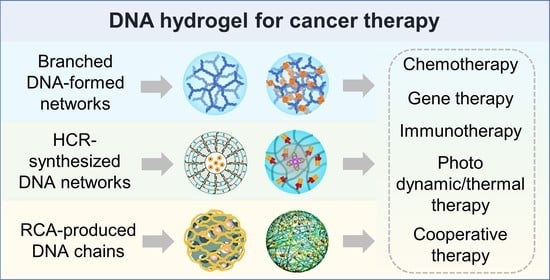
Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy
Abstract
1. Introduction
2. The Preparation and Drug Delivery Applications of DNA Hydrogels
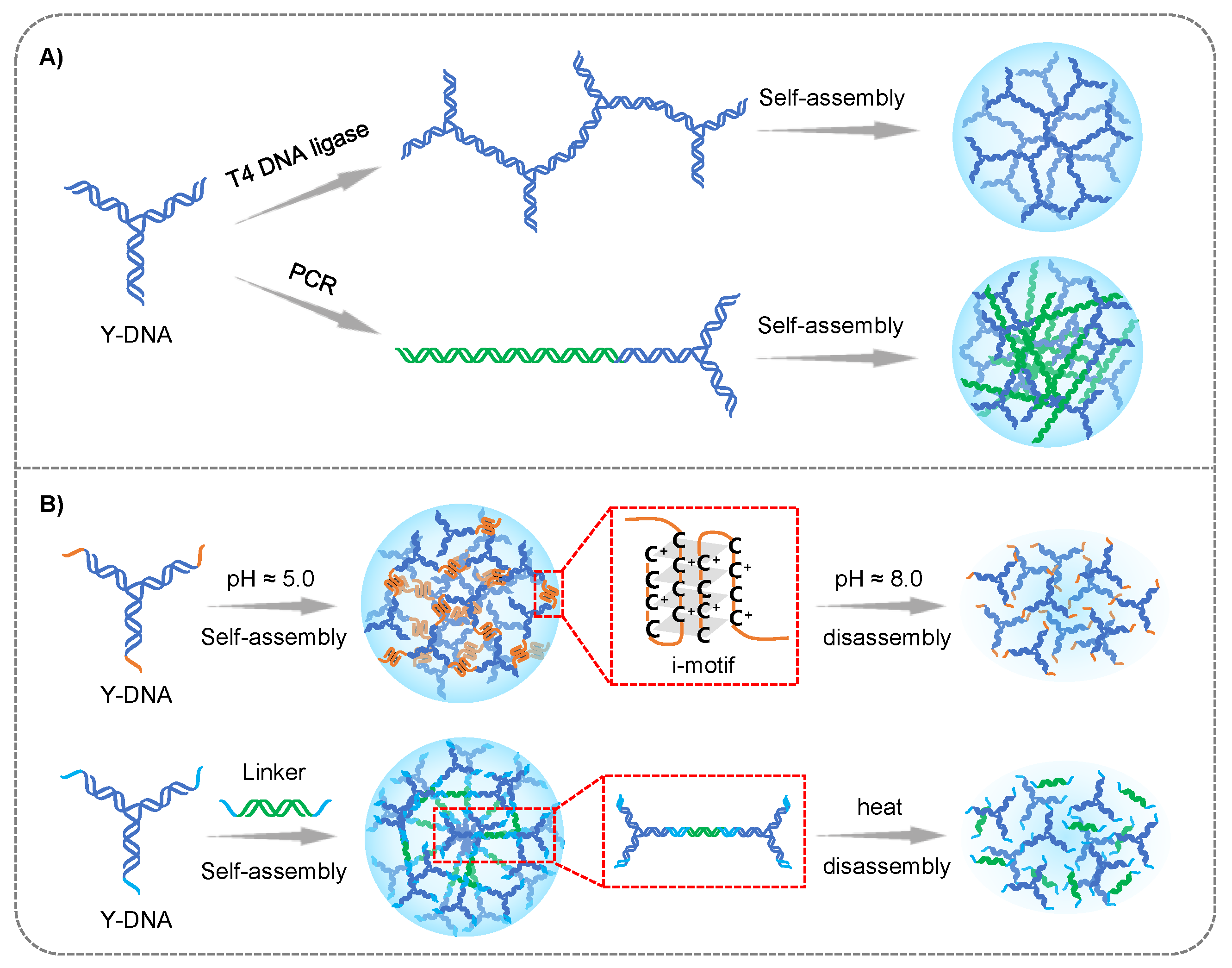
2.1. Branched DNA Formed Hydrogel
2.1.1. Branched DNA
2.1.2. Application in Cancer Therapy
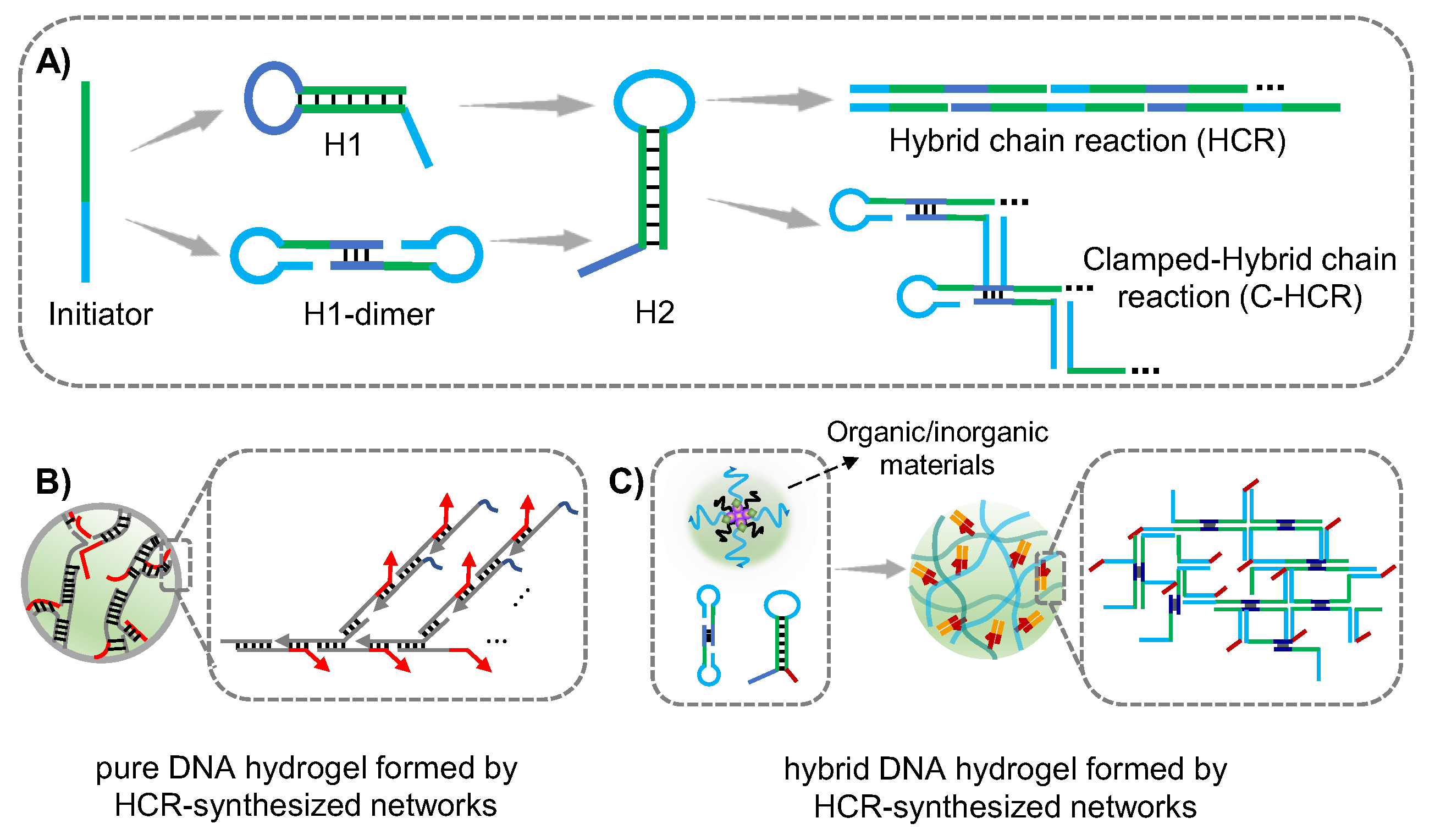
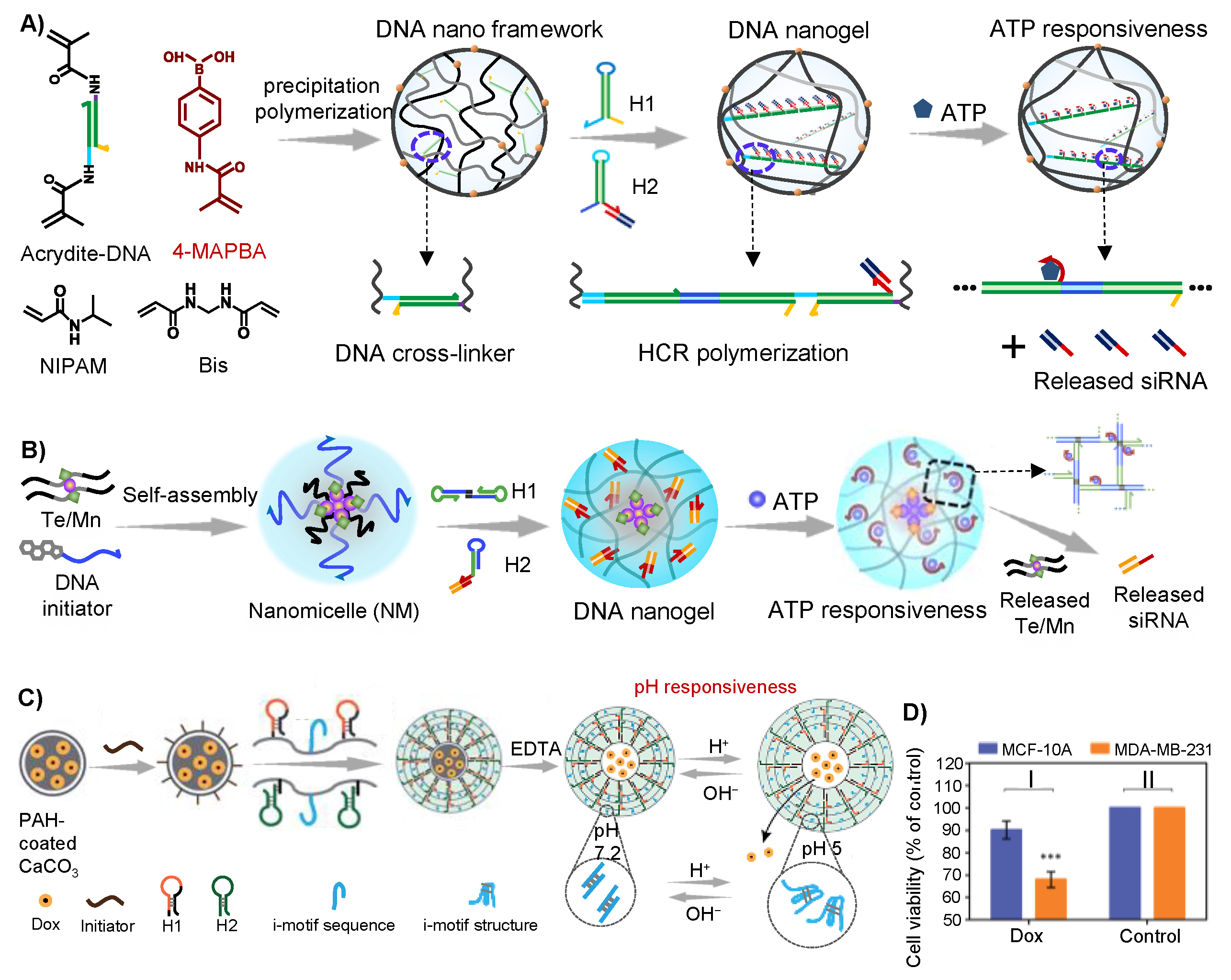
2.2. HCR-Synthesized DNA Networks Formed Hydrogel
2.2.1. HCR-Synthesized DNA Networks
2.2.2. Application in Cancer Therapy
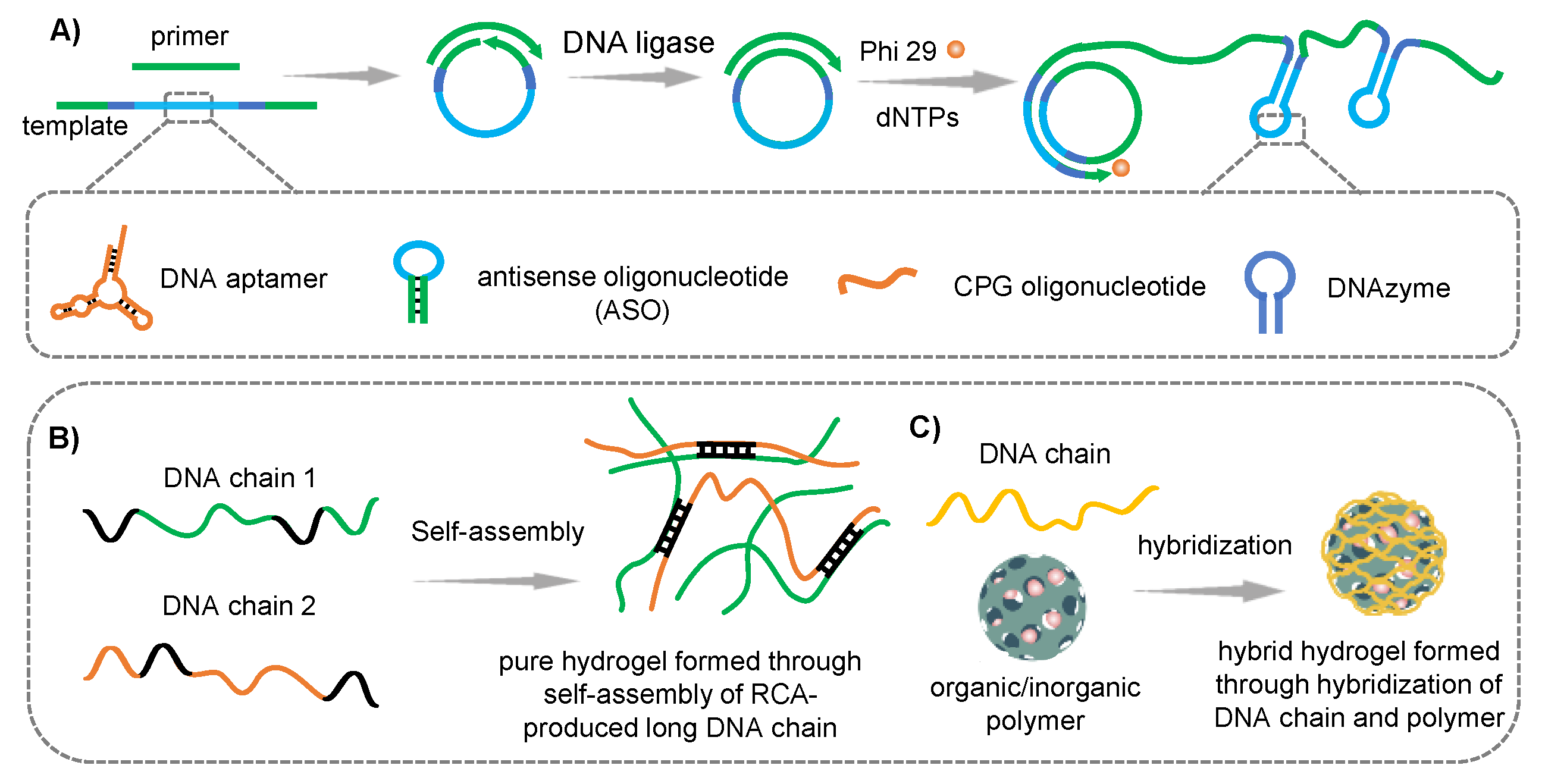
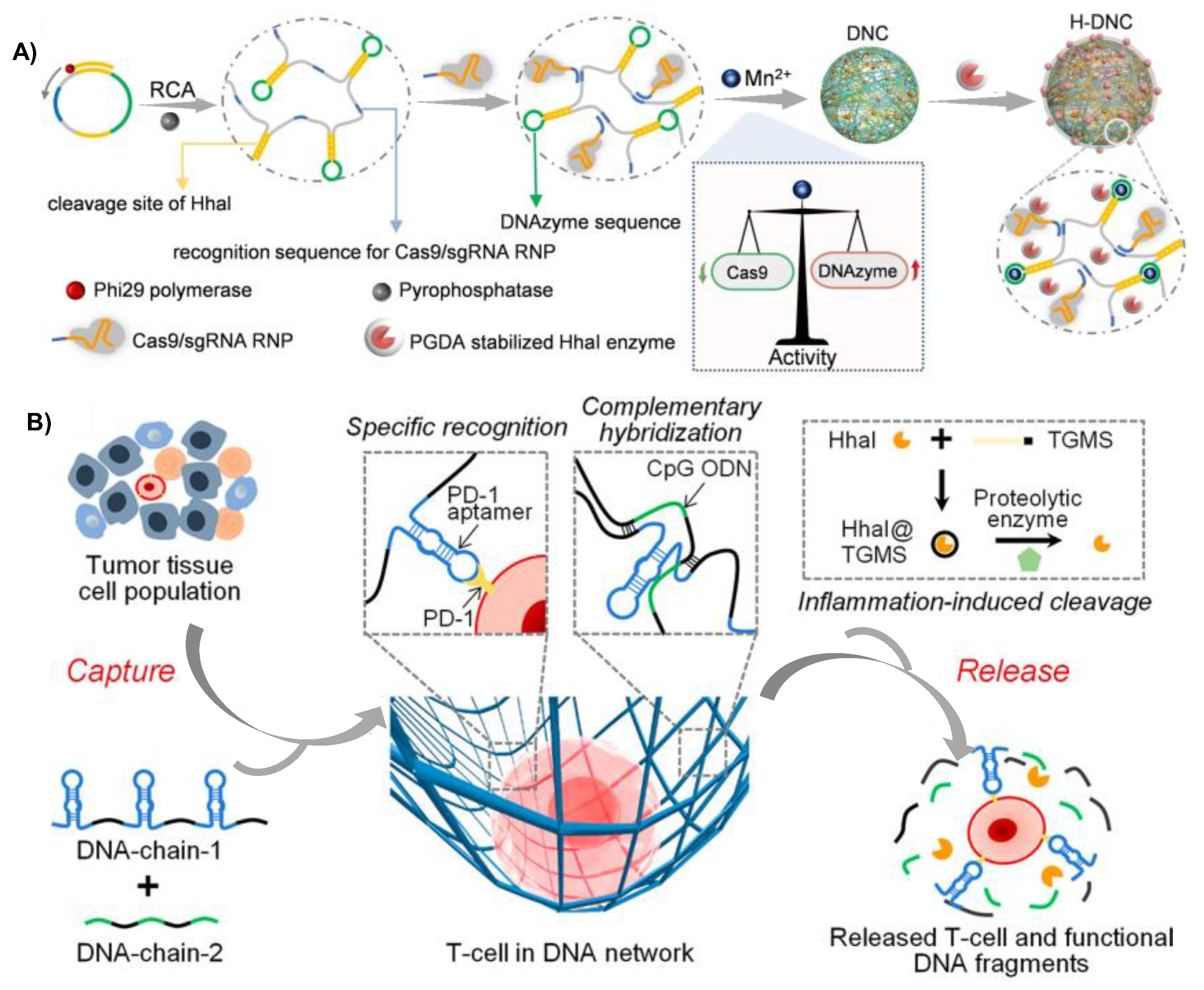
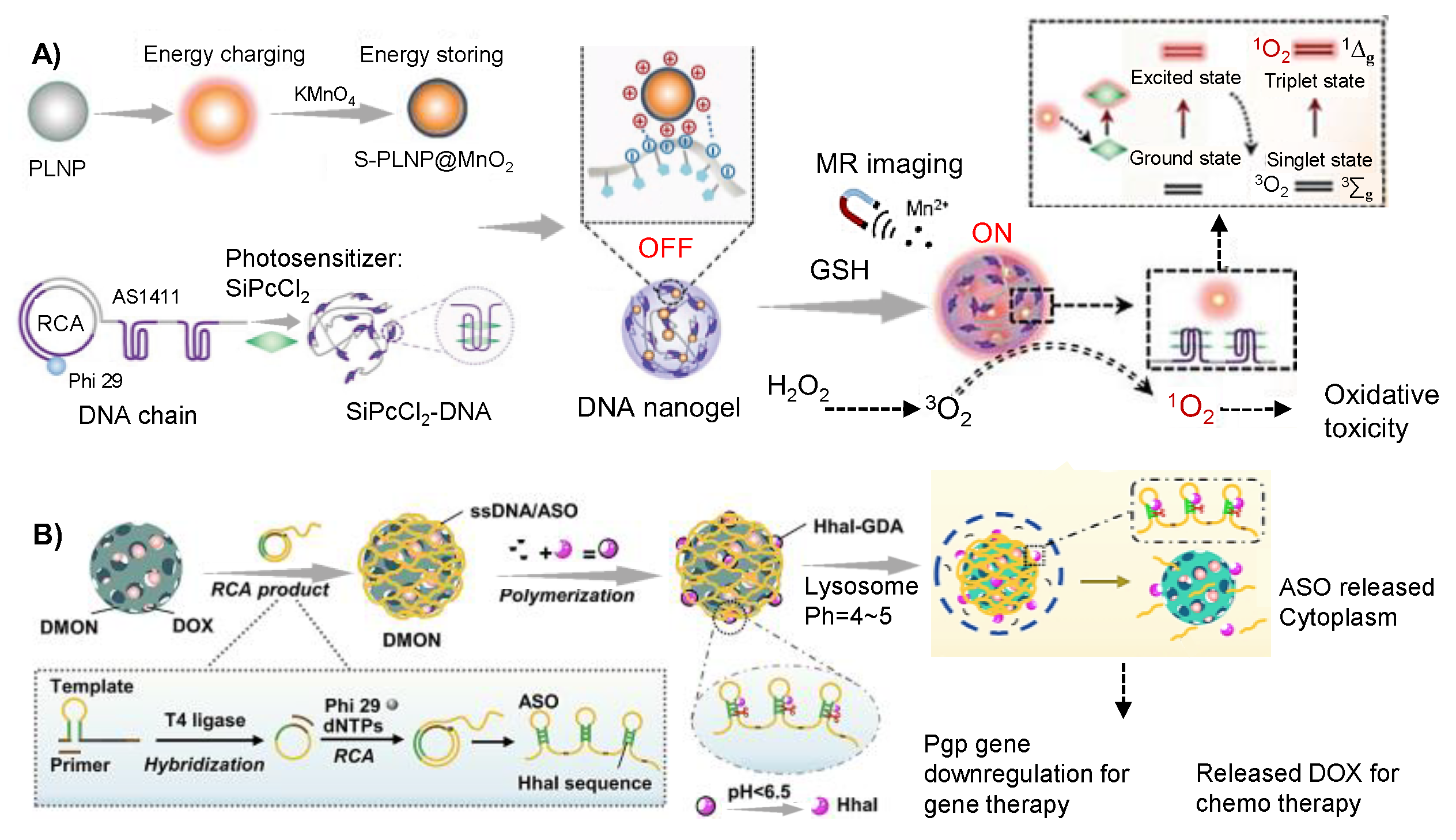
2.3. RCA-Produced DNA Chain-Based Hydrogel
2.3.1. RCA-Produced DNA Chain
2.3.2. Application in Cancer Therapy
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Delivery. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Jen, A.C.; Wake, M.C.; Mikos, A.G. Review: Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996, 50, 357–364. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Diaz, A.; Puiggali, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Seliktar, D.; Black, R.A.; Vito, R.P.; Nerem, R.M. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann. Biomed. Eng. 2000, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Rouabhia, M.; Goulet, F.; Berthod, F.; Moulin, V.; Germain, L. Tissue-engineered human skin substitutes developed from collagen-populated hydrated gels: Clinical and fundamental applications. Med. Biol. Eng. Comput. 1998, 36, 801–812. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater 2019, 8, e1900670. [Google Scholar] [CrossRef]
- Sanmartín-Masiá, E.; Poveda-Reyes, S.; Gallego Ferrer, G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int. J. Polym. Mater. Po. 2016, 66, 280–288. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Sayed, A.Z.; Abd El-Wahab, H.; Sayah, M.M. Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int. J. Biol. Macromol. 2020, 159, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.; Elkony, A.M.; El-Bahy, S.M. Methylene blue uptake by gum arabic/acrylic amide/3-allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt semi-IPN hydrogel. Int. J. Biol. Macromol. 