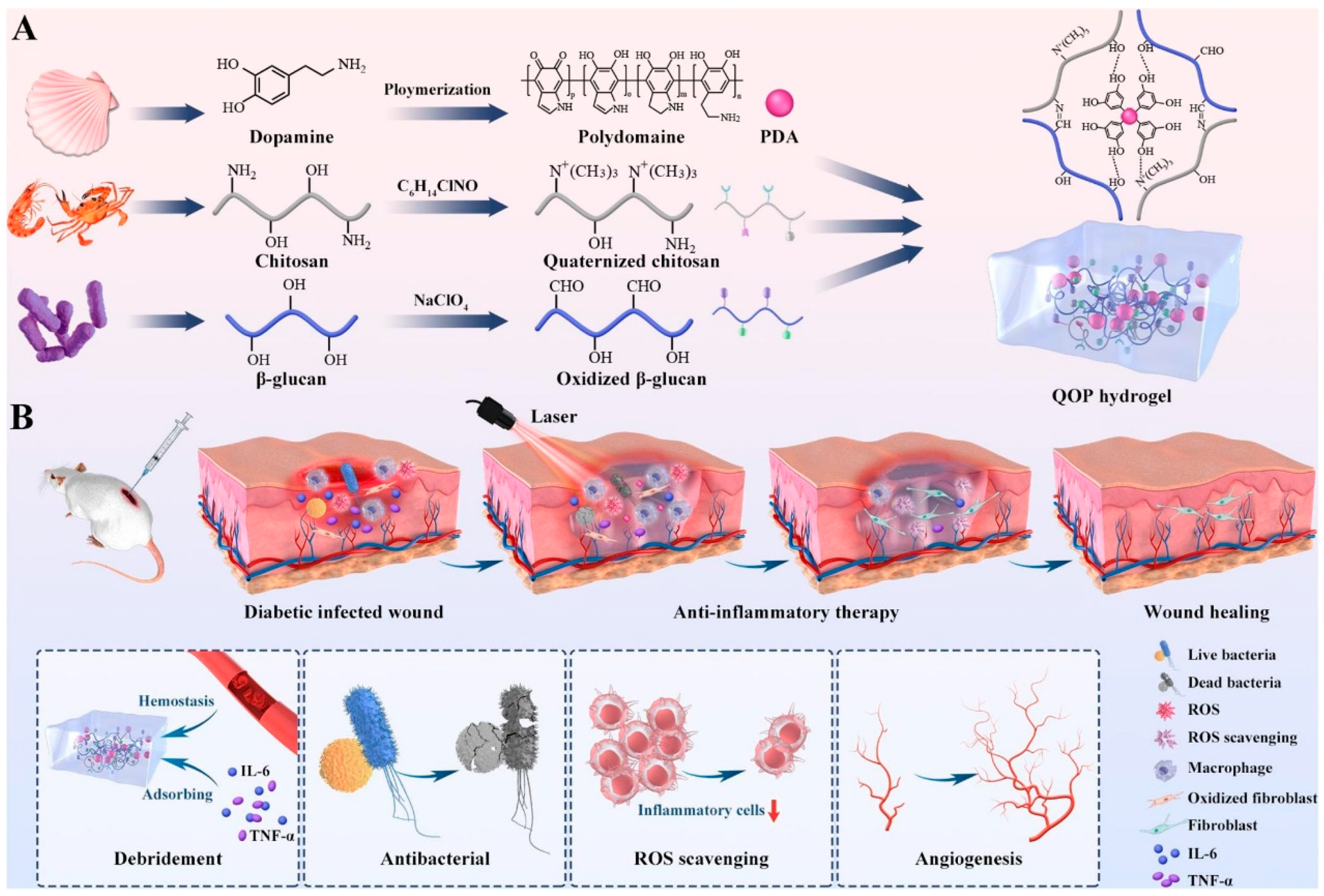
| Technology | Methods/Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Biological coating technology | Plasma spraying Chemical deposition Biomimetic mineralization Laser cladding Electrochemical deposition layer-by-layer self-assembly; .etc | High repeatability; Controlled process; Simple process; High deposition efficiency. | Unstable coating uniformity; Low bond strength. | [53,58,61] |
| Electrospinning technology | Electrostatic atomization of polymeric fluids for jet spinning in a strong electric field | For the repair of tissues and organs; Small pore size; High porosity; Good fibre homogeneity. | Fibre structure regulation; Low spinning efficiency; Limited variety of raw materials. | [70,76,80] |
| 3D printing technology | Based on digital model files Using bondable materials Constructs objects by printing layer by layer | Save material; High precision; High level of complexity; Distributed production; Low cost. | Difficult to produce on a large scale; Limited variety of materials; Equipment maintenance problems. | [83,84] |
| Injectable scaffolds | Formation of hydrogel materials by physical/chemical crosslinking | Good biocompatibility; Protects cells; Easy to modify. | Poor mechanical properties; Difficult to disinfect. | [87,90,92,96] |
| Particle to pore | Solution pouring/particle leaching | Pore size/porosity can be adjusted independently; Wide applicability; Modifiable solid core surface. | The scaffold is brittle; Pore-forming agents tend to remain/agglomerate; Organic solvents are often used; Inability to apply soft tissue. | [104,105,106] |
| Phase separation/Freeze drying method | Emulsion freeze-drying Solution freeze-drying Hydrogel freeze-drying | The scaffold is elastic; The penetration of the hole is good; Easy to introduce active substances; Achieving controlled microarchitectures; Easy to produce industrially. | Not easy preparation of large diameter stents; Avoid high temperature; Large pore size. | [107,108,109,110] |
| Gas foaming | Physical foaming method Chemical foaming method | No organic solvents are used (Physical); The reaction temperature is low (Physical); High porosity (Chemical); Good connectivity (Chemical). | Uncontrolled porosity and pore size (Physical); Low connectivity rate (Physical); Blowing agent requirements are high (Chemical); Closed porous structure. | [111,112] |
| Microspheres aggregation | Through heat treatment, the contact of the microspheres is linked together by chain motion | Good hole connection; Aperture easy to control; Controlled release; Regulation of drug release rate. | Small aperture; Low porosity. | [113] |
| Type | Technology | Combining Materials | References | |
|---|---|---|---|---|
| Chitosan and its derivative hydrogels | Physical and chemical crosslinking methods | - | [187,188,189,190,191] | |