2021, 186, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yi, H. Facile Micromolding-Based Fabrication of Biopolymeric-Synthetic Hydrogel Microspheres with Controlled Structures for Improved Protein Conjugation. Chem. Mater. 2015, 27, 3988–3998. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Abdel Hai, F.; Abd El-Wahab, H.; Aboelanin, H. Methylene blue removal using a novel hydrogel containing 3-Allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt. Adv. Polym. Technol. 2018, 37, 3561–3573. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Ende, M.T.A.; Peppas, N.A. Analysis of drug distribution in hydrogels using fourier transform infrared microscopy. Pharm. Res. 1995, 12, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Stile, R.A.; Burghardt, W.R.; Healy, K.E. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide)-Based Hydrogels That Support Tissue Formation in Vitro. Macromolecules 1999, 32, 7370–7379. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci., Pure Appl. Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Serpe, M.J. Stimuli-Responsive Assemblies for Sensing Applications. Gels 2016, 2, 8. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Hu, S.X.; Lv, Z.Y.; Lv, J.G.; Yu, W.T.; Xu, X.H.; Yang, D.Y. Bioinspired Mechanically Responsive Hydrogel upon Redox Mediated by Dynamic Coordination between Telluroether and Platinum Ions. Chem. Mater. 2020, 32, 2156–2165. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef] [PubMed]
- Lungu, R.; Paun, M.A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.V.; Paun, V.A.; Paun, V.P. Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels 2022, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Shao, Y.; Zhou, X.; Liu, Q.; Zhu, Z.; Zhou, B.; Dong, Y.; Stephanopoulos, N.; Gui, S.; Yan, H.; et al. Highly Permeable DNA Supramolecular Hydrogel Promotes Neurogenesis and Functional Recovery after Completely Transected Spinal Cord Injury. Adv. Mater. 2021, 33, e2102428. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.M.; Zhou, W.; Chen, Z.Z.; Hu, Y.; Yang, Y.; Zhang, G.Z.; Yang, Z.H. A Supramolecular Hydrogel Enabled by the Synergy of Hydrophobic Interaction and Quadruple Hydrogen Bonding. Gels 2022, 8, 244. [Google Scholar] [CrossRef]
- Park, N.; Um, S.H.; Funabashi, H.; Xu, J.; Luo, D. A cell-free protein-producing gel. Nat. Mater. 2009, 8, 432–437. [Google Scholar] [CrossRef]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA hydrogel-based gene editing and drug delivery systems. Adv. Drug Delivery. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef]
- Nagahara, S.; Matsuda, T. Hydrogel formation via hybridization of oligonucleotides derivatized in water-soluble vinyl polymers. Polym. Gels Netw. 1996, 4, 111–127. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yao, C.; Zhu, Y.; Yang, L.; Luo, D.; Yang, D. DNA Functional Materials Assembled from Branched DNA: Design, Synthesis, and Applications. Chem. Rev. 2020, 120, 9420–9481. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Ullyot, G.E.; Ullyot, B.H.; Slater, L.B. The metamorphosis of smith-kline & french laboratories to smith kline beecham: 1925–1998. Bull Hist Chem 2000, 25, 16–21. [Google Scholar]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.J.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Davis, F.F. Commentary—The origin of pegnology. Adv. Drug Deliv. Rev. 2002, 54, 457–458. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Neerooa, B.; Ooi, L.T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels 2021, 7, 60. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Hajikhani, M.; Nazari, L.; Aghelinejad, A.; Shahrousvand, M.; Irani, M.; Rostami, A. Preparation of colloidal nanoparticles PVA-PHEMA from hydrolysis of copolymers of PVAc-PHEMA as anticancer drug carriers. Nanotechnology 2022, 33, 14. [Google Scholar] [CrossRef]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Tennikova, T. Nanogels Capable of Triggered Release. In Tunable Hydrogels: Smart Materials for Biomedical Applications; Lavrentieva, A., Pepelanova, I., Seliktar, D., Eds.; Advances in Biochemical Engineering-Biotechnology; Springer International Publishing Ag: Cham, Switzerland, 2021; Volume 178, pp. 99–146. [Google Scholar]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Y.; Zhang, Z.; Huang, H.; Xu, Y.; Shen, F.; Wang, L.; Sun, L. Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels 2022, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiao, X.; Liu, S.; Hao, M.; Cheng, S.; Zhang, P.; Wen, Y. Functional DNA-based hydrogel intelligent materials for biomedical applications. J. Mater. Chem. B 2020, 8, 1991–2009. [Google Scholar] [CrossRef]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.C.; Xu, J.H.; Cui, C.; Liu, Y.; Hou, W.J.; Wang, Y.Y.; Zhang, L.Q.; et al. Self-assembly of DNA Nanohydrogels with Controllable Size and Stimuli-Responsive Property for Targeted Gene Regulation Therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Z.Y.; Wang, Y.; Zhang, B.D.; Liu, D.; Li, Y.M. Designable Immune Therapeutical Vaccine System Based on DNA Supramolecular Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 9310–9314. [Google Scholar] [CrossRef]
- Song, J.; Lee, M.; Kim, T.; Na, J.; Jung, Y.; Jung, G.Y.; Kim, S.; Park, N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018, 9, 4331. [Google Scholar] [CrossRef]
- Song, J.; Im, K.; Hwang, S.; Hur, J.; Nam, J.; Ahn, G.O.; Hwang, S.; Kim, S.; Park, N. DNA hydrogel delivery vehicle for light-triggered and synergistic cancer therapy. Nanoscale 2015, 7, 9433–9437. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Sun, J.; Ouyang, J.; Na, N. SiRNA-templated 3D framework nucleic acids for chemotactic recognition, and programmable and visualized precise delivery for synergistic cancer therapy. Chem. Sci. 2021, 12, 15353–15361. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lai, W.; Wang, F.; Li, L.; Fan, C.; Pei, H. Programming Drug Delivery Kinetics for Active Burst Release with DNA Toehold Switches. J. Am. Chem. Soc. 2019, 141, 20354–20364. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lv, Z.; Zhang, X.; Dong, Y.; Ding, X.; Li, Z.; Li, S.; Yao, C.; Yang, D. Supramolecular Self-Assembled DNA Nanosystem for Synergistic Chemical and Gene Regulations on Cancer Cells. Angew. Chem. Int. Ed. 2021, 60, 25557–25566. [Google Scholar] [CrossRef]