| Domain modification | - | [192,193,194] | ||
| 3D printing | - | [195,196,197,198,199,200] | ||
| Electrospinning and freeze-drying methods | Silk fibroin fiber; heparinized thermoplastic polyurethane co-electrospun fiber | [201] | ||
| Chitosan composite hydrogels | - | Natural materials | β-glycerophosphates (β-GP) | [208] |
| Fibrin | [207,209] | |||
| κ-carrageenan | [211] | |||
| Sulfonated sodium alginate | [212] | |||
| - | Artificial materials | Polyvinyl alcohol (PVA) | [187] | |
| Polydopamine modified carbon nanotubes | [214] | |||
| Hyaluronate-dopamine | [210] | |||
| Gelatin-methacryloyl (GelMA); 3-buten-1-amine (BA) | [215] | |||
| Poly-hedral oligomeric silsesquioxane (POSS) | [219] | |||
| Poly(ethylene glycol) diacrylate (PEGDA) | [220] | |||
| Aminopropyl triethoxy silane (APTES) | [221] | |||
| Polyeth-ylene glycol star octa-armed polyhedron oligosiloxane (POSS-PEG-CHO) | [222] | |||
| microfibrillated cellulose (MFC) | [223] | |||
| Chitosan hydrogels loaded with active ingredients | - | Genes | endothelial nitric oxide synthase(eNOS) | [225] |
| ZNF580 | [225,226,227,228] | |||
| Matrix metalloproteinase (MMP) | [237] | |||
| - | Pharmaceutical molecules | CO | [234] | |
| Thyroxine | [244] | |||
| Heparin | [150] | |||
| Cinnamaldehyde-tannic acid-zinc ace-tate nanospheres | [245] | |||
| Deoxyferriox-amine (DFO) | [246] | |||
| 1-phosphosphingosin (S1P) | [247] | |||
| Amphiphilic Pluronic F127 molecules | [248] | |||
| - | Stem cells | Mesenchymal stem cells (MSCs) | [253] | |
| Human placenta derived MSC (hP-MSCs) | [249] | |||
| Adipose-derived stem cells (ASCs) | [250] | |||
| Human amniotic stem cells (HAM-SCs) | [251] | |||
| Neural stem cells (NSCs) | [252] | |||
| - | Stem cell exosomes (derived from) | bone mesenchymal stem cells | [254,257] | |
| Human placenta derived MSC (hP-MSCs) | [255] | |||
| Human adipose-derived mesenchymal stem cells | [256] | |||
| - | Proteins | Bone morphogenetic protein-2 (BMP-2) | [258] | |
| LL-37 peptide | [259] | |||
| Substance P (SP) | [260] | |||
| Arginine | [261] | |||
| Glucose oxidase (GOx) | [262] | |||
| Collagen type I | [263] | |||
| - | Bioactive glass | [264,265,266] | ||
| - | Others | Ferromagnesium | [267] | |
| Mg2+ | [268,269] | |||
| Polydopamine nanoparticles (PDA NPs) | [270] | |||
| Hydrogen peroxide (H2O2) | [271] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373. https://doi.org/10.3390/gels9050373
Wang Q, Wang X, Feng Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels. 2023; 9(5):373. https://doi.org/10.3390/gels9050373
Chicago/Turabian StyleWang, Qiulin, Xiaoyu Wang, and Yakai Feng. 2023. "Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications" Gels 9, no. 5: 373. https://doi.org/10.3390/gels9050373
APA StyleWang, Q., Wang, X., & Feng, Y. (2023). Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels, 9(5), 373. https://doi.org/10.3390/gels9050373