- Li, F.; Yu, W.; Zhang, J.; Dong, Y.; Ding, X.; Ruan, X.; Gu, Z.; Yang, D. Spatiotemporally programmable cascade hybridization of hairpin DNA in polymeric nanoframework for precise siRNA delivery. Nat. Commun. 2021, 12, 1138. [Google Scholar] [CrossRef]
- Liao, W.C.; Lilienthal, S.; Kahn, J.S.; Riutin, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017, 8, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, T.; Lu, Y.; Reiff, M.; Mo, R.; Gu, Z. Cocoon-like self-degradable DNA nanoclew for anticancer drug delivery. J. Am. Chem. Soc. 2014, 136, 14722–14725. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, N.; Dong, Y.; Li, S.; Li, L.; Liu, Y.; Li, Z.; Yang, D. A Proton-Activatable DNA-Based Nanosystem Enables Co-Delivery of CRISPR/Cas9 and DNAzyme for Combined Gene Therapy. Angew. Chem. Int. Ed. 2022, 61, e202116569. [Google Scholar] [CrossRef]
- Yao, C.; Zhu, C.; Tang, J.; Ou, J.; Zhang, R.; Yang, D. T Lymphocyte-Captured DNA Network for Localized Immunotherapy. J. Am. Chem. Soc. 2021, 143, 19330–19340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Li, F.; Liu, C.; Huang, M.; Li, J.; Gao, F.; Ruan, X.; Yang, D. An Energy-Storing DNA-Based Nanocomplex for Laser-Free Photodynamic Therapy. Adv. Mater. 2022, 34, e2109920. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Tong, Z.; Zhang, R.; Yao, C.; Yang, D. Spatio-Temporal Controlled Gene-Chemo Drug Delivery in a DNA Nanocomplex to Overcome Multidrug Resistance of Cancer Cells. ACS Appl. Bio. Mater. 2022, 5, 3795–3805. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dong, Y.; Yao, C.; Yang, D. Construction of Branched DNA-based Nanostructures for Diagnosis, Therapeutics and Protein Engineering. Chem. Asian J. 2022, 17, e202200310. [Google Scholar] [CrossRef]
- Campolongo, M.J.; Tan, S.J.; Xu, J.; Luo, D. DNA nanomedicine: Engineering DNA as a polymer for therapeutic and diagnostic applications. Adv. Drug. Deliv. Rev. 2010, 62, 606–616. [Google Scholar] [CrossRef]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef]
- Hartman, M.R.; Yang, D.; Tran, T.N.; Lee, K.; Kahn, J.S.; Kiatwuthinon, P.; Yancey, K.G.; Trotsenko, O.; Minko, S.; Luo, D. Thermostable branched DNA nanostructures as modular primers for polymerase chain reaction. Angew. Chem. Int. Ed. 2013, 52, 8699–8702. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, L.; Fan, Q.; Liu, D. A pH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Ohtsuki, S.; Araie, Y.; Umeki, Y.; Endo, M.; Emura, T.; Hidaka, K.; Sugiyama, H.; Takahashi, Y.; Takakura, Y.; et al. Self-assembling DNA hydrogel-based delivery of immunoinhibitory nucleic acids to immune cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 123–130. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Pan, G.; Wang, P.; Li, Y.; Zhu, X.; Zhang, C. Injectable Drug-Conjugated DNA Hydrogel for Local Chemotherapy to Prevent Tumor Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 21441–21449. [Google Scholar] [CrossRef]
- Song, W.; Song, P.; Sun, Y.; Zhang, Z.; Zhou, H.; Zhang, X.; He, P. Self-Assembly of Multifunctional DNA Nanohydrogels with Tumor Microenvironment-Responsive Cascade Reactions for Cooperative Cancer Therapy. ACS Biomater. Sci. Eng. 2021, 7, 5165–5174. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, Z.; Wang, Y.; Zou, J.; Lin, Q.; Duan, Y. One-Step Self-Assembly of Multifunctional DNA Nanohydrogels: An Enhanced and Harmless Strategy for Guiding Combined Antitumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 46479–46489. [Google Scholar] [CrossRef]
- Tang, W.; Han, L.; Duan, S.; Lu, X.; Wang, Y.; Wu, X.; Liu, J.; Ding, B. An Aptamer-Modified DNA Tetrahedron-Based Nanogel for Combined Chemo/Gene Therapy of Multidrug-Resistant Tumors. ACS Appl. Bio. Mater. 2021, 4, 7701–7707. [Google Scholar] [CrossRef]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef]
- Lu, Z.; Shi, Y.; Ma, Y.; Jia, B.; Li, X.; Guan, X.; Li, Z. Fast and specific enrichment and quantification of cancer-related exosomes by DNA-nanoweight-assisted centrifugation. Anal. Chem. 2022, 94, 9466–9471. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, L.; Wang, S.; Liu, Y.; Wu, C.; Cui, C.; Sun, H.; Shi, M.; Jiang, Y.; Li, L.; et al. Molecular Recognition-Based DNA Nanoassemblies on the Surfaces of Nanosized Exosomes. J. Am. Chem. Soc. 2017, 139, 5289–5292. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Hu, Q.; Bai, H.; Zhang, S.; Shen, Y.; Tang, G.; Ping, Y. Redox-Activated Light-Up Nanomicelle for Precise Imaging-Guided Cancer Therapy and Real-Time Pharmacokinetic Monitoring. ACS Nano 2016, 10, 11385–11396. [Google Scholar] [CrossRef]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abdullah, R.; Hu, X.; Bai, H.; Fan, H.; He, L.; Liang, H.; Zou, J.; Liu, Y.; Sun, Y.; et al. Engineering of Bioinspired, Size-Controllable, Self-Degradable Cancer-Targeting DNA Nanoflowers via the Incorporation of an Artificial Sandwich Base. J. Am. Chem. Soc. 2019, 141, 4282–4290. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Han, S.; Park, Y.; Kim, H.; Nam, H.; Ko, O.; Lee, J.B. Double Controlled Release of Therapeutic RNA Modules through Injectable DNA-RNA Hybrid Hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 55554–55563. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.; Wright, G.; Wang, A.Z.; Gu, Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016, 28, 8912–8920. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Z.; Zuo, D.; Li, L.; Li, F.; Yang, D. A Synergistic DNA-polydopamine-MnO2 Nanocomplex for Near-Infrared-Light-Powered DNAzyme-Mediated Gene Therapy. Nano Lett. 2021, 21, 5377–5385. [Google Scholar] [CrossRef]


| Type of DNA Hydrogels | Formulation | Strategy Advantages | Strategy Limitations | Delivered Drugs | Application in Cancer Therapy | Ref. |
|---|---|---|---|---|---|---|
| Non-enzyme-mediated branched hydrogel | stimuli responsiveness (pH, temperature) | controllable symmetry; multivalency; enzyme-free | high concentration for preparing hydrogel | ASOs; CPG | immunotherapy | [45,46] |
| Enzyme-mediated branched hydrogel | strand extension (ligase) | controllable symmetry; multivalency; short reaction time | high concentration for preparing hydrogel; high cost | Camptothecin; DOX; transcribed siRNA | chemotherapy; chemo-photo thermal synergistic therapy gene therapy | [47,48] |
| Pure hydrogel formed by HCR-synthesized networks | linear/clamped amplification | isothermal amplification; enzyme-free; convenient operation | high requirements for sequence design; require reaction carrier (AuNP, cell membrane, …) for HCR | DOX; siRNA | chemo-gene synergistic therapy; chemotherapy | [49,50] |
| Hybrid hydrogel formed by HCR-synthesized networks | hybrid with other organic/inorganic material before HCR amplification | isothermal amplification; enzyme-free; high stability | high requirements for sequence design; lower initiation efficiency influenced by complex conformation | siRNA; DOX | gene therapy; chemotherapy | [51,52,53] |
| Pure hydrogel formed by RCA-produced long DNA chain | physical crosslinking after RCA reaction | isothermal amplification; convenient operation | high requirements for sequence (template and primer) design; high cost low stability | DOX; DNAzyme and CRISPR/Cas9 system; siRNA; CPG | chemotherapy; gene therapy; immunotherapy | [54,55,56] |
| Hybrid hydrogel formed by RCA-produced long DNA chain | hybrid with other organic/inorganic material after RCA amplification | isothermal amplification high stability | high requirements for sequence (template and primer) design high cost; complex operation for hybridization | SiPcCl2; DOX; ASOs | gene-photo thermal synergistic therapy; chemo-gene synergistic therapy | [57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Song, W.; Li, F. Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy. Gels 2023, 9, 239. https://doi.org/10.3390/gels9030239
Li J, Song W, Li F. Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy. Gels. 2023; 9(3):239. https://doi.org/10.3390/gels9030239
Chicago/Turabian StyleLi, Jing, Wenzhe Song, and Feng Li. 2023. "Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy" Gels 9, no. 3: 239. https://doi.org/10.3390/gels9030239
APA StyleLi, J., Song, W., & Li, F. (2023). Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy. Gels, 9(3), 239. https://doi.org/10.3390/gels9030239